Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties†
Abstract
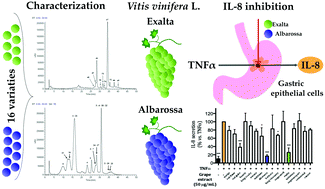
Several studies have shown that fresh grape and its derivatives contain phenolic compounds exhibiting antioxidant and health promoting effects, particularly in relation to the cardiovascular system. In this study, two methods were developed to characterize sixteen varieties of table and wine grapes: (1) a LC-MS method to identify major and minor phenolic compounds; and (2) a HPLC-DAD method to quantify the most representative compounds. Sixty-seven molecules belonging to different classes of phenolic compounds were identified: anthocyanins, flavan-3-ols, flavonols, stilbenes and organic acids. In parallel, the free radical scavenging activity and anti-inflammatory activities of the 16 grape varieties were evaluated. The results showed a good correlation between the total phenolic content and the biological activity. Extracts from Exalta and Albarossa grape varieties were the most active in reducing IL-8 release by gastric epithelial cells (IC50 = 8.48 μg mL−1 and 6.68 μg mL−1, respectively), a biomarker of inflammatory processes. The observed biological activities were mainly associated with skin and seed extracts/portions. The interest in studying table grapes and their non-fermented derivatives as sources of healthy compounds has increased in the last few years and our findings suggest that table grapes and their fresh derivatives, in addition to wine, could be involved in the health promoting effects of the Mediterranean diet.


 Please wait while we load your content...
Please wait while we load your content...