Effects of high pressure homogenization and addition of oil on the carotenoid bioaccessibility of carrot juice
Jianing
Liu
 a,
Jinfeng
Bi
a,
Jinfeng
Bi
 *a,
Xuan
Liu
*a,
Xuan
Liu
 *a,
Baiqing
Zhang
b,
Xinye
Wu
a,
Chandi Kanchana Deepali
Wellala
a and
Biao
Zhang
a
*a,
Baiqing
Zhang
b,
Xinye
Wu
a,
Chandi Kanchana Deepali
Wellala
a and
Biao
Zhang
a
aInstitute of Food Science and Technology, Chinese Academy of Agricultural Sciences (CAAS), Key Laboratory of Agro-Products Processing, Ministry of Agriculture and Rural Affairs, Beijing, China. E-mail: bjfcaas@126.com; liuxuancaas@126.com
bCollege of Food Science, Shenyang Agricultural University, Shenyang, China
First published on 20th December 2018
Abstract
Food processing and dietary lipids are considered as important factors for carotenoid bioaccessibility. The effects of high pressure homogenization (HPH) combined with oil or emulsion on carotenoid retention and bioaccessibility during digestion were investigated. The results illustrated that HPH decreased the area-based diameter (D[3,2]), and negative correlations were found between the total carotenoid bioaccessibility (TCB) and D[3,2] of carrot juice. The bioaccessibility of total carotenoids, β-carotene and α-carotene of the homogenized samples was below 6%, while the addition of 2% oil, 10% oil or emulsion increased the carotenoid bioaccessibility (up to 14.08% for α-carotene). The carotenoid retention rate (CRR) of the homogenized samples was higher than that of the homogenized samples with oil or emulsion in each digestion phase. The CRR in the small intestine phase had a significant negative correlation with TCB, and therefore, a high TCB could be achieved despite a low CRR in the small intestine. Oil added as an emulsion had a slightly higher volume of free fatty acids released compared with oil added as such.
1. Introduction
Carrot is one of the popular vegetables grown throughout the world and may protect human beings against certain types of cancer and cardiovascular disease owing to its high content of carotenoids.1 Carotenoid bioaccessibility (transfer of carotenoids from digestate to micelles) can be defined as the amount of the ingested compound available in the gastrointestinal tract for absorption.2 On account of their lipophilic nature and specific localization in plant-based tissues, carotenoid bioaccessibility is generally quite low in raw fruit and vegetables.3 In carrots, carotenoids are located in the chromoplasts of cells, and as a consequence, their release from food is restricted by the surrounding cell membranes and cell walls, affecting their bioaccessibility.4 Unlike some plants with soft tissues such as mangoes, carrots are composed of tissues with robust cell walls, and therefore, the liberation of carotenoids from the carrot tissue seems to be a major limiting factor for their bioaccessibility.5 However, mastication could not compensate for the low β-carotene bioaccessibility in raw carrots. Mechanical treatments such as juicing and blending, which could disrupt the tissue to small fractions, were essential.6 Carrot juice is one of the main processed products of carrots, and not from concentrate (NFC) carrot juice is becoming increasingly popular, since its taste, flavor and nutrition are close to those of fresh carrots.High pressure homogenization (HPH) is a non-thermal processing technology based on the application of mechanical effects such as high shear, cavitation and turbulence,7 which can be used to enhance the physical stability and inactivate the microorganisms present in fruit and vegetable juice.8,9 As a mechanical treatment, HPH might have the potential to enhance the release of carotenoids from chromoplasts, their solubilization in oil and further delivery. Mutsokoti et al.10 reported that HPH could effectively facilitate the transfer of carotenoids from carrot and tomato-based matrices to the oil phase. Svelander et al.11 reported that HPH didn't affect the carotene retention but increased the micellar incorporation of α-carotene and β-carotene in carrot emulsions. The carotenoid stability throughout the digestion is worth investigating, since carotenoid bioaccessibility and utilization are closely related to carotenoid retention in the small intestine and micelle fractions. Moreover, the effects of HPH on carotenoids also depend on carotenoid speciation. HPH could not increase the lycopene bioaccessibility of tomato emulsions due to the formation of fibrous cell structures which could entrap lycopene.11 Colle et al.12 also proposed that higher homogenization pressure caused the breakdown of cell aggregates in tomatos, but improved the strength of the fiber network, which decreased the lycopene bioaccessibility.
In addition, lipids play an important part in human diet and carotenoids are digested and absorbed along with lipids. Carotenoids need to be released from the food matrix, solubilized into the lipid phase, and incorporated into mixed micelles with certain hydrolysates of lipids before absorption.10,13 Oil is popular in both Oriental and Western diets, and is digested directly or in the form of emulsion and other processed products. In human diet, lipids are mostly present as oil-in-water emulsions, including soups and sauces.14 Adding oil directly or in the form of emulsion might have different influences on carotenoid bioaccessibility. When oil is added directly, it implies that emulsification still has to take place during digestion. When oil is added as an emulsion, the oil droplets and the emulsifier applied are also important factors for the system.3 The addition of 5% lipid before the in vitro digestion of tomato pulp could significantly enhance the lycopene bioaccessibility.15 Lipkie et al.16 reported that increasing the oil percentage contributed to a higher carotenoid bioaccessibility. The fatty acyl chain length also influenced the carotenoid bioaccessibility and long chain triglycerides generally caused a more obvious increment in carotenoid bioaccessibility, while medium chain triglycerides resulted in a less obvious increment in carotenoid bioaccessibility.3 Nevertheless, Colle et al.17 proposed that the type of lipid was of minor significance for the carotenoid bioaccessibility compared to the process applied (HPH and microwave heating).
To our knowledge, studies regarding the effects of HPH combined with oil addition on carotenoid retention and bioaccessibility during digestion are limited; more specifically, the comparisons between oil added as such and oil added as emulsion have not been reported. Therefore, the objective of this study was to investigate the effects of HPH parameters and oil addition on the carotenoid bioaccessibility of carrot juice. Carotenoid concentration in each digestion phase was determined to evaluate the carotenoid retention rate (CRR) of HPH-treated carrot juice. The addition of oil and emulsion was included in this study to analyze the effects of oil forms on the carotenoid stability and bioaccessibility of HPH-treated carrot juice during digestion.
2. Materials and methods
2.1 Materials
Fresh carrots (Daucus carota L.) were purchased from Xiaoying market, Beijing, China and were stored at 4 °C for the experiments. Corn oil was purchased from a local supermarket. Uric acid, sodium salt, sodium DL-lactate, mucin from porcine stomach, pepsin from porcine gastric mucosa (≥250 units per mg), lipase from porcine pancreas (type II), β-carotene and α-carotene (HPLC grade) were purchased from Sigma-Aldrich (St Louis, USA). Methyl-t-butyl-ether (MTBE), methanol (MeOH) and hexane were HPLC grade and were purchased from Fisher Chemical (Whippany, USA). Other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).2.2 Preparation of carrot juice
Carrots were washed, sliced, and blended with distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) for 2 min using a blender (JYL-C51V, Joyoung, China) to obtain crude carrot juice. Then the crude juice was ground using a colloid mill (JM-30A, Langtong, China).
2, w/w) for 2 min using a blender (JYL-C51V, Joyoung, China) to obtain crude carrot juice. Then the crude juice was ground using a colloid mill (JM-30A, Langtong, China).
2.3 HPH treatment
The colloid milled juice was homogenized using a homogenizer (JN-02HC, Guangzhou Juneng, China). A circulating water bath system (HL-01AS, Guangzhou Juneng, China) was connected to the homogenizer to control the temperature. Parameters were as follows: a pressure of 20 MPa, 60 MPa and 180 MPa; pass of 1, 2 and 3, and an inlet temperature of 25 °C, 50 °C and 70 °C.2.4 Addition of oil and emulsion
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min and homogenized at 150 MPa 3 times. The carrot juice was mixed with the prepared emulsion (w/w, 1
000 rpm for 1 min and homogenized at 150 MPa 3 times. The carrot juice was mixed with the prepared emulsion (w/w, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using the Ultra-turrax at 6000 rpm for 5 s before digestion.
1) using the Ultra-turrax at 6000 rpm for 5 s before digestion.
Carrot juice treated with HPH was referred to as HCJ, and the abbreviations of the samples treated with HPH and addition of 2% oil, 10% oil and emulsion were HCJ-2, HCJ-10 and HCJ-E, respectively. The corresponding non-homogenized carrot juice, and non-homogenized carrot juice with the addition of 2% oil, 10% oil and emulsion were referred to as NHCJ, NHCJ-2, NHCJ-10 and NHCJ-E, respectively.
2.5 ζ-Potential measurement
The particle charge (ζ-potential) was measured using a Zetasizer (Nano ZS, Malvern, UK). Homogenized samples were diluted with 10 mM phosphate buffer (pH 6.5). Samples after mouth, stomach and intestine phases were diluted with 10 mM phosphate buffer at pH values of 6.8, 2.5 and 7.0, respectively.2.6 Particle size measurement
The particle size parameter was measured as the samples passed through different phases of in vitro digestion using a laser particle size analyzer (S3500, Microtrac, USA). The area-based diameter (D[3,2]) was evaluated and expressed as μm.2.7 Microstructural analysis
The treated carrot juice (2 mL) was stained with 0.1 mL Nile red solution at a concentration of 1 mg mL−1 in ethanol and observed using a confocal laser scanning microscope (LSM 880, Carl Zeiss, Germany). Observations were carried out under a 10× objective lens, at an excitation of 561 nm.2.8 In vitro digestion
The treated carrot juice was passed through an in vitro digestion that included the mouth, stomach, and small intestine phases as described by Liu et al.18 An automatic titration unit (Metrohm, USA, Inc.) was used to maintain the pH at 7.0 by adding 0.25 M NaOH solution over 2 h in the small intestine phase. The volume of NaOH required to maintain the pH at 7.0 during the small intestine phase was recorded. In the following analysis, the initial carrot juice represents the juice immediately after HPH treatments before digestion.2.9 Carotenoid retention rate analysis
The procedure described by Knockaert et al.,19 with some modifications, was adopted to extract carotenoids from the initial carrot juice and the carrot juice after each digestion phase. The organic phase which contained carotenoids was separated from the water phase using a separating funnel. A rotary evaporator (RE-3000, Yarong, China) was used to dry the organic phase and the residue was redissolved using hexane with 0.1% BHT to 5 mL. Total carotenoid content (TCC) was spectrophotometrically measured at 450 nm using hexane and 0.1% BHT as the blank solution. The following equation described by Knockaert et al.19 was used to calculate the TCC: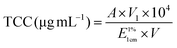 | (1) |
To identify and quantify the carotenoid monomer, the samples were filtered (0.2 μm pore size) and stored at −18 °C until further analysis. Each sample was separated and analyzed on a HPLC system equipped with a reversed phase C30-column (250 mm × 4.6 mm, 5 μm, YMC Europe, Germany), a binary HPLC pump (1525, Waters, USA) and an UV/Visible detector (2489, Waters, USA). Mobile phase A consisted of 81% MeOH, 15% MTBE and 4% ultra-pure grade water, and B consisted of 6% MeOH, 90% MTBE and 4% ultra-pure grade water. The gradient program was built in 45 min from 100% A to 44% A at a flow rate of 1 mL min−1. The detection wavelength was set as 450 nm and the injection volume was 10 μL. The concentrations of α-carotene and β-carotene were calculated from the α-carotene and β-carotene standard curves, respectively, and expressed as μg mL−1 of the juice. CRR was calculated as follows and expressed as a percentage:
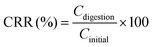 | (2) |
2.10 Carotenoid bioaccessibility
Bioaccessible carotenoids are considered as the amount of carotenoids available for absorption, namely, the carotenoids in mixed micelles.2 After the small intestine phase, raw digesta was centrifuged at 8000 rpm and 4 °C for 1 h. The supernatant was collected and assumed to be the micelle fraction, in which carotenoids were solubilized. Carotenoid bioaccessibility was calculated as follows and expressed as a percentage: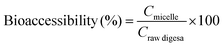 | (3) |
2.11 Statistical analysis
All the experiments were conducted in triplicate and the results were expressed as mean ± standard deviation. Data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple comparison or Pearson's correlation analysis using SPSS.19.0 software (IBM, USA). The level of significance was set at P < 0.05.3. Results and discussion
3.1 Particle size and macroscopic appearance analyses
D[3,2] of HCJ decreased significantly with the increasing homogenizing pressure and pass, respectively (Table 1). This might be attributed to the stronger shear rate induced by the increasing pressure and pass, which caused the stronger disruption of particles.20 This was in line with the result reported by Colle et al.12 that the volumetric percentage of the small particles in tomato pulp increased as the pressure increased. The increment of inlet temperature caused no significant increase of D[3,2] for HCJ, which might be due to the effect of HPH offsetting the heat-induced protein coagulation.21 NHCJ had no significant change of D[3,2] during digestion, which suggested that the carrot tissue fragments remained intact and the gastric condition was insufficient to further break down the fragments in NHCJ since it had a fairly high D[3,2] in the initial phase. For HCJ at 180 MPa, D[3,2] increased during the three digestion phases compared to the initial phase and this might be due to the fact that the initial D[3,2] was relatively low, and therefore, the addition of a simulated saliva fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) had a pronounced impact on the particle size.| Addition methods | Digestion phases | Non-homogenization | Homogenization pressure (1 pass and 25 °C) | Homogenization pass (60 MPa and 25 °C) | Inlet temperature (60 MPa and 1 pass) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 20 MPa | 60 MPa | 180 MPa | 2 passes | 3 passes | 50 °C | 70 °C | |||
| The results were expressed as mean ± standard deviation. Different lowercase letters indicate significant differences (P < 0.05) between the different phases of the same addition method within the same column. Different uppercase letters indicate significant differences (P < 0.05) between different addition methods in the same phase within the same column. | |||||||||
| 0% oil | Initial | 387.00 ± 1.13aA | 218.50 ± 4.67aA | 80.02 ± 0.05aA | 30.92 ± 0.08cA | 46.58 ± 0.33aA | 35.29 ± 0.07aBC | 84.75 ± 0.20aA | 86.60 ± 0.68aA |
| Mouth | 374.35 ± 3.46aA | 146.95 ± 4.17bA | 78.32 ± 0.40abA | 37.39 ± 0.79bA | 32.34 ± 0.21aA | 26.15 ± 0.40aA | 68.67 ± 1.27bA | 65.90 ± 6.90cA | |
| Stomach | 342.60 ± 28.28aA | 73.58 ± 4.70cA | 63.48 ± 10.59bA | 36.84 ± 0.64bA | 38.58 ± 6.39aA | 33.55 ± 5.62aA | 65.76 ± 7.06bA | 76.96 ± 0.46abA | |
| Intestine | 382.20 ± 36.77aA | 196.05 ± 38.40abA | 85.22 ± 4.67aA | 40.44 ± 1.53aA | 45.46 ± 9.86aA | 36.75 ± 6.50aA | 85.83 ± 4.99aA | 73.14 ± 0.09bcAB | |
| 2% oil | Initial | 368.15 ± 39.53aA | 219.70 ± 13.29aA | 79.83 ± 2.45abA | 30.68 ± 3.92bA | 46.97 ± 2.89aA | 31.79 ± 1.24aC | 74.89 ± 2.29bB | 87.80 ± 1.75aA |
| Mouth | 374.65 ± 3.32aA | 147.00 ± 5.37bA | 76.19 ± 1.32abA | 37.56 ± 0.80abA | 31.60 ± 0.87bA | 19.64 ± 0.73bBC | 68.58 ± 0.69cA | 64.39 ± 6.03cA | |
| Stomach | 343.65 ± 3.18aA | 73.52 ± 14.71cA | 66.13 ± 10.06bA | 37.05 ± 0.10abA | 38.53 ± 3.41abA | 30.85 ± 0.55aA | 61.94 ± 1.02dAB | 77.28 ± 0.23bA | |
| Intestine | 380.80 ± 13.58aA | 196.45 ± 10.25aA | 85.10 ± 5.38aA | 40.61 ± 3.51aA | 45.56 ± 7.57aA | 33.56 ± 4.74aA | 87.49 ± 2.43aA | 72.00 ± 0.95bcB | |
| 10% oil | Initial | 386.75 ± 27.65aA | 221.50 ± 3.11aA | 79.87 ± 3.50abA | 29.31 ± 2.57bA | 48.34 ± 2.68aA | 39.35 ± 0.80aA | 74.58 ± 5.01bB | 87.84 ± 0.22aA |
| Mouth | 375.85 ± 3.46abA | 147.05 ± 6.15cA | 76.21 ± 2.60abA | 38.41 ± 0.04aA | 32.81 ± 2.39cA | 20.38 ± 0.26cB | 70.61 ± 0.77bA | 63.90 ± 5.03cA | |
| Stomach | 335.15 ± 4.60bA | 81.12 ± 11.31dA | 66.35 ± 8.21bA | 38.03 ± 0.13aA | 39.23 ± 2.67bA | 34.14 ± 1.85bA | 62.10 ± 1.45cAB | 77.89 ± 0.49bA | |
| Intestine | 385.00 ± 18.95aA | 175.25 ± 1.91bA | 84.52 ± 3.41aA | 40.84 ± 4.64aA | 45.99 ± 1.13aA | 38.12 ± 1.41aA | 87.59 ± 1.46aA | 72.60 ± 0.23bA | |
| Emulsion | Initial | 359.25 ± 7.00aA | 209.40 ± 2.83aA | 77.58 ± 0.93abA | 28.95 ± 1.15bA | 45.29 ± 1.39aA | 37.04 ± 2.43aAB | 67.88 ± 3.61aB | 68.24 ± 0.99aB |
| Mouth | 352.80 ± 4.95aB | 141.25 ± 2.76cA | 74.42 ± 0.28bA | 37.46 ± 4.07aA | 29.19 ± 2.48bA | 19.08 ± 0.21cC | 54.65 ± 2.45bB | 53.85 ± 6.39bA | |
| Stomach | 351.25 ± 12.23aA | 76.53 ± 4.78dA | 59.18 ± 1.34cA | 36.66 ± 2.07aA | 23.59 ± 1.48cB | 31.68 ± 2.21bA | 52.99 ± 4.41bB | 71.91 ± 3.34aA | |
| Intestine | 427.45 ± 88.18aA | 162.35 ± 2.62bA | 85.89 ± 6.43aA | 39.17 ± 1.56aA | 41.91 ± 2.38aA | 35.46 ± 0.66abA | 75.13 ± 0.37aB | 68.25 ± 3.95aB | |
Most of the D[3,2] of HCJ-2, HCJ-10 and HCJ-E had similar variations during each digestion phase. Therefore, oil and emulsion could not markedly alter the particle size of carrot juice during digestion. D[3,2] of HCJ-2 at 50 °C or 70 °C showed different variations in the stomach and small intestine. Thus, it was deduced that the structural changes of particle-related substances (protein, lipid and polysaccharides) induced by high temperature could alter the particle size of carrot juice during digestion.
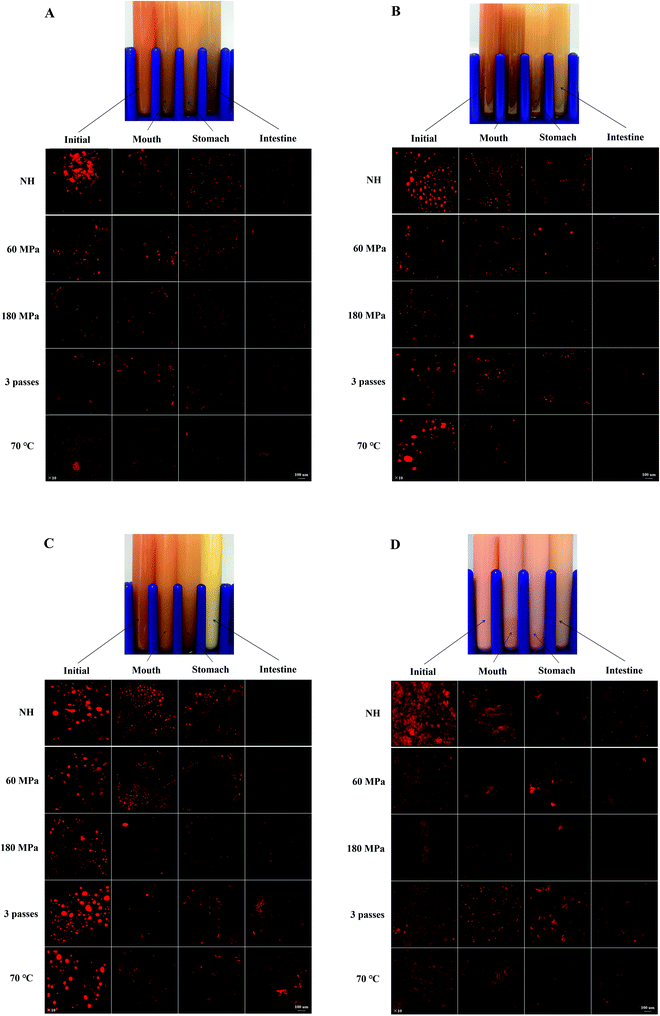
Macroscopic appearances of carrot juice with various treatments are shown in Fig. 1. Since all the samples subjected to the same HPH treatment had an identical macroscopic appearance, the sample homogenized at 60 MPa, 1 pass and 25 °C was taken as a representative. The different colors of the digestive juice manifested the different concentrations of carotenoids and the different interactions among the main substances in the digestive juice.
3.2 ζ-Potential and microstructural analysis
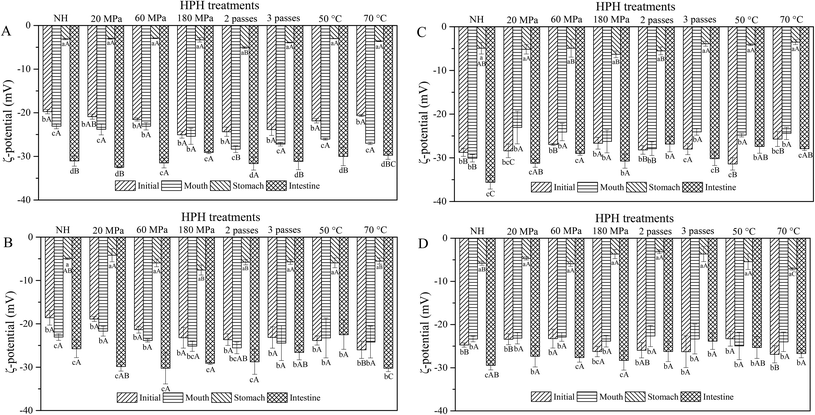
The obtained ζ-potential values are presented in Fig. 2. Biopolymer molecules, such as pectin from the cell wall, could be released after HPH treatment, and particles might be surrounded with a protective coat of negatively charged pectin, resulting in an overall negative surface charge.22 All the HCJ samples showed a similar trend that negative charge increased during the mouth phase, which might be due to the presence of mucin in SSF.18 In the stomach phase, the ζ-potential of HCJ increased up to values between −5.07 mV and −2.93 mV, which was due to the lower ionization degree of the surface active molecules at the oil–water interface induced by the low pH value.23 The ζ-potential of HCJ decreased significantly in the intestine phase, which was the lowest among the four phases. This was probably caused by the anionic lipid hydrolysis; moreover, the complex structure of bile salts and phospholipids might also have contributed to the results.24 In the intestine phase, the visible particles of HCJ under confocal microscopy became less and smaller (Fig. 1A), which contradicted with the results of D[3,2]. Considering the ζ-potential of HCJ in the intestine phase was the lowest among four phases, carotenoids in particles might be strongly encapsulated by pectin molecules in the presence of bile salts and SIF, contributing to the less and smaller particles under confocal microscopy.During digestion, almost all the ζ-potentials of HCJ-2 and HCJ-10 was maintained in the mouth phase but increased in the stomach phase. Compared with HCJ, the results suggested that oil somehow changed the ζ-potential of particles in carrot juice although the origin of the effect is unknown. Confocal microscopy indicated that the droplets in the mouth phase were bigger than those in the stomach phase (Fig. 1B and C), which showed that droplet flocculation had occurred and this was consistent with the result obtained by Li et al.25 It has been shown that lipid droplets might interact with polymers in the saliva, which could facilitate droplet coalescence and flocculation.26 Polymers such as pectin were involved in the above lipid flocculation; moreover, depletion flocculation and bridging flocculation might be two main phenomena.27
The ζ-potential of HCJ-E showed no significant difference in the mouth phase but increased significantly in the stomach phase as HCJ, HCJ-2 and HCJ-10. Theoretically, lipid droplets in carrot juice with emulsion were probably surrounded by a Tween coating, which was a nonionic surfactant; thus the system was not expected to cause a strong negative charge. However, the results suggested that HCJ-E had strong negative charge during digestion. Mun et al.28 reported that lipid droplets coated by a nonionic surfactant might have a negative charge, which was caused by anionic impurities, such as free fatty acids (FFA) or other oily ingredients. In addition, as mentioned before, pectin could also cause a negative charge in carrot juice. The ζ-potential of HCJ-E increased significantly in the stomach phase, which was the highest among the four phases, and this result was in accordance with a previous study using an emulsion-based system.25 Lipid droplets of HCJ-E were comparatively small and distributed uniformly throughout the initial samples (Fig. 1D). The droplets showed a decreasing trend from the mouth to the stomach phase, which suggested that the droplets were resistant to flocculation under gastric conditions. In the intestine phase, the digestion of lipid and the encapsulation by pectin molecules in the presence of bile salts and SIF might contribute to the less and smaller particles under confocal microscopy.
3.3 Lipid digestion
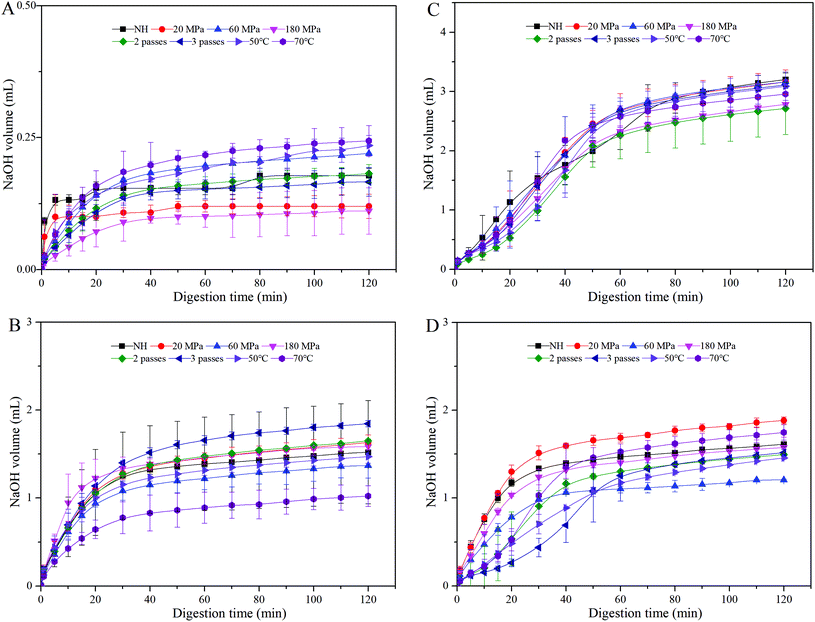
It was presumed that carotenoid bioaccessibility could be enhanced by improving lipid digestion, since both processes were closely related.29 Therefore, the volume of NaOH required to neutralize FFA released was recorded (Fig. 3). HCJ showed a slight increment in the volume of NaOH solution added to the reaction chamber in the intestine phase (Fig. 3A), which might be due to the digestion of a minute amount of lipid from the carrot and some components in the reagents (such as bile salts or lipase). For all the samples, there was a rapid increase in FFA released during the first 20 minutes, followed by a much slower increase at later time periods, which was similar to the result of Li et al.25 It indicated a rapid interaction between lipase and the lipid droplet surface, and further hydrolyzation. The volume of NaOH solution added to HCJ-2 was between 0.5 mL and 2 mL (Fig. 3B), while that added to HCJ-E was between 1 mL and 2 mL (Fig. 3D), which suggested that oil added as an emulsion had a slightly higher volume of FFA released. This might be induced by the lipid flocculation mentioned in section 3.2.The volume of NaOH solution added to HCJ-10 was between 2.3 mL and 3.2 mL (Fig. 3C), which had a relatively smaller variation range. Moreover, Devraj et al.30 proposed that lipid digestion might be inhibited in long chain fatty emulsions owing to the accumulation of long chain fatty acids at the surface of lipid droplets. These long chain fatty emulsions could form a crystalline shell around the lipid droplets limiting the access of lipase to the triglycerides, thereby inhibiting lipid digestion.
3.4 Carotenoid retention rate
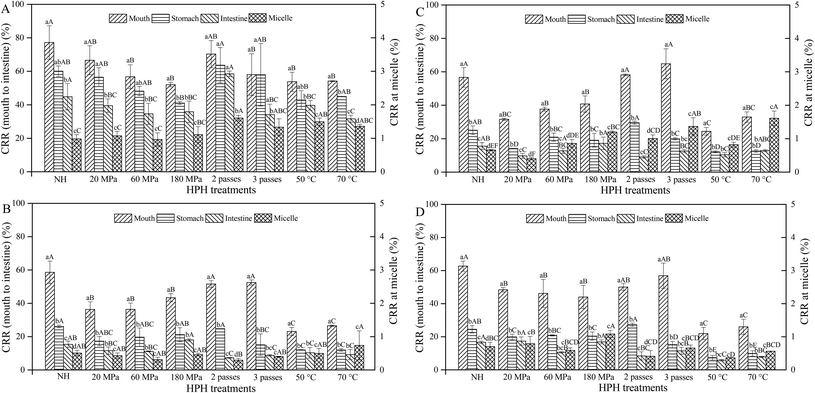
Generally, the CRR of HCJ, HCJ-2, HCJ-10 and HCJ-E showed a decreasing trend during the digestion. The ranges of CRR for the mouth, stomach and small intestine phases and the micelle fraction were 21.95%–77.22%, 7.38%–63.66%, 5.8%–58.52% and 0.29%–1.61%, respectively. The CRR of HCJ at 2 passes was relatively higher compared with other treatments, ranging from 1.61% to 70.31%, while the CRR of HCJ with other HPH processes generally showed no significance in the same digestion phase (Fig. 4A). The CRR of HCJ at micelles was significantly lower than that of other phases which was less than 2%, indicating that very little carotenoids transferred into micelles. But more passes and higher inlet temperature showed an increasing trend of the CRR in micelles.HCJ-2 and HCJ-10 at 50 °C and 70 °C had a significantly lower CRR compared with other treatments in the mouth phase (Fig. 4B and C). The CRR of HCJ-2 and HCJ-10 in the stomach and intestinal phases was significantly lower than that in the mouth phase, which might be caused by the acidic conditions and the instability of carotenoids in the stomach phase. Generally, HCJ-10 had a higher CRR in micelles than HCJ-2, which suggested that the oil concentration influenced carotenoid micellization. Since lipid hydrolysis led to the appearance of lipid digestion products (monoacylglycerols and free fatty acids), which could form mixed micelles with bile salts,23 carotenoids encapsulated in micelles were enhanced as more lipid digestion products were involved in the formation of micelles.
For HCJ-E, the CRR in the stomach and intestinal phases was also significantly lower than that in the mouth phase (Fig. 4D). The variation trend of the CRR during digestion was similar to that of HCJ-2 and HCJ-10. Therefore, it was speculated that different oil forms had little significant effect on the CRR during digestion. It is worth noting that the CRR of HCJ was the highest compared with that of HCJ-2, HCJ-10 and HCJ-E in each digestion phase. Since carotenoids are lipophilic, the presence of oil might make more carotenoids exposed to the environment, causing the decreasing transfer stability of carotenoids during digestion. Additionally, the present results showed that nonionic emulsifiers (Tween) could not improve the transfer stability of carotenoids during digestion. In a further study, ionic emulsifiers could be adopted to study their effects on carotenoid transfer stability. It is worth exploring a suitable emulsifier to enhance the carotenoid transfer stability during digestion for further increasing carotenoid bioaccessibility.
3.5 Carotenoid bioaccessibility
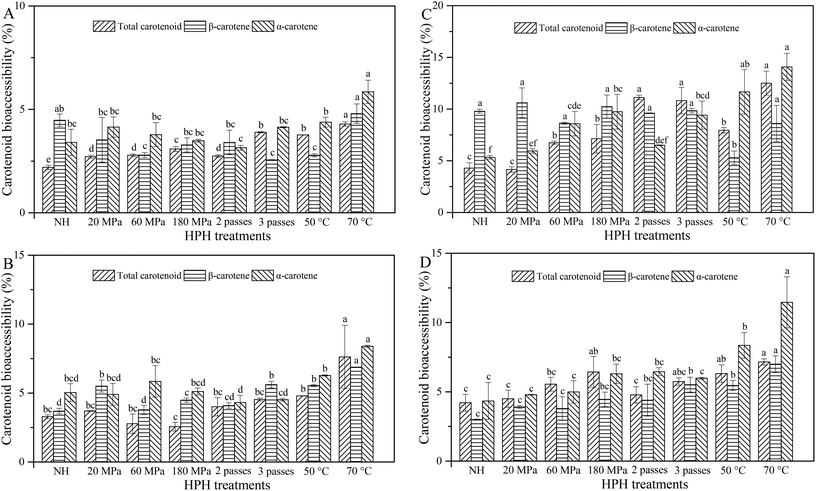
In order to be absorbed through the intestinal epithelium, carotenoids need to be first released from the food matrix, solubilized into the lipid phase and incorporated into mixed micelles.10 Bioaccessible carotenoids are considered as the amount of carotenoids available for intestinal absorption, namely the carotenoids in mixed micelles.2 Carotenoid bioaccessibility is calculated as the ratio of carotenoid concentration in micelles to the carotenoid concentration in raw intestine digesta.2,18 Blending caused cell breakage, resulting in a good release of β-carotene, and this was a prerequisite for high β-carotene bioaccessibility.6 As shown in Fig. 5A, HCJ showed higher values of total carotenoid bioaccessibility (TCB) than NHCJ (2.2%). TCB was higher for HCJ at 180 MPa (3.08%) compared with 20 MPa (2.72%) and 60 MPa (2.78%), and this might be due to the intensity with which HPH facilitated the release of carotenoids from carrot tissues and their encapsulation in micelles. The bioaccessibility of total carotenoids, β-carotene and α-carotene increased significantly when the inlet temperature was 70 °C. As mentioned by Lemmens et al.,3 thermal processing of fruit and vegetable-based products prior to consumption is important to open up the structural organization in which the carotenoids are embedded, thus increasing their liberation from the raw material. Therefore, it was deduced that HPH at 70 °C increased the liberation of carotenoids from carrot juice, contributing to the following higher TCB. In summary, carotenoid bioaccessibility of HCJ was lower than 6%, and this was similar to the result obtained by Mashurabad et al.,31 who reported that micellization of β-carotene and α-carotene was 4.8% and 4.6% in carrot puree, respectively. On one hand, this was probably caused by the physical state and specific localization of carotenoids in carrots. Furthermore, carotenoids occur as membrane-bound crystalline structures within the chromoplasts in carrots.32 Carotenoids from the crystalline chromoplast in carrots had lower liberation and bioaccessibility compared with those from the globular-tubular chromoplast in papayas and mangoes.33 It was speculated that HPH could increase the release of carotenoids to some extent, but carotenoids might not be fully released. On the other hand, carotenoids are lipophilic; thus, they need to be solubilized into the lipid phase and incorporated into mixed micelles with certain hydrolysates of lipid.10,13 Little lipid in carrot juice also caused a barrier for TCB, and this was the reason why the addition of oil or emulsion was considered.Generally, HCJ-2 had a higher bioaccessibility of total carotenoids, β-carotene and α-carotene compared with HCJ (Fig. 5B), which signified that oil could play a crucial role as a carotenoid carrier during digestion. The bioaccessibility of total carotenoids, β-carotene and α-carotene for HCJ-2 increased significantly when the inlet temperature was 70 °C. The study of Colle et al.17 showed that the fatty acyl chain length of oil was of minor importance for carotenoid bioaccessibility when the thermal treatment was conducted afterwards. The β-carotene bioaccessibility was higher for HCJ-2 at 3 passes compared with 1 and 2 passes. For pressure and pass variation, the α-carotene bioaccessibility of HCJ-2 showed no significant difference. Overall, the intense HPH treatment (3 passes or 70 °C) contributed to the increasing carotenoid bioaccessibility of carrot juice. Similarly, Knockaert et al.34 reported that HPH improved the lycopene bioaccessibility and HPH combined with 5% olive oil resulted in the highest amount of bioaccessible lycopene. However, the values of carotenoid bioaccessibility for HCJ-2 were generally lower than 10%. Except for the above-mentioned limitations for carotenoid bioaccessibility (physical state and specific localization), pectin released from cell wall disruption might also be an important factor, especially in the presence of lipid. Pectin had inhibition effects on lipid digestion by binding with calcium, interacting with bile salts, altering the digestive medium viscosity, changing the interface between oil and water phases, and inhibiting lipase activity.13 Thus pectin was supposed to have potential effects on carotenoid digestion and restrict the carotenoid bioaccessibility, since they need to be solubilized into the lipid phase, and incorporated into mixed micelles before absorption.10,13
The addition of 10% oil could increase the bioaccessibility of total carotenoids, β-carotene and α-carotene compared with 2% oil addition (Fig. 5C), and this result was in line with that of Lipkie et al.16 who reported that an increasing carotenoid bioaccessibility was observed with the enhancing oil concentration. However, Mashurabad et al.31 reported that the addition of 0.5–10% olive oil increased the micellization of carotene in carrots, while the carotenoid micellization remained similar after the addition of 2.5% oil, suggesting saturation. The present result proved that HPH also influenced the carotenoid bioaccessibility of the samples with oil, causing a higher carotenoid bioaccessibility with enhancing oil concentration. HCJ-10 showed higher values of TCB at 60 MPa and 180 MPa compared with those at 20 MPa and NHCJ-10. HCJ-10 at 2 and 3 passes had higher TCB than that at 1 pass, and HCJ-10 at 70 °C had higher TCB than those at 25 °C and 50 °C. The β-carotene bioaccessibility for HCJ-10 showed no significant difference except for HCJ-10 at 50 °C. HCJ-10 showed higher α-carotene at 180 MPa compared with that at 20 MPa. HCJ-10 at 2 and 3 passes had higher α-carotene bioaccessibility than that at 1 pass, and HCJ-10 at 70 °C and 50 °C had higher α-carotene bioaccessibility than that at 25 °C, up to 14.08% and 11.68%, respectively. Therefore, HPH combined with 10% oil addition showed a more obvious effect on α-carotene bioaccessibility compared with β-carotene bioaccessibility. The results of Victoria-Campos et al.35 and Huo et al.36 showed that the addition of oil was supposed to be more important for carotene than xanthophyll. With 10% oil addition, HPH conducted at 70 °C could not significantly increase the carotenoid bioaccessibility compared with other treatments. Overall, the results suggested that the addition of oil seemed to be more important than the HPH treatments for carotenoid bioaccessibility.
HCJ-E showed higher values of TCB at 180 MPa compared with those at 20 MPa and 60 MPa, but HCJ-E showed no significant difference at different passes (Fig. 5D). The bioaccessibility of total carotenoids, β-carotene and α-carotene for HCJ-E increased significantly when the inlet temperature was 70 °C. HCJ-E had significantly higher TCB than HCJ-2 at 60 MPa, 180 MPa and 3 passes (P < 0.05). In the stomach phase, carotenoids need to be released from the raw material and dispersed in the lipid phase, where a fine emulsion is formed. In the intestine phase, the carotenoids solubilized in the emulsion need to be incorporated into mixed micelles, together with bile salts, FFA and monoglycerides.3 Therefore, oil that was added as such and oil that was added as emulsion were different because emulsification had taken place for samples with oil in the stomach phase. It was deduced that oil that was added as an emulsion could increase the carotenoid bioaccessibility at higher pressure and more passes in some way. Moreover, the interactions between the emulsifier and carotenoids might also affect the carotenoid bioaccessibility,37 which resulted in different carotenoid bioaccessibilities for HCJ-2 and HCJ-E. A higher carotenoid bioaccessibility is the prerequisite of carotenoid bioavailability and carotenoid bioavailability is defined as the fraction of the ingested carotenoids that is available for utilization in normal physiological functions or for storage in the human body.3 Therefore, a further study might be focused on the final phase of the carotenoid biological activity, namely carotenoid bioavailability to better improve carotenoid utilization in the human body.
3.6 Correlation analysis
For HCJ-10 and HCJ-E, significant negative correlations could be found between TCB and initial D[3,2] (R2 = −0.68 for HCJ-10 and R2 = −0.72 for HCJ-E, P < 0.05). Lower D[3,2] contributed to higher TCB, which indicated that particle size reduction could be considered as an effective way to increase TCB in carrot juice which contained oil. The lower D[3,2] revealed that the cell wall disruption was stronger; therefore, the release of carotenoids from raw materials increased and the carotenoids in the micelle fraction were also enhanced. Moreover, regarding small particles, the surface area for the digestive enzyme to get access to lipid droplets was larger, thus contributing to a higher carotenoid bioaccessibility since they were digested along with lipids. The regression fitting curve could be modeled as TCB = 10.435 × e−0.003×D[3,2] for HCJ-10 (R2 = 0.64) and TCB = 10.396 × D[3,2]−0.148 (R2 = 0.51). R2 values were not very high, which was because homogenization pressure, pass and inlet temperature had different influencing pathways and patterns on TCB. But it was undeniable that a correlation could be found between TCB and D[3,2] of carrot juice, so the focus might be on the reduction of particle size to facilitate the carotenoid bioaccessibility in the further study.The CRR in the small intestine phase had a significant negative correlation with TCB (P < 0.01). This suggested that in spite of the low CRR in the small intestine, a high TCB could be achieved. Benllochtinoco et al.38 and Hornero-Méndez et al.32 proved that thermal treatment decreased the carotenoid content but increased the carotenoid bioaccessibility. These similar phenomena might be attributed to the same cause: the rupture of carrot tissue, which facilitated carotenoid release and its solubilization in mixed micelles.39 The regression fitting curve could be modeled as TCB = 15.087 × CRR−0.422 (R2 = 0.33), and TCB showed a asymptotic trend to the x axis when the CRR was high, signifying that a high CRR at a certain range could contribute to a low TCB. The low CRR in the small intestine showed the poor stability of carotenoids during digestion, while the ultimate goal was not only to increase the carotenoid micellization but also to enhance the carotenoid stability during digestion. In thefurther research, the focus might be on carotenoid stability during digestion so as to enhance the carotenoid content in micelles and the small intestine, simultaneously.
4. Conclusions
The results illustrated that HPH could decrease the D[3,2] of carrot juice, and the negative correlations could be found between TCB and D[3,2] for the HCJ-10 and HCJ-E. The bioaccessibility of total carotenoids, β-carotene and α-carotene was higher at 60 MPa, 1 pass and 70 °C than that in other treatments. The bioaccessibility of total carotenoids, β-carotene and α-carotene of HCJ was lower than 6%, but the addition of oil or emulsion could increase the carotenoid bioaccessibility (up to 14.08% for α-carotene). The CRR in the small intestine phase had a significant negative correlation with TCB, and therefore, a high TCB could be achieved despite the low CRR in the small intestine. Overall, the results highlighted the potential of HPH combined with the addition of oil or emulsion to boost the carotenoid bioaccessibility. However, the ultimate goal was not only to increase the carotenoid bioaccessibility but also to enhance the carotenoid stability during digestion. In the further research, the focus might be on carotenoid stability during digestion aiming at increasing the carotenoid content in micelles and the small intestine simultaneously.Furthermore, it was speculated that carotenoids in carrot juice are encapsulated by pectin during digestion via ζ-potential and confocal microscopy analyses. The effects of pectin on the digestion of carotenoids might be multifaceted. Pectin might not only have a potential influence on carotenoid bioaccessibility by inhibiting the lipid digestion, but also have encapsulating and stabilizing effects on carotenoids due to its emulsification. Thus, further studies are required to prove the effects of pectin on carotenoid bioaccessibility with or without the addition of oil.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31671868) and the National Key Research and Development Program of China (No. 2016YFD0400302-3).References
- K. D. Sharma, S. Karki, N. S. Thakur and S. Attri, J. Food Sci. Technol., 2012, 49(1), 22–32 CrossRef CAS PubMed.
- O. F. O'Connell, L. Ryan and N. M. O'Brien, Nutr. Res., 2007, 27(5), 258–264 CrossRef.
- L. Lemmens, I. Colle, S. V. Buggenhout, P. Palmero, A. V. Loey and M. Hendrickx, Trends Food Sci. Technol., 2014, 38(2), 125–135 CrossRef CAS.
- P. Palmero, A. Panozzo, I. Colle, C. Chigwedere, M. Hendrickx and A. V. Loey, Food Chem., 2016, 199, 423–432 CrossRef CAS PubMed.
- D. Y. Low, B. D'Arcy and M. J. Gidley, Food Res. Int., 2015, 67, 238–246 CrossRef CAS.
- L. Lemmens, B. S. Van, A. M. Van Loey and M. E. Hendrickx, J. Agric. Food Chem., 2010, 58(24), 12769–12776 CrossRef CAS PubMed.
- B. Wei, C. Cai, B. Xu, Z. Jin and Y. Tian, Food Chem., 2018, 240, 165–173 CrossRef CAS PubMed.
- M. T. K. Kubo, P. E. D. Augusto and M. Cristianini, Food Res. Int., 2013, 51(1), 170–179 CrossRef CAS.
- A. Bevilacqua, M. R. Corbo and M. Sinigaglia, Food Control, 2012, 24(1–2), 109–115 CrossRef CAS.
- L. Mutsokoti, A. Panozzo, A. P. Pallares, S. Jaiswal, A. V. Loey, T. Grauwet and M. Hendrickx, Food Chem., 2017, 232, 124–134 CrossRef CAS PubMed.
- C. A. Svelander, P. Lopez-Sanchez, P. D. Pudney, S. Schumm and M. A. Alminger, J. Food Sci., 2011, 76(9), H215–H225 CrossRef CAS PubMed.
- I. Colle, S. V. Buggenhout, A. V. Loey and M. Hendrickx, Food Res. Int., 2010, 43(8), 2193–2200 CrossRef CAS.
- B. Cervantespaz, J. J. Ornelaspaz, S. Ruizcruz, C. Riosvelasco, V. Ibarrajunquera, E. M. Yahia and A. A. Gardea-Béjar, Food Res. Int., 2017, 99(Pt 2), 917 CrossRef CAS PubMed.
- S. H. E. Verkempinck, L. Salvia-Trujillo, L. G. Moens, C. Carrillo, A. M. V. Loey, M. E. Hendrickx and T. Grauwet, J. Funct. Foods, 2018, 41, 135–147 CrossRef CAS.
- C. Ijp, B. S. Van, L. Lemmens, A. M. Van Loey and M. E. Hendrickx, Food Res. Int., 2012, 45(1), 250–255 CrossRef.
- T. E. Lipkie, F. F. De Moura, Z. Y. Zhao, M. C. Albertsen, P. Che, K. Glassman and M. G. Ferruzzi, J. Agric. Food Chem., 2013, 61(24), 5764–5771 CrossRef CAS PubMed.
- I. J. P. Colle, L. Lemmens, S. V. Buggenhout, K. Met, A. M. V. Loey and M. E. Hendrickx, Food Res. Int., 2013, 51(1), 32–38 CrossRef CAS.
- X. Liu, J. Bi, H. Xiao and D. J. McClements, J. Food Sci., 2016, 81(3), N754–N761 CrossRef CAS PubMed.
- G. Knockaert, L. Lemmens, S. Van Buggenhout, M. Hendrickx and A. Van Loey, Food Chem., 2012, 133(1), 60–67 CrossRef CAS.
- E. Dumay, D. Chevalier-Lucia, L. Picart-Palmade, A. Benzaria, A. Gràcia-Julià and C. Blayo, Trends Food Sci. Technol., 2013, 31(1), 13–26 CrossRef CAS.
- L. Y. Zhou, Y. Y. Wang, X. S. Hu, J. H. Wu and X. J. Liao, Innovative Food Sci. Emerging Technol., 2009, 10(3), 321–327 CrossRef CAS.
- S. Croak and M. Corredig, Food Hydrocolloids, 2006, 20(7), 961–965 CrossRef CAS.
- L. Salvia-Trujillo, S. H. Verkempinck, L. Sun, A. M. Van Loey, T. Grauwet and M. E. Hendrickx, Food Chem., 2017, 229, 653–662 CrossRef CAS PubMed.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48(2), 92–100 CrossRef CAS PubMed.
- Q. Li, T. Li, C. Liu, T. Dai, R. Zhang, Z. Zhang and D. J. McClemnets, Food Biophys., 2017, 12(2), 172–185 CrossRef.
- M. H. Vingerhoeds, T. B. J. Blijdenstein, F. D. Zoet and G. A. V. Aken, Food Hydrocolloids, 2005, 19(5), 915–922 CrossRef CAS.
- E. Capuano, Crit. Rev. Food Sci. Nutr., 2017, 57(16), 3543–3564 CrossRef CAS PubMed.
- S. Mun, E. A. Decker and D. J. Mcclements, Langmuir, 2005, 21(14), 6228–6234 CrossRef CAS PubMed.
- D. M. Deming and J. W. Erdman, Pure Appl. Chem., 1999, 71(12), 2213–2223 CAS.
- R. Devraj, H. D. Williams, D. B. Warren, A. Mullertz, C. J. Porter and C. W. Pouton, Int. J. Pharm., 2013, 441(1–2), 323–333 CrossRef CAS PubMed.
- P. C. Mashurabad, R. Palika, Y. W. Jyrwa, K. Bhaskarachary and R. Pullakhandam, J. Food Sci. Technol., 2017, 54(2), 1–9 Search PubMed.
- D. Hornero-Méndez and M. I. Mínguez-mosquera, Innovative Food Sci. Emerging Technol., 2007, 8(3), 407–412 CrossRef.
- R. M. Schweiggert, D. Mezger, F. Schimpf, C. B. Steingass and R. Carle, Food Chem., 2012, 135(4), 2736–2742 CrossRef CAS PubMed.
- G. Knockaert, S. K. Pulissery, I. Colle, B. S. Van, M. Hendrickx and A. V. Loey, Food Chem., 2012, 135(3), 1290–1297 CrossRef CAS PubMed.
- C. I. Victoria-Campos, J. D. J. Ornelas-Paz, E. M. Yahia and M. L. Failla, J. Agric. Food Chem., 2013, 61(15), 3642–3653 CrossRef CAS PubMed.
- T. Huo, M. G. Ferruzzi, S. J. Schwartz and M. L. Failla, J. Agric. Food Chem., 2007, 55(22), 8950–8957 CrossRef CAS PubMed.
- A. Degrou, S. Georgé, C. M. G. C. Renard and D. Page, Food Chem., 2013, 136(2), 435–441 CrossRef CAS PubMed.
- M. Benllochtinoco, A. Kaulmann, J. Cortereal, D. Rodrigo, N. Martíneznavarrete and T. Bohn, Food Chem., 2015, 187, 254–262 CrossRef CAS PubMed.
- J. Francisco, R. B. Lilian, Z. Adriana and V. Gustavo, Trends Food Sci. Technol., 2017, 67, 195–206 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |