Interactive influence of extracellular polymeric substances (EPS) and electrolytes on the colloidal stability of silver nanoparticles†
Ishara
Fernando
 ab,
Dan
Lu
bc and
Yan
Zhou
*bc
ab,
Dan
Lu
bc and
Yan
Zhou
*bc
aInterdisciplinary Graduate School, Nanyang Technological University, 639798, Singapore
bNanyang Environment & Water Research Institute, Advanced Environmental Biotechnology Centre, Nanyang Technological University, 1 Cleantech Loop, CleanTech One, 637141, Singapore. E-mail: zhouyan@ntu.edu.sg; Tel: +65 6790 6103
cSchool of Civil & Environmental Engineering, College of Engineering, Nanyang Technological University, 639798, Singapore
First published on 14th November 2019
Abstract
The colloidal stability of silver nanoparticles (AgNPs) was evaluated using time-resolved dynamic light scattering, electrophoretic mobility and dissolved silver concentration in the presence of common monovalent or divalent electrolytes and extracellular polymeric substances (EPS). The colloidal stability of AgNPs was significantly affected by the electrolyte. A relatively lower critical coagulation concentration (CCC) was recorded for AgNPs in the divalent electrolytes. Three types of EPS namely, soluble EPS (SB-EPS), loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS) extracted from activated sludge, were added into electrolytes that contain AgNPs to investigate the potential different impacts on AgNP transformation. Overall, the presence of all the types of EPS reduced the aggregation rate and increased the CCC values in NaNO3 and low concentrations of Ca(NO3)2 (0.05–10 mM) solutions. When the NaNO3 concentration was higher than 12 mM, the attachment efficiency of AgNPs was below one, suggesting that the EPS adsorbed on the AgNPs resulted in steric repulsion and stabilizing the AgNP suspension effectively. However, the presence of EPS increased the rate of aggregation of AgNPs at higher Ca(NO3)2 concentrations (10–40 mM), which can be due to the aggregation of the dissolved EPS via intermolecular bridge linking of the AgNPs and aggregates together. Among the three types of EPS used in the study, LB-EPS effectively stabilized the AgNPs irrespective of the electrolyte mainly due to the lower presence of the hydrophilic dissolved organic matter in LB-EPS. These results provide important insights into understanding the interactive impact of EPS and ions on AgNP transformation.
Environmental significanceThe colloidal stability of silver nanoparticles (AgNPs) is a prominent factor affecting the transport and fate of AgNPs in the aquatic environment. Different factors affect the colloidal stability of AgNPs depending on the matrix they are present in. Electrolytes and extracellular polymeric substances (EPS) in water can be considered as two such factors affecting the transformations of AgNPs. The current study assesses the interactive influence of electrolytes and EPS on the transport of AgNPs when present together in biological wastewater treatment systems. Our results present significant insights into the colloidal stability of AgNPs in the presence of both organic and inorganic substances. |
1. Introduction
Silver nanoparticles (AgNPs) are among the most widely used types of engineered NPs due to their unique physical and chemical properties and especially their antibacterial activity. Wastewater treatment plants are the most prominent distribution pathways among the multiple pathways where AgNPs enter aquatic matrices.1 Many research studies focused on understanding the factors including the solution pH, ionic strength, valence of the electrolyte and particle size which govern the transport and fate of AgNPs in aquatic environments.2 For example, increasing ionic strength can result in enhanced aggregation as divalent cations are more effective in facilitating aggregation than monovalent cations.3When AgNPs are discharged into wastewater treatment systems, they interact with organic matter, for instance, humic substances (HS), like humic acids (HA) and fulvic acids (FA), and non-humic substances consisting of biological macromolecules like proteins and polysaccharides. Organic matter adsorbed onto NPs can change the surface charge of the particles, thereby altering the colloidal stability.4 Different structures and components of organic matter can result in diverse impacts on the aggregation of NPs. Some studies have reported that protein molecules are effective in stabilizing AgNPs5 whereas polysaccharides hinder the stability of AgNPs.6,7 On the other hand, aromatic-rich HA are effective in stabilizing NPs compared to aliphatic-rich HA.8
According to previous studies, a considerable amount of NPs entering biological wastewater treatment systems are adsorbed by activated sludge, which reduces the concentration of NPs in effluents.9,10 The predicted average concentration of Ag in the sludge is in the range of 7–39 mg kg−1.11 AgNPs adsorbed onto the sludge will be embedded in the sludge and form new products such as silver sulphide (Ag2S).9,12 However, the removal mechanism of NPs by the activated sludge is not comprehensively investigated.
Extracellular polymeric substances (EPS) are a heterogeneous mixture in activated sludge, which are continuously secreted by microorganisms during their growth and metabolic activities.13 EPS are mainly a mixture of polysaccharides and proteins with different functional groups including amide, amino, carboxyl, hydroxyl and phosphoryl groups.14–16 EPS in biological wastewater treatment systems consist of different EPS fractions, predominantly soluble EPS (SB-EPS), loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS).17 EPS play an important role in protecting bacterial cells against environmental stress.18 The composition of EPS secreted by bacteria will be changed in order to respond to the changes in matrices that they are present in hence their adhesion capabilities. When present in wastewater treatment systems, EPS tend to interact with different types of electrolytes present in wastewater19,20 which could probably play a role in the cohesiveness of microbial aggregates as evaluated in different studies,21 by bridging the negatively charged sites of EPS to create stable, intermolecular and cell-EPS links. Meanwhile, they may also interact with NPs in wastewater. It is highly possible that the interaction will start from SB-EPS, and then LB-EPS and finally TB-EPS given the layered structure of EPS in biomass. When present in the solution, NPs tend to release ions (i.e. AgNPs release ionic Ag) which are harmful to bacteria. The distribution and the composition of EPS vary in such a way to provide maximum protection against toxic substances.22 To the best of our knowledge, only a few studies attempted to understand the role of EPS in the aggregation and the colloidal stability of NPs23,24 and they barely considered AgNPs. A complex environment with the presence of both EPS and ions and their interactive behavior are even rarely investigated.
The objective of this study is to explore the impact of EPS on the colloidal stability and the aggregation kinetics of AgNPs in the presence of NaNO3 or Ca(NO3)2 through time resolved dynamic light scattering (TR-DLS). In addition, several other techniques such as Fourier transform infrared (FTIR) spectroscopy, electrophoretic mobility (EPM) and dissolved ionic Ag concentration were used to quantitatively and qualitatively analyze the constituents and the functional groups of fractionalized EPS components, i.e. SB-EPS, LB-EPS and TB-EPS. This study provides an overview with fundamental understanding on the colloidal stability of AgNPs in the presence of both inorganic and organic matter.
2. Materials and methods
2.1. AgNP synthesis
The uncoated AgNPs used during the experiments were synthesized via reducing silver nitrate using sodium borohydride as detailed in our previous work.252.2. EPS extraction
Raw waste activated sludge from a local wastewater treatment plant in Singapore was obtained. It was settled at 4 °C for 24 h to concentrate first (11.2 ± 0.3 g VS per L) and stored at 4 °C overnight prior to use. EPS extracted from the sludge samples were analyzed according to the study of Zhou et al.26 Initially, 15 ml of the sludge sample was centrifuged for 15 min at 4 °C and 4000g and the supernatant was separated as SB-EPS. The remaining sludge pellet was re-suspended in 0.05% sodium chloride (NaCl) solution. Then the mixture was vortexed and incubated in a water bath at 70 °C for 1 min, followed by centrifugation at 4 °C for 10 min at a speed of 4000g. Then the supernatant was separated as LB-EPS. The residue of the suspension pellet was re-suspended to its original volume by adding 0.05% NaCl solution, placed in a 60 °C water bath for 30 min, and centrifuged at 4000g for 15 min at 4 °C. After that, the supernatant was separated as TB-EPS. All the EPS fractions were filtered through 0.45 μm membrane filters. Na+ and Cl− ions in the solution were removed via ultracentrifugation of the EPS solution using 3 kDa membrane centrifugal filters (Millipore Inc.) at 5000g for 30 min at 4 °C.2.3. Characterization of AgNPs and EPS
The total Ag concentration of the synthesized AgNPs was determined using the acid digestion method via mixing the AgNP stock solution with 70% (w/v%) nitric acid at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, incubating in an oven for 12 h at 60 °C, and diluting with DI water followed by the measurement of the dissolved ionic Ag using an inductively coupled plasma mass spectrometer (ICP-MS) (Perkin Elmer Instruments, USA).27
1 ratio, incubating in an oven for 12 h at 60 °C, and diluting with DI water followed by the measurement of the dissolved ionic Ag using an inductively coupled plasma mass spectrometer (ICP-MS) (Perkin Elmer Instruments, USA).27
The dissolved Ag concentration in the parent solution and the experimental samples was measured using ICP-MS after ultra-centrifugation. Ultra-centrifugation was carried out using 10 kDa Amicon ultra-15 centrifugal filter units (Merck, Millipore) at 7000g for 30 min at 4 °C. The liquid phase was collected and diluted with 1% (w/v%) HNO3 before being loaded onto an ICP-MS.28
TEM images of the AgNPs were obtained using TEM grids (Cu, 200 mesh, Latech Ltd. Singapore) covered with an aliquot of the sample mixture, ultracentrifuged for 1 h at 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to directly deposit the AgNPs onto the grid. Then the grids were rinsed using DI water and dried overnight. The above prepared Cu grids were imaged using a JEOL 2010 HR (JEOL Ltd., Japan) instrument at 200 kV electron generation and with a magnification between 8000× and 20
000 rpm to directly deposit the AgNPs onto the grid. Then the grids were rinsed using DI water and dried overnight. The above prepared Cu grids were imaged using a JEOL 2010 HR (JEOL Ltd., Japan) instrument at 200 kV electron generation and with a magnification between 8000× and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×.
000×.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235) was used to determine the protein content in the samples.31 Major soluble organic fractions with different chemical functions and sizes in EPS were quantified using size-exclusion chromatography, combined with organic carbon and nitrogen detection (LC-OCD-OND) as described in the study of Xiao et al.32 The biopolymers, building blocks, high molecular weight (HMW) proteins, low molecular weight (LMW) neutrals, LMW acids and HMW polysaccharides were quantified using LC-OCD-OND.33 LMW polysaccharide and LMW protein concentrations were obtained by subtracting HMW polysaccharide and HMW protein concentrations from the total polysaccharide and protein concentrations determined using the spectrometry method, respectively.
235) was used to determine the protein content in the samples.31 Major soluble organic fractions with different chemical functions and sizes in EPS were quantified using size-exclusion chromatography, combined with organic carbon and nitrogen detection (LC-OCD-OND) as described in the study of Xiao et al.32 The biopolymers, building blocks, high molecular weight (HMW) proteins, low molecular weight (LMW) neutrals, LMW acids and HMW polysaccharides were quantified using LC-OCD-OND.33 LMW polysaccharide and LMW protein concentrations were obtained by subtracting HMW polysaccharide and HMW protein concentrations from the total polysaccharide and protein concentrations determined using the spectrometry method, respectively.
2.4. Stability experiments
The variation in the intensity weighted average hydrodynamic diameter of the experimental solutions with time was measured using a Zetasizer Nano (ZEN 3600, Malvern, UK). The aggregation kinetics of AgNPs was recorded at different NaNO3 (0–500 mM) and Ca(NO3)2 (0–40 mM) concentrations in the presence and absence of EPS using time resolved DLS. The range of electrolyte concentrations was selected to mimic the levels of electrolytes in wastewater.34 Nitrate(V) was selected as the anion to avoid the potential precipitation with AgNPs compared to other anions commonly available in the aquatic environment. Since the dissolved organic carbon (DOC) concentration in wastewater treatment systems varies within the range of 10–90 mg L−1,35 all the working solutions of the different EPS fractions were maintained at 50 mg L−1 TOC during the AgNP aggregation experiments. The solution pH and the temperature were maintained at 8.0 and 25 °C, respectively.To initiate the experiments, aliquots of pure AgNP solution were added to the electrolyte solution or mixture of EPS and electrolyte solution to a final volume of 1.2 mL. The final concentration of AgNPs in the experimental samples was kept at 5 mg L−1. A cuvette containing the solution mixture was vortexed for 5 s to ensure proper mixing prior to the measurement. The hydrodynamic diameter of AgNPs in the mixture was recorded for a period of 60 min. The scattered light intensity during the measurement was detected using a photodetector at a scattering angle of 173°, with each autocorrelation function being accumulated over a period of 10 s with a stability period of 3 s. All the experiments were performed three times under the same conditions and the results present the average of the three values.
The EPM of the samples in the absence and presence of EPS was measured using a Malvern Zetasizer Nano. An incubation time of one hour was used during the measurement to allow the sample to be stabilized to meet the measurement quality criteria. The dissolved ionic Ag concentration of the experimental samples was quantified after one hour of incubation using the method mentioned previously.28
The detailed methods used in obtaining the FTIR spectrum and the adsorption experiments are mentioned in the ESI.†
2.5. Determination of aggregation kinetics
Aggregation kinetics of AgNPs are expressed as the initial rate of increase in the hydrodynamic diameter with time as measured using TR-DLS. The aggregation rate constant (k) is proportional to the initial rate of increase in the hydrodynamic diameter and inversely proportional to the initial concentration of AgNPs in the solution which is denoted as (N0).36,37 It can be determined by eqn (1). | (1) |
The initial increase in the hydrodynamic diameter of AgNPs with time was calculated until the time that the hydrodynamic diameter becomes 1.5 times the initial value. The method proposed by Huangfu et al.38 was used in determining these time points. Linear least square regression analysis was used to calculate Dh(t)/dt. The relationship used in this study has been tested and widely used in studying the aggregation kinetics of different types of NPs.16,36,39,40
The attachment efficiency (AE) (α) in the range of 0 to 1 was calculated by normalizing the k obtained under different solution conditions by kfast obtained under favourable or fast aggregation conditions.36 The AE is used as a tool in quantifying the aggregation kinetics of NPs according to the DLVO theory.41 The described relationship is shown in eqn (2) mentioned below.
 | (2) |
Since the initial AgNP concentration in the solutions was maintained constant during the measurements under different solution conditions, eqn (2) can be shortened by neglecting N0, and obtaining the AE as shown in eqn (3).
 | (3) |
When calculating the AE in the presence of different EPS fractions, (dDh(t)/dt)t→0,fast was obtained from the average values of (dDh(t)/dt)fast in the diffusion limited regime where the ionic strength is above the critical coagulation concentration (CCC) in the presence of different electrolytes.
2.6. Statistical analysis
Pearson's correlation was utilized to assess the linear correlation between the AE, adsorbed carbon and the dissolved organic matter using the software, SPSS version 19.0.42 The degree of correlation among the considered factors was evaluated using the Pearson's correlation coefficient (R), which is in the range of −1 to +1 with +1 denoting a perfect positive correlation, −1 denoting a perfect negative correlation and 0 denoting no correlation. A two-tailed t-test for the null hypothesis was carried out to determine the p value where the regression slope is zero. A statistically significant correlation is obtained with a probability (p value) less than 0.05.3. Results and discussion
3.1. AgNP and EPS characterization
| Diameter (nm) | pH | Zeta potential (mV) | |
|---|---|---|---|
| TEM | DLS | ||
| 22 ± 0.674 | 32.72 ± 2.214 | 8.02 | −28.4 ± 1.6 |
| EPS | TOC (mg L−1) | PS/TOC (mg PS/mg TOC) | PN/TOC (mg PN/mg TOC) | Volume of EPS added to make 50 mg L−1 TOC in 10 ml of experimental solution (ml) |
|---|---|---|---|---|
| SB | 69.45 | 0.56 | 0.74 | 7.20 |
| LB | 118.395 | 0.40 | 0.68 | 4.22 |
| TB | 151.35 | 0.32 | 0.60 | 3.30 |
| EPS | Bio polymers | PN | HMW PN | LMW PN | PS | HMW PS | LMW PS | HA | Building blocks | LMW neutrals | LMW acids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PN – protein, PS – polysaccharide, HA – humic acid. | |||||||||||
| SB | 17.19 | 18.52 | 8.08 | 10.43 | 12.33 | 9.11 | 3.21 | nq. | 18.64 | 2.93 | 0.60 |
| LB | 6.97 | 28.82 | 2.86 | 25.96 | 15.10 | 4.11 | 10.99 | nq. | 14.38 | 18.73 | 3.02 |
| TB | 3.81 | 32.50 | 2.36 | 30.14 | 15.34 | 1.45 | 13.89 | nq. | 15.20 | 24.43 | 4.28 |
3.2. Aggregation kinetics of AgNPs in different electrolyte solutions
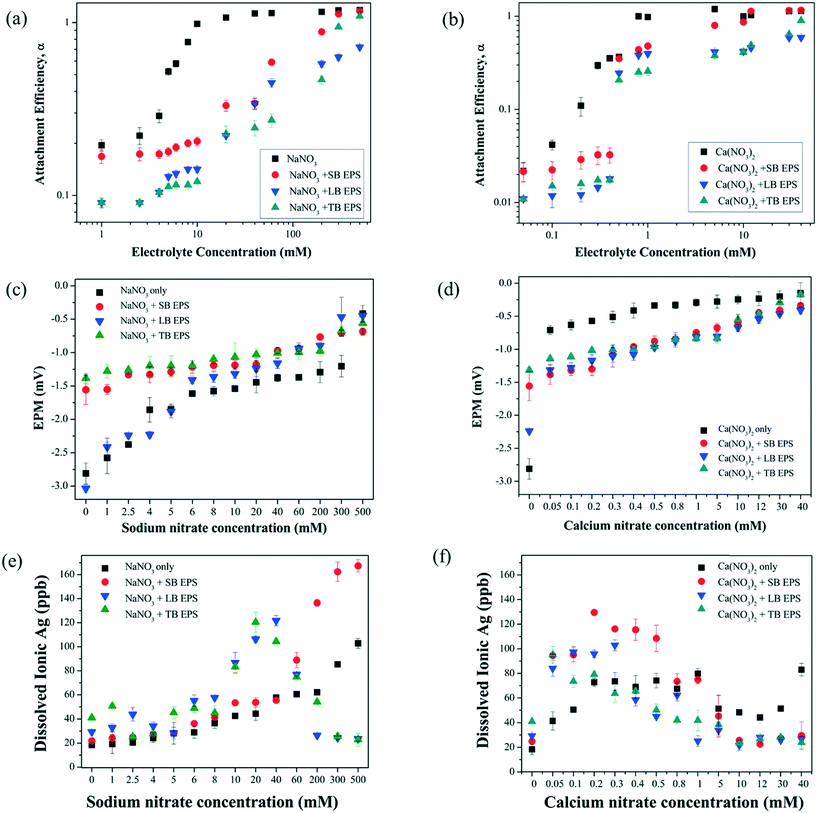
The AE values of AgNPs as a function of the electrolyte concentration (NaNO3 or Ca(NO3)2) are presented in Fig. 1. The hydrodynamic diameter profiles of AgNPs in the presence of the electrolyte are presented in Fig. S3.† At a relatively low concentration of the electrolyte (i.e., <10 mM for NaNO3 and <0.8 mM for Ca(NO3)2), the increase in the electrolyte concentration elevated the degree of charge screening16 and led to an increase in the rate of aggregation, as revealed by the increase in the AE. This is known as the reaction limited regime (α < 1). At higher electrolyte concentrations, i.e. 10–500 mM for NaNO3 and 10–40 mM for Ca(NO3)2, the charge of AgNPs was completely screened and the energy barrier between AgNPs was eliminated, enabling the NPs to undergo diffusion limited aggregation (α ≥ 1). | ||
| Fig. 1 Change in the attachment efficiencies of AgNPs as a function of NaNO3 and Ca(NO3)2 concentrations. | ||
This observed aggregation behavior of AgNPs in electrolyte solution is consistent with the DLVO theory. The aggregation kinetics reaches the maximum in the diffusion limited regime and is independent of the electrolyte concentration. The CCC of an electrolyte is the concentration at which the reaction limited and diffusion limited regimes intersect.37 The CCC for the AgNPs was determined to be 12 mM in NaNO3, which was much higher than that in Ca(NO3)2 (0.8 mM). According to the Schulze–Hardy rule, for a negatively charged surface, the CCC ratio of the cations of valences 2 and 1 should be in the range of  −6 to
−6 to 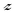 −2, where
−2, where 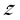 is the higher valence among the cations.43,44 The ratio of CCC values we obtained (0.8/12) is proportional to
is the higher valence among the cations.43,44 The ratio of CCC values we obtained (0.8/12) is proportional to  −3.9068, where
−3.9068, where  is the valence of the calcium counterion, which is in accordance with the Schulze–Hardy rule.
is the valence of the calcium counterion, which is in accordance with the Schulze–Hardy rule.
3.3. Effects of SB-EPS, LB-EPS and TB-EPS on the colloidal stability of AgNPs in monovalent cations
To explore the effects of different EPS fractions on the rate of aggregation of AgNPs, the AE in the presence of SB-EPS, LB-EPS and TB-EPS as a function of NaNO3 concentration was examined (Fig. 2(a)). Overall, the addition of EPS resulted in a much lower AE compared to the EPS-free solution, indicating that EPS hindered the aggregation of AgNPs. These results are consistent with previous research studies exploring the effect of biological macromolecules such as NOM, alginate and BSA on the stability of different types of NPs.4,16,36,45 Interestingly, only minor difference in the EPM of AgNPs was observed in the absence of EPS and presence of SB-EPS in the NaNO3 solution, whereas a considerable effect was observed in the presence of LB-EPS and TB-EPS as shown in Fig. 2(c). These results indicate that despite a significantly affected AE, SB-EPS did not have a considerable effect on the EPM. Hence, the change in the rate of aggregation of AgNPs under these conditions can be due to some other reasons, which can be mainly due to the steric repulsion induced from the adsorption of EPS molecules onto the AgNPs, which effectively stabilized the system. The percentage mass fractions of SB-EPS, LB-EPS and TB-EPS adsorbed onto the AgNPs as a function of NaNO3 concentration are presented in Fig. S4(a).† The results obtained for the adsorption demonstrate the presence of steric hindrance induced by the adsorbed EPS during the aggregation of AgNPs in the NaNO3 solution.The dissolved ionic Ag concentration of the experimental samples gradually increased with the electrolyte concentration in the absence of EPS (Fig. 2(e)). The trend observed in the ionic Ag concentration in the presence of SB-EPS is similar to the EPS free condition. However, the amount of ionic Ag released in the presence of SB-EPS is higher than in the absence of EPS, especially when NaNO3 concentration is high. In the presence of LB-EPS and TB-EPS, the ionic Ag concentration increased up to 120 ppb, which doubled the ionic Ag concentration compared to the other two cases, and then decreased to the lower values with the further increase in the electrolyte concentration. The decrease observed in the ionic Ag concentration can be attributed to the coating effect induced by the higher amount of DOC (Tables 2 and 3), present in the LB-EPS and TB-EPS solutions which inhibited the dissolution of AgNPs to ionic Ag.
In the presence of SB-EPS, LB-EPS and TB-EPS, the CCC increased from 12 mM (no EPS) to 250 mM, 982.4 mM and 376.7 mM for NaNO3, respectively (Fig. 2(a)), suggesting that LB-EPS stabilized AgNPs more effectively than SB-EPS and TB-EPS. This again can be reflected in the aggregation profiles of AgNPs in the presence and absence of SB-EPS, LB-EPS and TB-EPS (Fig. S5†), which shows the change in the hydrodynamic diameter of the solution with time. According to Fig. S5,† the hydrodynamic diameter of the AgNPs remained almost constant in the presence of LB-EPS, in a constant electrolyte concentration, where as it changed differently in the presence of SB-EPS and TB-EPS. Under these conditions, the adsorbed EPS (Fig. S4†) on the hydrophobic AgNP surfaces induce long range steric repulsive forces, hence promoting the AgNP stability.
3.4. Effects of SB-EPS, LB-EPS and TB-EPS on the colloidal stability of AgNPs in divalent cations
Fig. 2(b) shows the variation in the AE of the AgNPs in the presence and absence of EPS fractions as a function of the Ca(NO3)2 concentration. At a relatively low Ca(NO3)2 concentration, the AgNPs were stable in the presence of different EPS fractions. This stabilization can be due to the steric effect induced by the adsorbed EPS on the AgNPs as shown in Fig. S4(b).† Nevertheless, the AE of the AgNPs increased with the increasing Ca(NO3)2 concentration in the presence of EPS. When the Ca(NO3)2 concentration is increased above 10 mM, the AE was greater than 1 in the presence of SB-EPS, which further increased with the Ca(NO3)2 concentration. The AE was almost constant up to 0.4 mM and beyond 5 mM Ca(NO3)2 in the presence of LB-EPS and TB-EPS. The CCC values of AgNPs in Ca(NO3)2 changed from 0.8 mM (no EPS) to 11 mM, 1452.3 mM, and 44.1 mM in the presence of SB-EPS, LB-EPS and TB-EPS, respectively. Even though the CCC values increased compared to the no EPS conditions, the value obtained in the LB-EPS again shows a drastic difference. Furthermore, the CCC value obtained in the presence of LB-EPS in Ca(NO3)2 was higher than that in NaNO3. This shows that irrespective of the electrolyte, LB-EPS stabilized the AgNP suspension effectively than the other EPS fractions. The representative aggregation profiles of AgNPs in the presence and absence of different EPS fractions in Ca(NO3)2 solutions are shown in Fig. S6,† which also shows a similar observation to in the presence of NaNO3. Even though the change in the hydrodynamic diameter of AgNPs in the presence of LB-EPS and TB-EPS is similar and lower compared to the SB-EPS and EPS free conditions, the variation in the hydrodynamic diameter of LB-EPS is less compared to that of TB-EPS.The enhanced aggregation of AgNPs in the presence of SB-EPS compared to the other types of EPS in Ca(NO3)2 solutions could be due to the composition of SB-EPS with the formation of intermolecular bridges between Ca2+ and COO− in the macromolecules.16 This phenomenon was verified through the results obtained for the z-average diameters of AgNPs in different EPS fractions in the presence of varying Ca(NO3)2 concentrations. As shown in Fig. S7(b),† the z-average diameters of AgNPs in the presence of EPS increased with the Ca(NO3)2 concentration over 12 mM.
The EPM values became less negative as the concentration of Ca(NO3)2 increased (Fig. 2(d)), which was probably due to the charge screening reduced from the compression of the double layer or the neutralization of charge with the adsorption of Ca2+ (ref. 46) on the AgNP surface. Upon mixing with EPS, a similar increase in EPM with a Ca2+ concentration was observed, but with a smaller amount in the absence of EPS. This indicates the complexation of Ca2+ and EPS, forming EPS functional groups such as ionic carboxylates and phosphoryl which are negatively charged47 and increasing the negative EPM of AgNPs. Decreasing EPM values suggest that electrostatic forces may be prominent among the interactions between negatively charged AgNPs and EPS, which is similar to the observations by Mosley et al.48 Hence, the impact of EPS on the aggregation of AgNPs can be due to the combined impact of interparticle bridging and electrostatic forces, while the stability induced by EPS on the AgNPs can be due to the cumulative effect of electrostatic and steric repulsion.
The dissolved ionic Ag concentration in the Ca(NO3)2 solution in the absence and presence of EPS (Fig. 2(f)) increased with the electrolyte concentration and started to decrease at some point. This point of trend reversal was different in various EPS types. When comparing this result with the other results obtained using different techniques, the release of ionic Ag was inhibited at higher electrolyte concentrations in the presence of EPS. This may also be a consequence of the enhanced aggregation of AgNPs followed by coating with biological macromolecules present in the matrix.
3.5. Impact of the AgNP–EPS interactions on the FTIR spectrum
The FTIR spectra of the three types of EPS in the absence and presence of AgNPs were analyzed to qualitatively assess the impact of biochemical components of the EPS (Fig. 3). From Fig. 3(a), it can be observed that the peak at 3353 cm−1 (the red line) is assigned to –OH and –NH2.49–51 The peaks at 2920 and 2854 cm−1 can be attributed to asymmetric stretching vibrations and symmetric stretching of –CH2, respectively.52–54 The peak at 1643 cm−1 is assigned to the stretching vibration of –COOH.49,55 The broad peak at 1355 cm−1 is the overlapped peak of the symmetric deformation of –CH3 and –CH2 in the protein and symmetric stretching of –COOH.56 The peak at 1064 cm−1 is derived from the symmetric stretching of the phosphodiester backbone of nucleic acids.56 Upon mixing of SB-EPS with AgNPs (the blue line), some of the above-mentioned peaks disappear, and the intensities of the peaks at 2920, 1643 and 1064 cm−1 increased, which could be caused by the increased symmetric vibrations of CH2, vibrations of the C–N bond in the proteins and the stretching vibrations of O–H. This observation suggests that the stability of AgNPs induced by the SB-EPS can be linked mainly to the presence of hydroxyl groups in the solution.In the FTIR spectrum obtained for LB-EPS (the red line in Fig. 3(b)), peaks are observed at 3709 cm−1 (–OH and –NH2),49–51 2932 cm−1 (asymmetric stretching vibrations of –CH2),52,53 1639 cm−1 (stretching vibration of –COOH),49,55 1260 cm−1 (stretching vibration of C–O),57 and 1022 cm−1 (stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and asymmetric stretching vibration of –C–O–C–)58,59 (Fig. 3(b)). Due to the interaction with AgNPs (blue line), the peak at 3709 cm−1 disappears and the intensities at 2932 and 1639 cm−1 increased. The peak at 1022 cm−1 in LB-EPS blue-shifts to 979 cm−1 and the peak at 1260 cm−1 red-shifts to 1419 cm−1, suggesting that the functional groups in the LB-EPS attach onto the surface of AgNPs forming attractive coulombic interactions6 between the AgNPs and LB-EPS.
C and asymmetric stretching vibration of –C–O–C–)58,59 (Fig. 3(b)). Due to the interaction with AgNPs (blue line), the peak at 3709 cm−1 disappears and the intensities at 2932 and 1639 cm−1 increased. The peak at 1022 cm−1 in LB-EPS blue-shifts to 979 cm−1 and the peak at 1260 cm−1 red-shifts to 1419 cm−1, suggesting that the functional groups in the LB-EPS attach onto the surface of AgNPs forming attractive coulombic interactions6 between the AgNPs and LB-EPS.
In Fig. 3(c) (red line), the peaks at 2917 and 2829 cm−1 belong to the asymmetric stretching vibration and symmetric stretching of –CH2.52–54 The peak at 1742 cm−1 was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of ester groups in lipids and fatty acids.56 The peak at 1555 cm−1 was attributed to the stretching vibration of C–N and deformation vibration of N–H of amide II in the protein.55,59 The peaks at 1467, 1261, and 969 cm−1 are assigned to asymmetric deformation of CH3 and CH2 of proteins,56,60 stretching vibration of C–O arising from polysaccharides and nucleic acids.57 Most of these peaks disappeared due to the interaction with AgNPs. 1006 cm−1 (red shifted peak at 969 cm−1) with increased intensity can be observed in the mixture of AgNPs and TB-EPS, which could be caused by the bonding of C–O of the carboxylic groups and the C–O–C in the polysaccharides in the TB-EPS onto the AgNPs.
O stretch of ester groups in lipids and fatty acids.56 The peak at 1555 cm−1 was attributed to the stretching vibration of C–N and deformation vibration of N–H of amide II in the protein.55,59 The peaks at 1467, 1261, and 969 cm−1 are assigned to asymmetric deformation of CH3 and CH2 of proteins,56,60 stretching vibration of C–O arising from polysaccharides and nucleic acids.57 Most of these peaks disappeared due to the interaction with AgNPs. 1006 cm−1 (red shifted peak at 969 cm−1) with increased intensity can be observed in the mixture of AgNPs and TB-EPS, which could be caused by the bonding of C–O of the carboxylic groups and the C–O–C in the polysaccharides in the TB-EPS onto the AgNPs.
When comparing the interactions of the three types of the EPS with the AgNPs, the functional groups in the TB-EPS were more tolerant to the presence of AgNPs owing to the protective role of the outer layers.61 The differences between the FTIR spectra suggest that the different functional groups present in the EPS fractions interacted differently with AgNPs.
To investigate the correlation between DOM abundance and AE as well as the adsorbed carbon, the Pearson's correlation coefficients were calculated (Table 4). The amount of adsorbed carbon (AC) was strongly positive (p < 0.01) correlated to the concentrations of biopolymers, HMW PN, PN, LMW PN, PS, HMW PS, LMW PS, building blocks, LMW neutrals, LMW acids, TN and TOC. This reveals that the adsorption of carbon onto the AgNPs strongly depends on the amount of DOMs present in the EPS. Among them, PS showed the highest correlation (R = 0.869, p < 0.01) followed by building blocks. On the other hand, the AE had a strong negative correlation with the PN, LMW PN, PS, LMW PS, building blocks, LMW neutrals, LMW acids and TOC. This reveals the fact that the AE is reduced due to the presence of the dissolved organic matter, while decreasing the potential for the aggregation of AgNPs. The AE shows a weak or moderate negative correlation with the TN concentration (R = −0.216, p < 0.05). In addition, the AE shows a strong positive correlation with the electrolyte concentration, which depicts that the AgNP aggregation is facilitated by the electrolytes present in the matrix. There was nearly no correlation between the AE and the biopolymers, HMW PN and HMW PS, which is reasonable that they are less reactive to interact with the AgNPs due to their inherently neutral behavior.62
| Biopolymer | PN | HMW PN | LMW PN | PS | HMW PS | LMW PS | |
|---|---|---|---|---|---|---|---|
| AE | — | −0.445** | — | −0.433** | −0.436** | — | −0.415** |
| AC | 0.573** | 0.789** | 0.547** | 0.689** | 0.869** | 0.589** | 0.607** |
| Electrolyte conc. | Building blocks | LMW neutrals | LMW acids | TN | TOC | |
|---|---|---|---|---|---|---|
| **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level. “—” denotes that correlation is insignificant (p > 0.05). | ||||||
| AE | 0.505** | −0.359** | −0.397** | −0.395** | −0.216* | −0.435** |
| AC | — | 0.828** | 0.541** | 0.524** | 0.658** | 0.709** |
The improved stabilization of AgNPs in the presence of LB-EPS irrespective of the electrolyte was observed (Fig. 2(a) and (b)). This can be due to several factors, such as the increase in the concentration of protein compared to SB-EPS and the change in the hydrophobic–hydrophilic interactions which is governed by the different fractions of organic matter. The predominant factor was due to the lowest amount of building blocks present in the LB-EPS compared to the other three types of EPS. Building blocks in the EPS denote the hydrophilic DOC with a molecular weight of 300–500 g mol−1.33,63 Therefore, the low amount of hydrophilic parts (Table 3) present in the LB-EPS helps in improving the stability of AgNPs and reducing the aggregation.64 This is confirmed by the correlation between the building blocks and the AE and AC, which is significantly negative and positive, respectively.
When present together with the biomass in wastewater treatment systems, AgNPs tend to interact with the SB-EPS first as they would be available in abundance due to their solubility. According to the results in this study, interactions with the SB-EPS would result in the release of ionic Ag. The amount of ionic Ag released in the presence of SB-EPS is higher than that released in the absence of EPS (Fig. 2(e) and (f)). The released ionic Ag and the remaining AgNPs would then interact with the LB-EPS. Even though the amount of ionic Ag released in the presence of LB-EPS is lower than that of SB-EPS (Fig. 2(e) and (f)), the cumulative amount of ionic Ag released in the presence of both SB-EPS and LB-EPS can adversely affect the biomass. Due to the dissolved organic compounds present and the stabilizing capability in LB-EPS, the adverse impacts of the ionic Ag affecting the bacteria will be reduced as shown in the higher CCC, lower AE (Fig. 2(a) and (b)), FTIR (Fig. 3(b)) and correlation results (Table 4). Hence, with the prior interaction of LB-EPS, the TB-EPS, which is attached to the cell wall, would have minimal interaction with the ionic Ag released and AgNPs. Furthermore, a higher proportion of hydrophobic groups, i.e., protein related N–H, was present in the TB-EPS (Table 2 and 3(c)), which explains its role in increasing the surface hydrophobicity of the sludge65 with a higher concentration of protein-like substances protecting the bacteria.
On the other hand, in the presence of Na+ and low concentration of Ca2+, EPS stabilizes the AgNPs with the extracellular protein playing a dominant role in the stabilization process. Therefore, under these conditions, EPS may enhance the toxicity of AgNPs towards the bacteria and decrease the removal efficiency of AgNPs. However, at higher Ca2+ concentrations, dissolved exopolysaccharide molecules form polysaccharide aggregates through intermolecular bridging via calcium complexation. Subsequently, these aggregates bridge the AgNP aggregates together resulting in an increase in the overall size of the aggregates reducing the toxicity towards the bacteria and increasing the removal efficiency of the AgNPs. The concentration of electrolytes in wastewater usually falls within the higher range of the studied concentration range.34 When present together with EPS and electrolytes in wastewater treatment systems, AgNPs tend to aggregate due to the reduced dissolution by electrolytes. The AgNP aggregates formed will then be coated by the EPS present which further stabilizes the AgNPs and reduces their capability of releasing ionic Ag. The decrease in the release of ionic Ag reduces the toxicity of AgNPs towards the bacteria. The trapped AgNPs may finally end up in the downstream sludge system and eventually anaerobic digestion. Hence the presence of EPS in activated sludge will help in removing the AgNPs from wastewater and reduce their negative impact towards the environment. However, the concentration of EPS and the characteristics of wastewater will determine the final fate of AgNPs present in wastewater.
4. Conclusions
The presence of EPS and various types of cations in wastewater treatment systems would have different impacts on the transformations of AgNPs. This study reports the impact of EPS on the colloidal stability of AgNPs in the presence of electrolytes, NaNO3 and Ca(NO3)2. The results demonstrate that EPS effectively stabilize the AgNPs in the presence of NaNO3 and low concentration of Ca(NO3)2. However, this enhanced the rate of aggregation at a higher Ca(NO3)2 concentration as a result of the aggregation of the dissolved EPS with AgNPs through intermolecular bridging, connecting the AgNPs and forming aggregates. Among the three types of EPS, LB-EPS showed promising effects in improving the stabilization of AgNPs, mainly due to the less hydrophilic constituents present therein. Hence, the results of this study are useful in understanding the impact of EPS on the aggregation and colloidal stability of negatively charged NPs, which is important in assessing the transformations of NPs, eventually determining their transport and final fate in wastewater treatment systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the assistance received during the experiments from the Central Environmental Science & Engineering Laboratory (CESEL) and Dr. Qian Tingting in FTIR analysis at Nanyang Technological University, Singapore.References
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47, 3866–3877 CrossRef CAS.
- W. Zhou, Y.-L. Liu, A. M. Stallworth, C. Ye and J. J. Lenhart, Effects of pH, Electrolyte, Humic Acid, and Light Exposure on the Long-Term Fate of Silver Nanoparticles, Environ. Sci. Technol., 2016, 50, 12214–12224 CrossRef CAS.
- K. A. Huynh and K. L. Chen, Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions, Environ. Sci. Technol., 2011, 45, 5564–5571 CrossRef CAS PubMed.
- R. F. Domingos, N. Tufenkji and K. J. Wilkinson, Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid, Environ. Sci. Technol., 2009, 43, 1282–1286 CrossRef CAS.
- J.-T. Tai, C.-S. Lai, H.-C. Ho, Y.-S. Yeh, H.-F. Wang, R.-M. Ho and D.-H. Tsai, Protein–Silver Nanoparticle Interactions to Colloidal Stability in Acidic Environments, Langmuir, 2014, 30, 12755–12764 CrossRef CAS PubMed.
- S. S. Khan, A. Mukherjee and N. Chandrasekaran, Impact of exopolysaccharides on the stability of silver nanoparticles in water, Water Res., 2011, 45, 5184–5190 CrossRef CAS PubMed.
- S. Sudheer Khan, A. Mukherjee and N. Chandrasekaran, Interaction of colloidal silver nanoparticles (SNPs) with exopolysaccharides (EPS) and its adsorption isotherms and kinetics, Colloids Surf., A, 2011, 381, 99–105 CrossRef CAS.
- Y. Li, C. Yang, X. Guo, Z. Dang, X. Li and Q. Zhang, Effects of humic acids on the aggregation and sorption of nano-TiO2, Chemosphere, 2015, 119, 171–176 CrossRef CAS.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS.
- M. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Titanium nanomaterial removal and release from wastewater treatment plants, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS.
- S. A. Blaser, M. Scheringer, M. MacLeod and K. Hungerbühler, Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles, Sci. Total Environ., 2008, 390, 396–409 CrossRef CAS.
- B. Kim, C.-S. Park, M. Murayama and M. F. Hochella, Discovery and Characterization of Silver Sulfide Nanoparticles in Final Sewage Sludge Products, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS.
- Z. Wei, S. Huang, Y. Zhang, H. Li and S. Zhou, Characterization of extracellular polymeric substances produced during nitrate removal by a thermophilic bacterium Chelatococcus daeguensis TAD1 in batch cultures, RSC Adv., 2017, 7, 44265–44271 RSC.
- A. Omoike and J. Chorover, Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis, Geochim. Cosmochim. Acta, 2006, 70, 827–838 CrossRef CAS.
- M. Hoffman and A. W. Decho, in Microbial extracellular polymeric substances, Springer, 1999, pp. 217–230 Search PubMed.
- D. Lin, S. Drew Story, S. L. Walker, Q. Huang and P. Cai, Influence of extracellular polymeric substances on the aggregation kinetics of TiO2 nanoparticles, Water Res., 2016, 104, 381–388 CrossRef CAS PubMed.
- G.-H. Yu, P.-J. He, L.-M. Shao and P.-P. He, Stratification structure of sludge flocs with implications to dewaterability, Environ. Sci. Technol., 2008, 42, 7944–7949 CrossRef CAS.
- R. Weiner, S. Langille and E. Quintero, Structure, function and immunochemistry of bacterial exopolysaccharides, J. Ind. Microbiol., 1995, 15, 339–346 CrossRef CAS.
- C. Mayer, R. Moritz, C. Kirschner, W. Borchard, R. Maibaum, J. Wingender and H.-C. Flemming, The role of intermolecular interactions: studies on model systems for bacterial biofilms, Int. J. Biol. Macromol., 1999, 26, 3–16 CrossRef CAS.
- X.-M. Liu, G.-P. Sheng, H.-W. Luo, F. Zhang, S.-J. Yuan, J. Xu, R. J. Zeng, J.-G. Wu and H.-Q. Yu, Contribution of extracellular polymeric substances (EPS) to the sludge aggregation, Environ. Sci. Technol., 2010, 44, 4355–4360 CrossRef CAS.
- F. Ahimou, M. J. Semmens, G. Haugstad and P. J. Novak, Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness, Appl. Environ. Microbiol., 2007, 73, 2905–2910 CrossRef CAS.
- P. Lembre, C. Lorentz and P. Di Martino, Exopolysaccharides of the biofilm matrix: a complex biophysical world, The complex world of polysaccharides, 2012, pp. 371–392 Search PubMed.
- J. Labille, F. Thomas, M. Milas and C. Vanhaverbeke, Flocculation of colloidal clay by bacterial polysaccharides: effect of macromolecule charge and structure, J. Colloid Interface Sci., 2005, 284, 149–156 CrossRef CAS.
- B. Koukal, P. Rossé, A. Reinhardt, B. Ferrari, K. J. Wilkinson, J.-L. Loizeau and J. Dominik, Effect of Pseudokirchneriella subcapitata (Chlorophyceae) exudates on metal toxicity and colloid aggregation, Water Res., 2007, 41, 63–70 CrossRef CAS.
- I. Fernando and Y. Zhou, Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles, Chemosphere, 2019, 216, 297–305 CrossRef CAS.
- X. Zhou, Q. Wang, G. Jiang, P. Liu and Z. Yuan, A novel conditioning process for enhancing dewaterability of waste activated sludge by combination of zero-valent iron and persulfate, Bioresour. Technol., 2015, 185, 416–420 CrossRef CAS.
- I. Fernando, T. Qian and Y. Zhou, Long term impact of surfactants & polymers on the colloidal stability, aggregation and dissolution of silver nanoparticles, Environ. Res., 2019, 179, 108781 CrossRef CAS.
- I. Fernando and Y. Zhou, Concentration dependent effect of humic acid on the transformations of silver nanoparticles, J. Mol. Liq., 2019, 284, 291–299 CrossRef CAS.
- D. Li, Y. Zhou, Y. Tan, S. Pathak, M. bin Abdul Majid and W. J. Ng, Alkali-solubilized organic matter from sludge and its degradability in the anaerobic process, Bioresour. Technol., 2016, 200, 579–586 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. T. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- C. Le, C. Kunacheva and D. C. Stuckey, “Protein” measurement in biological wastewater treatment systems: a critical evaluation, Environ. Sci. Technol., 2016, 50, 3074–3081 CrossRef CAS.
- K. Xiao, Y. Chen, X. Jiang, Q. Yang, W. Y. Seow, W. Zhu and Y. Zhou, Variations in physical, chemical and biological properties in relation to sludge dewaterability under Fe (II)–Oxone conditioning, Water Res., 2017, 109, 13–23 CrossRef CAS PubMed.
- S. A. Huber, A. Balz, M. Abert and W. Pronk, Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography–organic carbon detection–organic nitrogen detection (LC-OCD-OND), Water Res., 2011, 45, 879–885 CrossRef CAS PubMed.
- L. Varden and F. Bou-Abdallah, Detection and separation of inorganic cations in natural, potable, and wastewater samples using capillary zone electrophoresis with indirect UV detection, Am. J. Anal. Chem., 2017, 8, 81 CrossRef CAS PubMed.
- A. Katsoyiannis and C. Samara, The fate of dissolved organic carbon (DOC) in the wastewater treatment process and its importance in the removal of wastewater contaminants, Environ. Sci. Pollut. Res., 2007, 14, 284–292 CrossRef CAS.
- K. L. Chen and M. Elimelech, Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions, J. Colloid Interface Sci., 2007, 309, 126–134 CrossRef CAS PubMed.
- K. L. Chen and M. Elimelech, Aggregation and Deposition Kinetics of Fullerene (C60) Nanoparticles, Langmuir, 2006, 22, 10994–11001 CrossRef CAS PubMed.
- X. Huangfu, J. Jiang, J. Ma, Y. Liu and J. Yang, Aggregation Kinetics of Manganese Dioxide Colloids in Aqueous Solution: Influence of Humic Substances and Biomacromolecules, Environ. Sci. Technol., 2013, 47, 10285–10292 CrossRef CAS PubMed.
- N. B. Saleh, L. D. Pfefferle and M. Elimelech, Influence of Biomacromolecules and Humic Acid on the Aggregation Kinetics of Single-Walled Carbon Nanotubes, Environ. Sci. Technol., 2010, 44, 2412–2418 CrossRef CAS.
- W. Zhang, U. S. Rattanaudompol, H. Li and D. Bouchard, Effects of humic and fulvic acids on aggregation of aqu/nC60 nanoparticles, Water Res., 2013, 47, 1793–1802 CrossRef CAS.
- B. Derjaguin and L. Landau, Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes, Prog. Surf. Sci., 1993, 43, 30–59 CrossRef.
- D. Lu, K. Xiao, Y. Chen, Y. N. A. Soh and Y. Zhou, Transformation of dissolved organic matters produced from alkaline-ultrasonic sludge pretreatment in anaerobic digestion: From macro to micro, Water Res., 2018, 142, 138–146 CrossRef CAS.
- J. T. G. Overbeek, The rule of Schulze and Hardy, Pure Appl. Chem., 1980, 52, 1151–1161 CAS.
- A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech and N. Tufenkji, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions, Environ. Sci. Technol., 2010, 44, 6532–6549 CrossRef CAS.
- A.-K. Ostermeyer, C. Kostigen Mumuper, L. Semprini and T. Radniecki, Influence of Bovine Serum Albumin and Alginate on Silver Nanoparticle Dissolution and Toxicity to Nitrosomonas europaea, Environ. Sci. Technol., 2013, 47, 14403–14410 CrossRef CAS.
- X. Liu, M. Wazne, Y. Han, C. Christodoulatos and K. L. Jasinkiewicz, Effects of natural organic matter on aggregation kinetics of boron nanoparticles in monovalent and divalent electrolytes, J. Colloid Interface Sci., 2010, 348, 101–107 CrossRef CAS.
- Q. Li and M. Elimelech, Organic fouling and chemical cleaning of nanofiltration membranes: measurements and mechanisms, Environ. Sci. Technol., 2004, 38, 4683–4693 CrossRef CAS PubMed.
- L. M. Mosley, K. A. Hunter and W. A. Ducker, Forces between colloid particles in natural waters, Environ. Sci. Technol., 2003, 37, 3303–3308 CrossRef CAS PubMed.
- Y. Zhou, S. Xia, J. Zhang, B. T. Nguyen and Z. Zhang, Insight into the influences of pH value on Pb (II) removal by the biopolymer extracted from activated sludge, Chem. Eng. J., 2017, 308, 1098–1104 CrossRef CAS.
- G. Guibaud, N. Tixier, A. Bouju and M. Baudu, Relation between extracellular polymers' composition and its ability to complex Cd, Cu and Pb, Chemosphere, 2003, 52, 1701–1710 CrossRef CAS PubMed.
- Y. Zhou, S. Xia, Z. Zhang, J. Zhang and S. W. Hermanowicz, Associated adsorption characteristics of Pb (II) and Zn (II) by a novel biosorbent extracted from waste-activated sludge, J. Environ. Eng., 2016, 142, 04016032 CrossRef.
- Y. Zhou, S. Xia, J. Zhang, Z. Zhang and S. W. Hermanowicz, Adsorption characterizations of biosorbent extracted from waste activated sludge for Pb(II) and Zn(II), Desalin. Water Treat., 2016, 57, 9343–9353 CrossRef CAS.
- M. Iqbal, A. Saeed and S. I. Zafar, FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste, J. Hazard. Mater., 2009, 164, 161–171 CrossRef CAS.
- G. Socrates, Infrared and Raman characteristic group frequencies: tables and charts, John Wiley & Sons, 2004 Search PubMed.
- G. Guibaud, S. Comte, F. Bordas and M. Baudu, Metal removal from single and multimetallic equimolar systems by extracellular polymers extracted from activated sludges as evaluated by SMDE polarography, Process Biochem., 2005, 40, 661–668 CrossRef CAS.
- B. Cao, L. Shi, R. N. Brown, Y. Xiong, J. K. Fredrickson, M. F. Romine, M. J. Marshall, M. S. Lipton and H. Beyenal, Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics, Environ. Microbiol., 2011, 13, 1018–1031 CrossRef CAS PubMed.
- L. Wei, Y. Li, D. R. Noguera, N. Zhao, Y. Song, J. Ding, Q. Zhao and F. Cui, Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism, J. Hazard. Mater., 2017, 321, 473–483 CrossRef CAS PubMed.
- G. Guibaud, S. Comte, F. Bordas, S. Dupuy and M. Baudu, Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel, Chemosphere, 2005, 59, 629–638 CrossRef CAS PubMed.
- Y. Liu, W. Lv, Z. Zhang and S. Xia, Influencing characteristics of short-time aerobic digestion on spatial distribution and adsorption capacity of extracellular polymeric substances in waste activated sludge, RSC Adv., 2018, 8, 32172–32177 RSC.
- F. Brian-Jaisson, M. Molmeret, A. Fahs, L. Guentas-Dombrowsky, G. Culioli, Y. Blache, S. Cérantola and A. Ortalo-Magné, Characterization and anti-biofilm activity of extracellular polymeric substances produced by the marine biofilm-forming bacterium Pseudoalteromonas ulvae strain TC14, Biofouling, 2016, 32, 547–560 CrossRef CAS.
- G. You, J. Hou, Y. Xu, C. Wang, P. Wang, L. Miao, Y. Ao, Y. Li and B. Lv, Effects of CeO2 nanoparticles on production and physicochemical characteristics of extracellular polymeric substances in biofilms in sequencing batch biofilm reactor, Bioresour. Technol., 2015, 194, 91–98 CrossRef CAS.
- D. K. Božanić, S. Dimitrijević-Branković, N. Bibić, A. Luyt and V. Djoković, Silver nanoparticles encapsulated in glycogen biopolymer: Morphology, optical and antimicrobial properties, Carbohydr. Polym., 2011, 83, 883–890 CrossRef.
- S. A. Huber, Evidence for membrane fouling by specific TOC constituents, Desalination, 1998, 119, 229–234 CrossRef CAS.
- M. Luo, Y. Huang, M. Zhu, Y.-N. Tang, T. Ren, J. Ren, H. Wang and F. Li, Properties of different natural organic matter influence the adsorption and aggregation behavior of TiO2 nanoparticles, J. Saudi Chem. Soc., 2018, 22, 146–154 CrossRef CAS.
- X. Guo, X. Wang and J. Liu, Composition analysis of fractions of extracellular polymeric substances from an activated sludge culture and identification of dominant forces affecting microbial aggregation, Sci. Rep., 2016, 6, 28391 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00861f |
| This journal is © The Royal Society of Chemistry 2020 |