Efficient removal of Pb2+ by Tb-MOFs: identifying the adsorption mechanism through experimental and theoretical investigations†
Received
23rd September 2018
, Accepted 16th November 2018
First published on 16th November 2018
Abstract
Nanotube-like Tb-based metal–organic frameworks (Tb-MOFs) were synthesized via the assembly of carboxylate-based ligands and Tb3+ under solvothermal conditions. The porous Tb-MOFs with high stability and considerable active functional groups make them ideal adsorbents in environment remediation. The factors influencing the adsorption property of Tb-MOFs toward Pb2+ ions were studied, comprising pH, ionic strength, adsorbent content, initial Pb2+ concentration and contact time. The Tb-MOFs exhibited excellent adsorption property with a maximum removal capacity of 547 mg g−1 and could maintain a high adsorption performance even after five cycles. Results from batch adsorption experiments and X-ray photoelectron spectroscopy (XPS) analysis imply that the formation of the inner-sphere complex (C–/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) between the nitrogenous groups of Tb-MOFs and Pb2+ is the primary adsorption mechanism. Furthermore, density functional theory (DFT) calculations confirm that the most favorable adsorption configuration varies with the reaction conditions and deprotonated functional groups tend to bond with Pb2+ at high pH. These results suggest that Tb-MOFs could be used as a promising adsorbent for the removal of Pb2+ ions from an aqueous environment.
N⋯Pb) between the nitrogenous groups of Tb-MOFs and Pb2+ is the primary adsorption mechanism. Furthermore, density functional theory (DFT) calculations confirm that the most favorable adsorption configuration varies with the reaction conditions and deprotonated functional groups tend to bond with Pb2+ at high pH. These results suggest that Tb-MOFs could be used as a promising adsorbent for the removal of Pb2+ ions from an aqueous environment.
Environmental significance
Lead is one of the world's leading environmental pollutants and has caused severe public health consequences. Reusable Tb-MOFs display highly efficient removal of Pb2+ from solutions. The mechanism of Pb2+ adsorption by Tb-MOFs was investigated by batch adsorption experiments, XPS analysis and DFT calculations. The inner-sphere complexes (C–/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) formed with stable adsorption configurations contribute greatly to the adsorption. These findings illustrate that Tb-MOFs can be regarded as promising adsorbents for the removal of Pb2+ from aqueous solutions in environmental cleanup. N⋯Pb) formed with stable adsorption configurations contribute greatly to the adsorption. These findings illustrate that Tb-MOFs can be regarded as promising adsorbents for the removal of Pb2+ from aqueous solutions in environmental cleanup.
|
Introduction
Heavy metal ions are prevalent in contaminated water because of their mass release and their non-biodegradable property, which may lead to severe environmental problems and hazards to human health.1,2 Lead with unquestionable toxicity has a relatively long biological half-life of 10 to 30 years in the ecosystem.3–5 Ingestion of lead can result in various diseases, including cancer, anaemia, liver failure and nephritis, even at a very low concentration.6,7 The maximum permissible levels for Pb2+ in potable water are 0.015 and 0.01 mg L−1 according to the Environmental Protection Agency (EPA) and World Health Organization (WHO), respectively.8 Therefore, lead treatment employment of efficient techniques including chemical precipitation, electrochemical techniques, flocculation, adsorption, and membrane separation has received considerable attention in various fields.3,8,9 Among these, adsorption is considered as one of the most effective techniques for the removal of heavy metal ions due to its high efficiency, ease of handling, and applicability even at low ion concentrations.3,9 Moreover, the regeneration capacity of the adsorbents endows the sustainability of the technique with regards to environmental protection. Conventional adsorbent materials (i.e., natural biosorbents, activated carbons, polymer particles, nanocomposites, mesoporous materials, etc.) are limited in real applications since they are not structurally and functionally tunable and contain limited active sites. These drawbacks of the conventional adsorbents increase the need of designing easily fabricated advanced adsorbents for Pb2+ capture.6,9–11
Metal–organic frameworks (MOFs) as a novel class of superior porous materials, have attracted wide attention due to their facile synthesis, large surface area, chemical stability and potential applications in purification and seperation.10,12 The outstanding performance of MOFs in these applications depends generally on specific functional groups and controllable pore size/shape in their structures. Recently, tremendous applications have been made for MOFs for their affluent active sites, such as amidogen, alkynyl, or sulfonic acid group, etc.10,12 However, the main disadvantages of MOF materials, which are their low chemical and mechanical stabilities compared with those of zeolites, may undoubtedly limit their use in large-scale industrial applications.13–15 Due to its coordination ability to organic functional groups, trivalent Ln3+ ions are deemed to be highly promising candidates for designing MOFs.16 MOFs constructed with Tb as the metal node had higher chemical stability and could be facilely functionalized with abundant functional groups.13,14,16 Additionally, compared with transition metal-based MOFs, the design and synthesis of innovative porous Ln-MOFs for metal ion adsorption and separation, particularly for Pb2+, are less explored because it is difficult to test the accessibility of the porous structures to control and predicate the overall crystal structures of Ln-MOFs.13,15 However, investigation on Ln-MOFs, particularly Tb-MOFs, would offer an opportunity to efficiently dispose the highly-toxic heavy metal ions present in contaminated water.
Selection of organic linkers for MOFs is essential to establish the skeleton structure and physiochemical characteristics. Hard-acid Ln3+ ions would preferably coordinate with hard-base carboxylate donor ligands according to the hard/soft-acids/bases (HSAB) theory.16,17 Due to the high oxophilicity of lanthanides, carboxylate ligands are believed to be the supreme linkers for building Ln-MOFs.16,18,19 4,4′,4′′-(1,3,5-triazine-2,4,6-triyltriimino) tris-benzoic acid (H3TATAB, shown in Fig. S1†) contains abundant N/O-containing groups. Amino, imine and carboxylic acid groups can be introduced into the Ln-MOFs, while the nitrogenous groups do not react with the constitutive metal ions. These unreacted N groups seem to be one of the most prospective ligands for heavy metal ion removal.14,20 Furthermore, in Ln-MOFs, Ln3+ ions can bridge with the deprotonated carboxylic acid groups through oxygen atoms, which can enhance the stability of the MOFs in aqueous solutions and retain the active sites for adsorption.19 The nitrogenous groups of H3TATAB have affinity for Pb2+ ions through complex formation or electrostatic interactions.21 However, due to the sophisticated adsorption mechanism and the lack of understanding of the nature of MOFs, people randomly utilize these materials as adsorbents in practice.
Taking advantage of the excellent properties of Ln-MOFs, H3TATAB and Tb3+ were selected to construct nanotube-like MOFs via solvothermal reactions. The Tb-MOFs were fabricated successfully under mild conditions and examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer–Emmett–Teller (BET), powder X-ray diffraction (XRD), Raman, and Fourier transform infrared spectroscopy (FT-IR). Specific porous structure and the abundant nitrogenous groups of the Tb-based MOFs make it suitable for removal of Pb2+ ions from contaminated water. Factors including pH, ionic strength, adsorbent content, initial Pb2+ concentration and contact time were investigated to understand the adsorption properties of Tb-MOFs toward Pb2+ ions. The reusability of Tb-MOFs was investigated by a simple solvent treatment for five repeated adsorption–desorption cycles. The reaction mechanism between Tb-MOFs and Pb2+ was clarified by a combination of batch adsorption experiments, X-ray photoelectron spectroscopy (XPS) analysis and density functional theory (DFT) calculations. The results suggest the potential application of Tb-MOFs for efficient elimination of Pb2+ ions from wastewater.
Experimental section
Synthesis of Tb-based MOFs
Tb-Based MOFs were successfully fabricated by using a solvothermal method in accordance with the previous research.13,14 In brief, Tb(NO3)3·6H2O (0.8 mmol), H3TATAB (0.4 mmol), Milli-Q water (16 mL) and DMF (24 mL) were added to a Teflon-lined stainless steel autoclave and heated at 100 °C for 72 h, and then cooled to room temperature. The as-prepared Tb-based MOFs were washed with Milli-Q water and ethyl alcohol several times.
Batch adsorption experimental systems
The adsorption of Pb2+ ions on Tb-MOFs was conducted in 10 mL polythene centrifugal tubes. Various volumes of Tb-MOFs (1.5 g L−1), Milli-Q water, NaCl and Pb2+ (360 mg L−1) were added into the tubes to obtain the required suspensions of 0.001, 0.01 or 0.1 mol L−1 ionic strength. 0.01–0.1 mol L−1 NaOH or HCl aqueous solutions were employed to adjust the pH value of the mixtures. To achieve adsorption equilibrium, the experiments were performed at 298, 308 or 318 K for 24 h and then, the suspensions were separated by centrifugation (8000 rpm, 20 min). Finally, the concentrations of Pb2+ were detected by spectrophotometry (λmax = 616 nm) with chlorophosphonazo III as the chromogenic reagent.
The regeneration of Tb-MOFs was performed by using 0.2 mM EDTA solution as the desorption solvent with solid/liquid 0.15 g L−1. After adsorption, the Tb-MOFs were separated and added in EDTA solution under stirring for 24 h. Then, they were separated by centrifugation and washed thoroughly with Milli-Q water several times. The recycled adsorbents could be extracted and used for Pb2+ ion removal again.
The adsorption percentage (%), distribution coefficient (Kd) and adsorption capacity (Cs, mg g−1) were calculated according to the following equations:22–24
| | 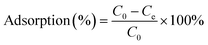 | (1) |
| | 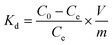 | (2) |
| | 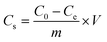 | (3) |
where
C0 and
Ce (mg L
−1) are the initial and the equilibrium concentrations of Pb
2+, respectively,
V (mL) is the suspension volume, and
m (g) represents the weight of Tb-MOFs. Experimental data are presented as the average of triplicate measurements and the relative errors were less than 5%.
Characterization
The morphology of the as-prepared Tb-MOFs was characterized by SEM (S-2500, Japan Hitachi) and TEM (JEM-2010) with EDS mapping. Powder XRD spectra were determined by a diffractometer (Philips X'Pert Pro Super X-ray) with Cu Kα source (λ = 1.54178 Å) and Mercury software (Mercury 3.6) was used to simulate the XRD curve of Tb-MOFs. The as-prepared Tb-MOFs were also investigated by FT-IR spectroscopy (Nicolet 8700, Thermo Scientific) in the range from 400 to 4000 cm−1 and Raman spectroscopy (RAMANLOG 6, SPEX company, USA) at 298 K. The zeta-potential values for Tb-MOFs were recorded over a range of pH via a Nanosizer ZS instrument (Malvern Instrument Co., UK) at 298 K. The specific surface area was measured under N2 atmosphere using a NOVA 4200e instrument (Quantachrome, FL, USA). Thermogravimetric analysis (TGA) was performed on a thermal gravimetric analyzer (Shimadzu, Kyoto, Japan) under N2 atmosphere by heating from room temperature to 800 °C at a heating rate of 20 °C per min. The XPS measurement was recorded using ESCALAB 250 (Thermo-VG Scientific, USA).
Computational details
Calculations for all possible adsorption structures were performed in the Gaussian 09 program using the density functional theory (DFT) with WB97XD functional.4,14 The 6-31G(d, p) basis sets were adopted for C, H, O, and N atoms, while the pseudopotential basis set of Lanl2dz was used for the Pb atom. One or two TATAB ligands were selected from the single crystal structure to investigate the adsorption styles of Pb2+ on MOFs. In order to simulate the probable real environment of MOFs, three or six O atoms of the carboxylate terminals (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups) were fixed and the other O atoms of the carboxylate (COO−) were saturated by H atoms.14 Considering the possible deprotonation of N atoms in Tb-MOFs, both deprotonated and undeprotonated TATAB models were used in calculations. All possible initial structures of Pb2+ binding on various sites of MOF were considered and optimized to local minima.4
O groups) were fixed and the other O atoms of the carboxylate (COO−) were saturated by H atoms.14 Considering the possible deprotonation of N atoms in Tb-MOFs, both deprotonated and undeprotonated TATAB models were used in calculations. All possible initial structures of Pb2+ binding on various sites of MOF were considered and optimized to local minima.4
Results and discussion
Characterizations of Tb-based MOFs
The morphology and nanostructure of the Tb-MOFs were characterized by SEM and TEM (Fig. 1). The Tb-MOFs display a uniform nanotube morphology with smooth and tidy surface (Fig. 1a–c). Fig. 1d–f clearly show that the Tb-MOFs have nanotube structures with multilayered walls. Closer examination reveals that the average diameter of the nanotubes is 25 nm and the length of nanotubes varies approximately between 200 and 1000 nm. EDS mapping was conducted to study the distribution of C, N, O and Tb elements (Fig. S2†). The EDS maps of Tb and N (Fig. 1h and i) have profiles similar to the TEM image (Fig. 1g), and the spots were homogeneously distributed.
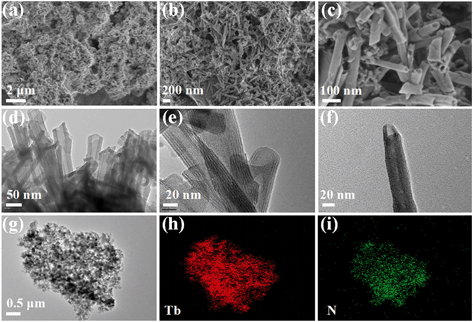 |
| | Fig. 1 SEM (a–c) and TEM images (d–g) of Tb-MOFs. EDS mapping (h and i) of Tb-MOFs. | |
Fig. 2a shows the XRD patterns of the as-prepared Tb-MOFs and the simulated Tb-MOFs curve using Mercury software.13,14 The Tb-MOFs belong to the monoclinic space group (P21/c) and exhibited a nanotube-like 3D framework. The patterns of Tb-MOFs agree well with the simulated curve, which indicates that Tb-MOF nanotubes with crystalline structure have been successfully synthesized. The structure of Tb-MOFs can be described as symmetry-related dinuclear Tb carboxylate clusters with Tb as the metal node and deprotonated H3TATAB as the ligands.13,14 The Tb-carboxylate chains with dinuclear clusters connected to carboxylate groups are shown in Fig. 2b. The dinuclear Tb cluster consists of two 8-coordinated Tb3+ ions bridged by oxygen atoms and are linked with the TATAB ligands (Fig. 2c). The XRD patterns at different pH values were similar to each other, suggesting that the Tb-MOFs are chemically stable over a broad pH range (pH 3.0–8.0).
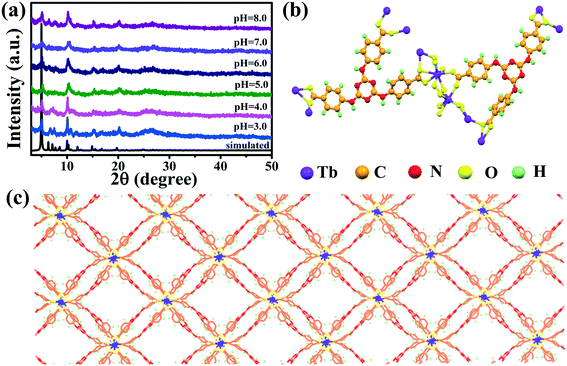 |
| | Fig. 2 XRD patterns of the Tb-MOFs powder (experimental and simulated curves) (a); the coordinated environments of Tb3+ (b); and the crystal structure of Tb-MOFs (c). | |
The FT-IR spectrum of Tb-MOFs is presented in Fig. 3a. A series of characteristic absorption bands in the range of 400–780 cm−1 can be assigned to the Tb–O lattice vibrations.25,26 The bands at 874, 1243, 1384, 1500 and 3410 cm−1 correspond to N–H, C–NH–C, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –NH stretching vibrations,21,27–29 respectively, and indicate the abundance of nitrogenous groups (such as amino and imine) on the surface of Tb-MOFs. The nitrogen-containing functional groups can offer plenty of active sites and thus contribute to the excellent adsorption performance of Tb-MOFs. The other absorption bands at 795 (C–H stretching), 1180 (C–O stretching), 1600 (aromatic ring stretching), 1660 (C
N and –NH stretching vibrations,21,27–29 respectively, and indicate the abundance of nitrogenous groups (such as amino and imine) on the surface of Tb-MOFs. The nitrogen-containing functional groups can offer plenty of active sites and thus contribute to the excellent adsorption performance of Tb-MOFs. The other absorption bands at 795 (C–H stretching), 1180 (C–O stretching), 1600 (aromatic ring stretching), 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), and 2930 cm−1 (C–H stretching) suggested that the integrated structure of TATAB still remains unchanged even after the formation of Tb-carboxylate chains.30–33
C stretching), and 2930 cm−1 (C–H stretching) suggested that the integrated structure of TATAB still remains unchanged even after the formation of Tb-carboxylate chains.30–33
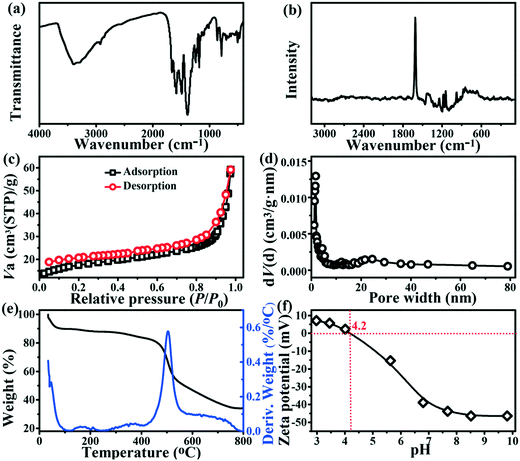 |
| | Fig. 3 FT-IR spectrum (a); Raman spectrum (b); N2 adsorption–desorption isotherm (c); pore size distribution curve (d); TAG (e) and the zeta potential (f) of Tb-MOFs. | |
Raman spectroscopy, a non-destructive technique, was used to characterize the structure of Tb-MOFs. As shown in Fig. 3b, the peak at 1613 cm−1 is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode of the benzene ring in the TATAB linker.34,35 The peak at 1414 cm−1 is associated with the vibration of triazine and the peak at 977 cm−1 is assigned to the C–N stretching mode.35 The strong broad peak at 665 cm−1 is attributed to the stretching vibration of Tb–O,36,37 suggesting high affinity between the Tb and O atoms. The peak at 422 cm−1 is attributed to the Tb–O stretching mode.38 The peaks at 579, 736, 1155 and 1306 cm−1 are attributed to the C–H stretching mode of benzene ring and N–H stretching mode of triazine.29 The obtained Raman spectra is further evidence that the functional groups of TATAB were still maintained in the Tb-MOF structures.
C stretching mode of the benzene ring in the TATAB linker.34,35 The peak at 1414 cm−1 is associated with the vibration of triazine and the peak at 977 cm−1 is assigned to the C–N stretching mode.35 The strong broad peak at 665 cm−1 is attributed to the stretching vibration of Tb–O,36,37 suggesting high affinity between the Tb and O atoms. The peak at 422 cm−1 is attributed to the Tb–O stretching mode.38 The peaks at 579, 736, 1155 and 1306 cm−1 are attributed to the C–H stretching mode of benzene ring and N–H stretching mode of triazine.29 The obtained Raman spectra is further evidence that the functional groups of TATAB were still maintained in the Tb-MOF structures.
The surface area of Tb-MOFs calculated from the N2 adsorption–desorption isotherm was 56.72 m2 g−1 (Fig. 3c). Since the average pore diameter was ∼1.75 nm (Fig. 3d), the Tb-MOFs could be regarded as microporous materials. However, the surface area of Tb-MOFs is not as large as other reported MOFs,13,14,39 which may due to the lower crystallinity and the relatively limited pore/size distribution of Tb-MOFs. In addition, the adsorption and desorption curves were not completely closed due to the slightly irregular mesoporosity of Tb-MOFs.40 TGA measurements were performed to study the thermal stability of Tb-MOFs (Fig. 3e). For a fresh sample of Tb-MOFs, the TGA curve shows the weight loss of moisture and ethanol from 40 to 100 °C. Then, the TGA curve does not show significant changes from 100 to 450 °C, which suggests the relative stability of Tb-MOFs, the compound decomposed quickly upon further heating to 500 °C due to the carbonization of H3TATAB. These results indicate that the Tb-MOFs have high stability in aqueous systems.
Zeta potential, an important parameter that characterizes the surface charge of Tb-MOFs, is shown in Fig. 3f. The pHPZC (pH at the point of zero charge) value of Tb-MOFs is ∼4.2. It can be seen that the zeta potential of Tb-MOFs shifts from positive to negative with an increase in pH. This is attributed to the deprotonation of the functional groups of Tb-MOFs at high pH values. The electrostatic attraction between the negatively charged Tb-MOFs and the heavy metal ions is the driving force for the adsorption but the electrostatic repulsion can hinder the metal ions from the liquid to adsorb on the surface of the solid. Hence, according to the zeta potential values, the range of pH for the removal of cations can be selected.
Effect of pH and ion strength
The pH value of a solution plays a vital role in the adsorption process; it not only affects the species of heavy metal ions but also the surface charge and protonation/deprotonation of the adsorbents.3 The effect of pH on the adsorption of Pb2+ by Tb-MOFs has been investigated in the range from 3.0 to 7.0 (Fig. 4a). Pb exists in the form of Pb2+ at pH < 7.0 (Fig. S3†). It can be observed that the adsorption of Pb2+ onto Tb-MOFs was strongly pH-dependent and Pb2+ removal increased with the increase in pH values. At pH > pHPZC, the positively charged cationic Pb2+ can be easily adsorbed on the negatively charged Tb-MOFs by electrostatic attraction. As pH increases, the deprotonation of the nitrogenous groups favors the adsorption of Pb2+via Pb⋯N complexation, thus considerably increasing Pb2+ removal. In contrast, at pH < pHPZC, the surface of Tb-MOFs is positively charged because the nitrogenous groups are excessively protonated and the abundant H+ ions are prone to compete with Pb2+ ions, which leads to the decrease in removal percentage at low pH.3
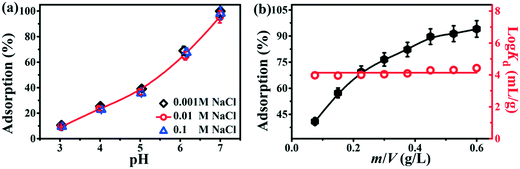 |
| | Fig. 4 Effect of pH and ion strength on the adsorption of Pb2+ by Tb-MOFs at m/V = 0.15 g L−1 (a). Effect of Tb-MOF content on Pb2+adsorption (b). [Pb2+] = 90 mg L−1, pH = 5.5. | |
In order to verify the practical usability, the interaction between Tb-MOFs and Pb2+ was investigated in solutions with different ionic strengths. As shown in Fig. 4a, the adsorption of Pb2+ on Tb-MOFs is weakly dependent on the ionic strength. It has been reported that the outer-sphere complexation depends on the ionic strength, whereas the inner-sphere complexation exhibits outstanding tolerance towards ionic strength changes because of the strong chemical bonding between the heavy metal ions and the active sites.21 The weak interference of ionic strength on Pb2+ adsorption suggests that adsorption mainly depends on the inner-sphere complexation (nitrogenous groups with lone pair of electrons binding to Pb2+ ions) rather than the outer-sphere surface complexation.
Effect of adsorbent content
Adsorbent content is an important factor that affects the adsorption efficiency of a material for a fixed original concentration of the heavy metal ions. Fig. 4b depicts the effect of the Tb-MOFs content on Pb2+ removal. A meaningful relationship is that the adsorption increases quickly as the adsorbent content increased. This can be interpreted in this study as the increase in the Tb-MOFs content leads to more active sites for Pb2+ removal. Additionally, the distribution coefficient Kd is usually applied to evaluate the interaction ability between the adsorbent and heavy metal ions.15,21 As shown in Fig. 4b, Kd is hardly dependent on the content of Tb-MOFs, which agrees with its physicochemical properties.11,15 Furthermore, the values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd for Tb-MOFs are above 4.0 mL g−1, which indicates good affinity of Tb-MOFs for Pb2+ ions.15,21 Therefore, the Tb-MOFs can be considered as an efficient adsorbent for Pb2+ removal from large volumes of wastewater.
Kd for Tb-MOFs are above 4.0 mL g−1, which indicates good affinity of Tb-MOFs for Pb2+ ions.15,21 Therefore, the Tb-MOFs can be considered as an efficient adsorbent for Pb2+ removal from large volumes of wastewater.
Adsorption kinetics
The adsorption kinetics of Pb2+ ions on Tb-MOFs is shown in Fig. 5a. The removal rate increases quickly within 10 min due to the large number of available sites at the initial stage. Then, it increases slowly as the concentration of Pb2+ ions and adsorption sites reduce until reaching the adsorption equilibrium within 50 min. To further study the adsorption mechanism of Tb-MOFs, the pseudo-second-order model and intraparticle diffusion model were used to evaluate the experimental data.3,23 The equations with fitting parameters are shown in Table S1.† It can be seen that the pseudo-second-order model depicts the whole adsorption process very well with high correlation coefficients (0.999). Adsorption of Pb2+ ions on Tb-MOFs is primarily by chemisorption involving the complexation of Pb2+ ions with functional groups of Tb-MOFs.11 The intraparticle diffusion model is not merely the limiting step because the straight line does not pass through the origin (Fig. 5b). The intercept (A) of intraparticle diffusion, an important parameter, reflects the thickness of the boundary layer that limits the diffusion rate of ions. The value of A increased with the increase in time (Table S1†), i.e., the larger the A values, the greater would be the boundary layer effect. This corresponds to the fact that the adsorption rate decreases gradually with the increase in reaction time. These results indicate that four steps with different limiting processes are possibly involved: (I) Pb2+ ions rapidly reach the surface of Tb-MOFs due to the presence of abundant active sites, resulting in instantaneous or external surface adsorption; (II) adsorption slows down, indicating the rate-limiting stage for Pb2+ ions diffusion into the interior of the Tb-MOFs nanotube; (III) adsorption declines quickly due to the relatively low residual Pb2+ concentration and the limited amounts of unoccupied active sites; and (IV) Pb2+ adsorption reaches a quite stable equilibrium in the final step. It should be noted that intraparticle diffusion is not the sole rate-limiting step and the chemical complex formation might also be involved.41
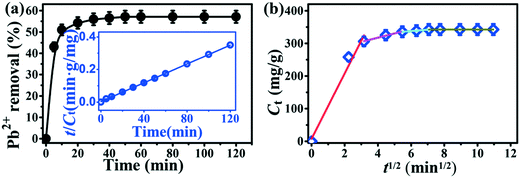 |
| | Fig. 5 Adsorption kinetics of Pb2+ on Tb-MOFs. Pseudo-second-order kinetic model (a) and intraparticle diffusion model (b). [Pb2+] = 90 mg L−1, pH = 5.5, m/V = 0.15 g L−1. | |
Adsorption isotherms and thermodynamics
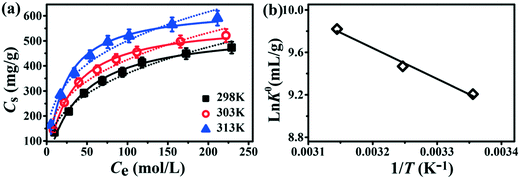
To further investigate the adsorption performance and the thermodynamic properties of Tb-MOFs, isotherm models, which can demonstrate the interactions between the Tb-MOFs and Pb2+ ions, were employed. The adsorption of Pb2+ ions increased promptly with an increase in original concentration (Fig. 6a), which is due to abundant active sites available on Tb-MOFs. Then, adsorption gradually increased and reached a plateau as the active sites were occupied completely. Additionally, the adsorption increased monotonically with the increase in temperature in the range from 298 to 313 K, indicating that the increase in the temperature is beneficial for Pb2+ removal by Tb-MOFs. The adsorption data could be simulated by the Langmuir and Freundlich models (Fig. 6a), which are shown as eqn (4) and (5), respectively.42| | 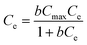 | (4) |
where Cmax (mg g−1) is the maximum adsorption capacity, b is the constant related to the heat adsorption, and 1/n and KF are the Freundlich constants. The fitting parameters obtained by Langmuir and Freundlich models are presented in Table 1. According to the regression coefficients (R2), the Langmuir model is more suitable for simulating the thermodynamic data than the Freundlich model, and suggests that the active sites of Tb-MOFs have equal adsorption performance and are uniformly distributed. Compared with the maximum Pb2+ adsorption capacity of other MOFs and adsorbents, Tb-MOFs exhibits higher adsorption capacity in spite of not having sufficiently high surface area (Table 2).
 |
| | Fig. 6 Isotherms of Pb2+ adsorbed onto Tb-MOFs (solid line: Langmuir model; dashed line: Freundlich model) (a). Liner plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0versus 1/T (b). K0versus 1/T (b). | |
Table 1 Parameters calculated from Langmuir and Freundlich models for Pb2+ adsorption onto Tb-MOFs
|
T (K) |
Langmuir |
Freundlich |
|
C
max (mg g−1) |
b (L mg−1) |
R
2
|
K
F
|
n
|
R
2
|
| 298 |
547 |
0.025 |
0.988 |
71 |
0.359 |
0.969 |
| 308 |
576 |
0.035 |
0.994 |
95 |
0.325 |
0.956 |
| 318 |
635 |
0.047 |
0.983 |
126 |
0.299 |
0.957 |
Table 2 Comparison of the maximum adsorption capacity of Pb2+ on Tb-MOFs with those of other adsorbents
| Adsorbents |
pH |
C
max (mg g−1) |
Ref. |
| MOF-derived magnetic inorganic sorbents |
4.9 |
345 |
3
|
| Magnetic aminofunctionalized aluminium MOF |
— |
492 |
4
|
| [Ag12(MA)8(mal)6.18·H2O]n MOF |
7.0 |
120 |
5
|
| g-C3N4 |
5.0 |
65.6 |
9
|
| MoS2@biochar |
5.0 |
189 |
11
|
| Zirconium-based porous MOF |
7.0 |
73 |
22
|
| Amino-functionalization of Cr-based MOFs |
6.0 |
88 |
42
|
| Bifunctional mesoporous organosilica |
6.0 |
22 |
43
|
| Tb-MOFs |
5.5 |
547 |
This study |
Temperature-dependent adsorption isotherms were plotted to calculate the thermodynamic parameters, namely, entropy change (ΔS0), enthalpy change (ΔH0) and Gibbs free energy change (ΔG0) as follows:11
| | 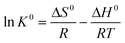 | (6) |
| | ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0 | (7) |
where
K0 represents the thermodynamic equilibrium constant,
R is the gas constant (8.314 J mol
−1 K
−1), and
T (K) is the temperature in Kelvin. ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0 was be obtained by plotting ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kdversus Ce
Kdversus Ce and extrapolating the curve to 0. The thermodynamic parameters can reveal the mechanism of Pb
2+ ion adsorption onto Tb-MOFs (
Fig. 6b and Table S2
†). The increase in positive Δ
H0 with increase in temperature suggests an endothermic adsorption process. A likely explanation for the positive value of Δ
H0 is that the Pb
2+ cations dissolve well in water, while the hydration sheath of Pb
2+ cations may hinder its adsorption on the Tb-MOFs surface. However, with the increase in temperature, the enhanced molecular motion may reduce the thickness of the hydration sheath. The potential barrier between Pb
2+ ions and Tb-MOFs is reduced, thus enhancing the adsorption of Pb
2+ ions on the Tb-MOFs. Δ
G0 becomes more negative with the increase in temperature, which implies that the adsorption process is spontaneous and higher temperature is beneficial to enhance the adsorption performance. Furthermore, the positive value for Δ
S0 reveals the random increase in the amount of Pb
2+ adsorbed on the Tb-MOFs at the solid/solution interface and the high affinity between the Tb-MOFs and Pb
2+.
23
Adsorption mechanism
XPS analysis was performed to identify the surface properties of the Tb-MOFs before and after Pb2+ ion adsorption. Fig. 7a shows the coexistence of Tb, N, O, and C elements in the Tb-MOF samples. After the adsorption of Pb2+ ions by Tb-MOFs (Tb-MOFs–Pb), new strong peaks appeared at 643.8, 437.9, 415.3, 138.6 and 19.5 eV, corresponding to Pb 4p, Pb 4f and Pb 5d. This directly proves that the Pb2+ ions are successfully adsorbed on Tb-MOFs.
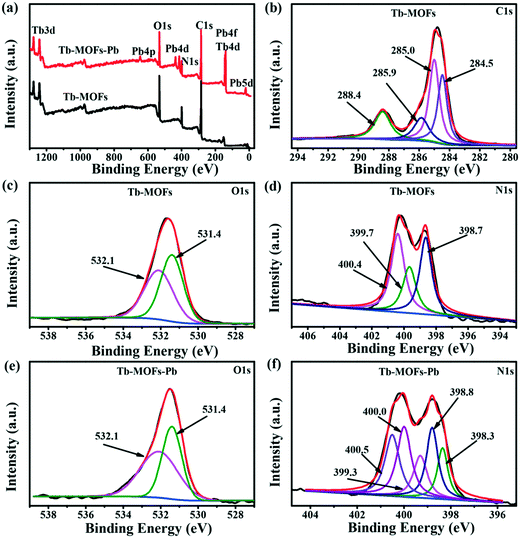 |
| | Fig. 7 XPS survey of Tb-MOFs before and after Pb2+ adsorption (a); high-resolution spectra of C 1s (b), O 1s (c), and N 1s (d) present in Tb-MOFs. (e) O 1s and (f) N 1s spectra of Tb-MOFs after Pb2+ adsorption i.e. Tb-MOFs–Pb. | |
The high-resolution spectrum can be applied to differentiate and identify the elemental composition on the surface of the adsorbent, which would help elucidating the adsorption mechanism. In the high-resolution C 1s curve of Tb-MOFs (Fig. 7b), the four different peaks centered at 288.4, 285.9, 285.0, and 284.5 eV can be ascribed to O–C–O, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and benzene ring,43–46 respectively, which are consistent with the FT-IR and Raman analyses. In the high-resolution O 1s spectrum of Tb-MOFs, the two peaks at 532.1 and 531.4 eV are attributed to Tb–O and C–O,47,48 respectively (Fig. 7c). Interestingly, after Pb2+ ions adsorbed on the Tb-MOFs, the deconvoluted O1s spectrum of Tb-MOF–Pb shows almost unchanged Tb–O and C–O peaks (Fig. 7e), indicating that the contribution of surface oxygenic functional groups in the ion removal process may have been overlooked. This phenomenon can be attributed to the saturated coordination of Tb atoms and the hydrophobic benzene hindering the heavy metal ions in the liquid from the surface oxygenic functional groups of the Tb-MOFs.14 The N 1s band (Fig. 7d) can be divided into three peaks of N species, namely, 400.4, 399.7, and 398.7 eV, corresponding to C–N, N–H, and C
N and benzene ring,43–46 respectively, which are consistent with the FT-IR and Raman analyses. In the high-resolution O 1s spectrum of Tb-MOFs, the two peaks at 532.1 and 531.4 eV are attributed to Tb–O and C–O,47,48 respectively (Fig. 7c). Interestingly, after Pb2+ ions adsorbed on the Tb-MOFs, the deconvoluted O1s spectrum of Tb-MOF–Pb shows almost unchanged Tb–O and C–O peaks (Fig. 7e), indicating that the contribution of surface oxygenic functional groups in the ion removal process may have been overlooked. This phenomenon can be attributed to the saturated coordination of Tb atoms and the hydrophobic benzene hindering the heavy metal ions in the liquid from the surface oxygenic functional groups of the Tb-MOFs.14 The N 1s band (Fig. 7d) can be divided into three peaks of N species, namely, 400.4, 399.7, and 398.7 eV, corresponding to C–N, N–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N.49–51 It is clearly seen that the binding energies of C
N.49–51 It is clearly seen that the binding energies of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N, and N–H increase after Pb2+ ions adsorbed on Tb-MOFs. This is because the shared bond between the N atom and Pb2+ ions can decrease the electron cloud density on the N atom, and hence increase the binding energy of the nitrogenous groups.52 Moreover, two new peaks at 399.3 and 398.3 eV can be probably attributed to amine (–N–) and imine (–N
N, C–N, and N–H increase after Pb2+ ions adsorbed on Tb-MOFs. This is because the shared bond between the N atom and Pb2+ ions can decrease the electron cloud density on the N atom, and hence increase the binding energy of the nitrogenous groups.52 Moreover, two new peaks at 399.3 and 398.3 eV can be probably attributed to amine (–N–) and imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) from the Pb–N– and Pb–N
) from the Pb–N– and Pb–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) bonding modes. Thus, the nitrogenous groups could act as active sites for coordination with Pb2+ ions. These results are consistent with the fact that the adsorption of Pb2+ ions on the Tb-MOFs is mainly ascribed to the complexation with nitrogenous groups via the inner-sphere mechanism (Pb–N– and Pb–N
bonding modes. Thus, the nitrogenous groups could act as active sites for coordination with Pb2+ ions. These results are consistent with the fact that the adsorption of Pb2+ ions on the Tb-MOFs is mainly ascribed to the complexation with nitrogenous groups via the inner-sphere mechanism (Pb–N– and Pb–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ).
).
Desorption mechanism and recycle performance
For the Pb2+ adsorption to be useful and economically favorable, the Tb-MOFs must have good adsorption capacity and should be regenerated. The regeneration agents were applied to recycle the spent Tb-MOFs. An effective, strong ligand and non-destructive regeneration approach was employed using the chelating agent (EDTA), which demonstrated both good ion desorption efficiency and capacity recovery due to its strong affinity to Pb2+ ions. Fig. 8a shows the regeneration ability of Tb-MOFs after treatment with EDTA. The Pb2+ adsorption capacity slightly reduced for EDTA since some active sites on Tb-MOFs occupied by Pb2+ ions could not be completely recovered. However, a high adsorption capacity could be maintained even after five adsorption–desorption cycles. XRD was employed to investigate the stability of the Tb-MOFs (Fig. 8b), and no significant changes could be observed in the XRD pattern, suggesting that Tb-MOFs maintains high structural stability after every cycle of regeneration. A minor amount of Tb ions was released in the solution and high adsorption rates were observed through the regeneration cycles, suggesting the stability of Tb-MOFs (Table S3†).14 Furthermore, the nanotube-like morphology of Tb-MOFs was maintained even after the fifth cycle of regeneration (Fig. S4†). Therefore, Tb-MOFs still kept a high structural stability after every cycle of regeneration. Based on practical applications, the Tb-MOFs show satisfactory adsorption performance after regeneration and can be applied potentially as an efficient adsorbent for Pb2+ ion removal from wastewater.
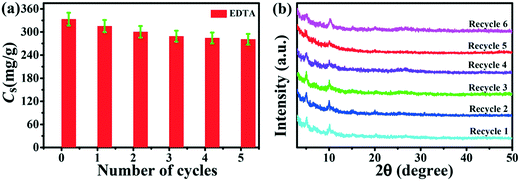 |
| | Fig. 8 Recycling of Tb-MOFs by Pb2+ removal using EDTA (a). XRD patterns of Tb-MOFs after each desorption cycle (b). | |
DFT calculation
To investigate the binding sites of adsorbed Pb2+ ions on the Tb-MOFs surface, DFT calculation was adopted to understand the adsorption process at the atomic level.53 One advantage of Tb-MOFs is that the structure offers enough space to adsorb Pb2+ ions without any orientation limitation. The Pb2+ ions can combine with the functional groups to form the inner-sphere complexes (Pb–N– and Pb–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). As binding energy Eb can indicate the possible interaction mechanism between the heavy metal ions and adsorbents, it was calculated as follows:54
). As binding energy Eb can indicate the possible interaction mechanism between the heavy metal ions and adsorbents, it was calculated as follows:54| | | Eb = ETb–MOFs + EPb − ETb-MOFs–Pb | (8) |
The results are shown in the Fig. 9 and Table S4.† The positive values for Eb indicate a favorable and stable reaction. Moreover, the high values of Eb (> 1 eV) imply that the adsorption of Pb2+ ions on Tb-MOFs is a chemical adsorption process and is consistent with the experimental analysis (inner-sphere complex).55Fig. 9a and b show the single ligand binding with Pb2+ ions. We can see that the Eb of [single(-H)-Tb-MOFs⋯Pb]+ is higher than that of [single-Tb-MOFs⋯Pb]2+, indicating that [single(-H)-Tb-MOFs⋯Pb]+ is more favorable. A similar situation also occurs in the double ligand adsorption process: Eb([double-Tb-MOFs⋯Pb]+) < Eb([double(-H)-Tb-MOFs⋯Pb]+) < Eb([double(-2H)-Tb-MOFs⋯Pb]). These results show that the deprotonated functional groups can further improve the adsorption performance, which is in agreement with the experimental results (the adsorption capacity increases with the increase in pH). It can be concluded that at low pH, [single-Tb-MOFs⋯Pb]2+ and [double-Tb-MOFs⋯Pb]+ are the possible forms and [double-Tb-MOFs⋯Pb]+ is more favorable. With the increase in pH, different forms such as [single(-H)-Tb-MOFs⋯Pb]+, [double(-H)-Tb-MOFs⋯Pb]+, and [double(-2H)-Tb-MOFs⋯Pb] may involve in the complexation and [double(-2H)-Tb-MOFs⋯Pb] is the most stable structure.
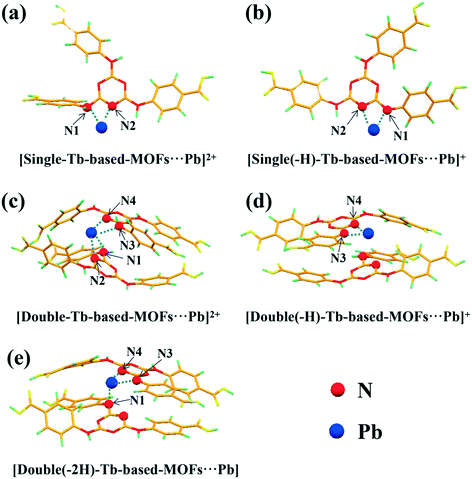 |
| | Fig. 9 The optimized geometries for Pb2+ ion adsorbed on Tb-MOFs: (a) the single ligand binding with Pb2+, (b) the single ligand binding (N1 removed a proton) with Pb2+, (c) double ligands binding with Pb2+, (d) double ligands binding (N3 removed a proton) with Pb2+, (e) double ligands binding (N1 and N3 removed two protons) with Pb2+. | |
Conclusions
Nanotube-like Tb-MOFs with satisfactory adsorption capacity, high structure stability and good recycle performance were successfully fabricated. The results suggest that Langmuir model is suitable for depicting the adsorption process, and Pb2+ adsorption on Tb-MOFs is an endothermic and spontaneous process. An adsorption capacity of 547 mg g−1 was achieved by Tb-MOFs for Pb2+ within 50 min with structure stability. The rate-limiting step controls the entire Pb2+ removal process and the adsorption mechanism could be explained by the formation of an inner-sphere complex (C–/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) between the nitrogenous groups and Pb2+. The adsorption configurations optimized by DFT suggest that the Tb-MOFs structure offers adequate space to adsorb Pb2+ ions and the deprotonated functional groups bond easily with Pb2+, forming stable adsorption complexes even at high pH. The adsorption mechanism of Tb-MOFs suggests its great potential in the removal of Pb2+ from contaminated water.
N⋯Pb) between the nitrogenous groups and Pb2+. The adsorption configurations optimized by DFT suggest that the Tb-MOFs structure offers adequate space to adsorb Pb2+ ions and the deprotonated functional groups bond easily with Pb2+, forming stable adsorption complexes even at high pH. The adsorption mechanism of Tb-MOFs suggests its great potential in the removal of Pb2+ from contaminated water.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
Financial support from National Natural Science Foundation of China (U1607102, 21876047), Science Challenge Project (TZ2016004), the Fundamental Research Funds for the Central Universities (2018ZD11), the Foundation of Basic Research for Application of Qinghai Province (2017-ZJ-706) are acknowledged.
Notes and references
- L. Ma, Q. Wang, S. Islam, Y. Liu, S. Ma and M. Kanatzidis, Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− ion, J. Am. Chem. Soc., 2016, 138, 2858–2866 CrossRef CAS PubMed.
- J. Efome, D. Rana, T. Matsuura and C. Lan, Metal–organic frameworks supported on nanofibers to remove heavy metals, J. Mater. Chem. A, 2018, 6, 4550–4555 RSC.
- D. Chen, W. Shen, S. Wu, C. Chen, X. Luo and L. Guo, Ion exchange induced removal of Pb(II) by MOF-derived magnetic inorganic sorbents, Nanoscale, 2016, 8, 7172–7179 RSC.
- R. Ricco, K. Konstas, M. Styles, J. Richardson, R. Babarao, K. Suzuki, P. Scopece and P. Falcaro, Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water, J. Mater. Chem. A, 2015, 3, 19822–19831 RSC.
- M. Salarian, A. Ghanbarpour, M. Behbahani, S. Bagheri and A. Bagheri, A metal-organic framework sustained by a nanosized Ag12 cuboctahedral node for solid-phase extraction of ultra traces of lead(II) ions, Microchim. Acta, 2014, 181, 999–1007 CrossRef CAS.
- J. Efome, D. Rana, T. Matsuura and C. Lan, Insight studies on metal-organic framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution, ACS Appl. Mater. Interfaces, 2018, 10, 18619–18629 CrossRef CAS PubMed.
- F. Rouhani and A. Morsali, Fast and selective heavy metal removal by a novel metal-organic framework designed with in-situ ligand building block fabrication bearing free nitrogen, Chem. – Eur. J., 2018, 24, 5529–5537 CrossRef CAS PubMed.
- F. Rouhani and A. Morsali, Goal-directed design of metal-organic frameworks for Hg(II) and Pb(II) adsorption from aqueous solutions: A conceptual review, Chem. – Eur. J., 2018, 24, 17170–17179 CrossRef CAS PubMed.
- R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat and A. Alsaedi, Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions, Chem. Eng. J., 2015, 260, 469–477 CrossRef CAS.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaed, T. Hayat and X. Wang, Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- H. Zhu, X. Tan, L. Tan, C. Chen, N. S. Alharbi, T. Hayate, M. Fang and X. Wang, Biochar derived from sawdust embedded with molybdenum disulfide for highly selective removal of Pb2+, ACS Appl. Nano Mater., 2018, 1, 2689–2698 CrossRef CAS.
- L. Zhu, D. Sheng, C. Xu, X. Dai, M. A. Silver, J. Li, P. Li, Y. Wang, Y. Wang, L. Chen, C. Xiao, J. Chen, R. Zhou, C. Zhang, O. K. Farha, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Identifying the recognition site for selective trapping of 99TcO4− in a hydrolytically stable and radiation resistant cation metal-organic framework, J. Am. Chem. Soc., 2017, 139, 14873–14876 CrossRef CAS PubMed.
- H. Zhang, D. Chen, H. Ma and P. Cheng, Real-time detection of traces of benzaldehyde in benzyl alcohol as a solvent by a flexible lanthanide microporous metal-organic framework, Chem. – Eur. J., 2015, 21, 15854–15859 CrossRef CAS PubMed.
- W. Liu, X. Dai, Z. Bai, Y. Wang, Z. Yang, L. Zhang, L. Xu, L. Chen, Y. Li, D. Gui, J. Diwu, J. Wang, R. Zhou, Z. Chai and S. Wang, Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation, Environ. Sci. Technol., 2017, 51, 3911–3921 CrossRef CAS PubMed.
- C. Xiao, M. A. Silver and S. Wang, Metal-organic frameworks for radionuclide sequestration from aqueous solution: a brief overview and outlook, Dalton Trans., 2017, 46, 16381–16386 RSC.
- J. Heine and K. MÜller-Buschbaum, Engineering metal-based luminescence in coordination polymers and metal-organic frameworks, Chem. Soc. Rev., 2013, 42, 9232–9242 RSC.
- X. Zhao, D. Tian, Q. Gao, H. Sun, J. Xu and X. Bu, A chiral lanthanide metal-organic framework for selective sensing of Fe(III) ions, Dalton Trans., 2016, 45, 1040–1046 RSC.
- Z. Wang, H. Liu, S. Wang, Z. Rao and Y. Yang, A luminescent terbium-succinate MOF thin film fabricated by electrodeposition for sensing of Cu2+ in aqueous environment, Sens. Actuators, B, 2015, 220, 779–787 CrossRef CAS.
-
S. Kaskel, The chemistry of metal-organic frameworks: Synthesis, characterization, and applications, Wiley, 2016, ISBN: 978-3-527-33874-0 Search PubMed.
- X. Tan, J. Hu, G. Montavon and X. Wang, Sorption speciation of nickel(II) onto Ca-Montmorillonite: Batch, EXAFS techniques and modeling, Dalton Trans., 2011, 40, 10953–10960 RSC.
- H. Zhu, J. Wu, M. Fang, L. Tan, C. Chen, N. Alharbi, T. Hayat and X. Tan, Synthesis of a core-shell magnetic Fe3O4-NH2@PmPD nanocomposite for efficient removal of Cr(VI) from aqueous media, RSC Adv., 2017, 7, 36231–36241 RSC.
- Ş. Tokalıoğlu, E. Yavuz, S. Demir and Ş. Patat, Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples, Food Chem., 2017, 237, 707–715 CrossRef PubMed.
- X. Tan, M. Fang, L. Tan, H. Liu, X. Ye, T. Hayat and X. Wang, Core-shell hierarchical C@Na2Ti3O7·9H2O nanostructures for the efficient removal of radionuclides, Environ. Sci.: Nano, 2018, 5, 1140–1149 RSC.
- J. Wu, H. Zhu, G. Liu, L. Tan, X. Hu, C. Chen, N. S. Alharbi, T. Hayat and X. Tan, Fabrication of core-shell CMNP@PmPD nanocomposite for efficient As(V) adsorption and reduction, ACS Sustainable Chem. Eng., 2017, 5, 4399–4407 CrossRef CAS.
- V. Kumar, O. Ntwaeaborwa, J. Holsa, D. Motaung and H. Swart, The role of oxygen and titanium related defects on the emission of TiO2:Tb3+ nano-phosphor for blue lighting applications, Opt. Mater., 2015, 46, 510–516 CrossRef CAS.
- D. Chen, E. Jordan and M. Gell, Sol-gel combustion synthesis of nanocrystalline YAG powder from metal-organic precursors, J. Am. Ceram. Soc., 2008, 91, 2759–2762 CrossRef CAS.
- S. Khan, S. Shahid, S. Kanwal and G. Hussain, Synthesis characterization and antibacterial activity of Cr (III), Co (III), Fe (II), Cu (II), Ni (III) complexes of 4-(2-(((2-hydroxy-5-nitrophenyl) diazenyl) (phenyl) methylene) hydrazinyl) benzene sulfonic acid based formazan dyes and their applications on leather, Dyes Pigm., 2018, 148, 31–43 CrossRef CAS.
- D. Kong, N. Qiao, H. Liu, J. Du, N. Wang, Z. Zhou and Z. Ren, Fast and efficient removal of copper using sandwich-like graphene oxide composite imprinted materials, Chem. Eng. J., 2017, 326, 141–150 CrossRef CAS.
- C. Müller, L. David, V. Chiş and S. Pînzaru, Detection of thiabendazole applied on citrus fruits and bananas using surface enhanced Raman scattering, Food Chem., 2014, 145, 814–820 CrossRef PubMed.
- Y. Zhao, H. Chen, J. Li and C. Chen, Hierarchical MWCNTs/Fe3O4/PANI magnetic composite as adsorbent
for methyl orange removal, J. Colloid Interface Sci., 2015, 450, 189–195 CrossRef CAS PubMed.
- Z. Jin, X. Wang, Y. Sun, Y. Ai and X. Wang, Adsorption of 4-n-nonylphenol and bisphenol-a on magnetic reduced graphene oxides: A combined experimental and theoretical studies, Environ. Sci. Technol., 2015, 49, 9168–9175 CrossRef CAS PubMed.
- J. Wang, J. Hao, D. Liu, S. Qin, C. Chen, C. Yang, Y. Liu, T. Yang, Y. Fan, Y. Chen and W. Lei, Flower stamen-like porous boron carbon nitride nanoscrolls for water cleaning, Nanoscale, 2017, 9, 9787–9791 RSC.
- Y. Sun, X. Wang, C. Ding, W. Cheng, C. Chen, T. Hayat, A. Alsaedi, J. Hu and X. Wang, Direct synthesis of bacteria-derived carbonaceous nanofibers as a highly efficient material for radionuclides elimination, ACS Sustainable Chem. Eng., 2016, 4, 4608–4616 CrossRef CAS.
- F. Fernández-Trillo, J. Hest, J. Thies, T. Michon, R. Weberskirch and N. Cameron, Reversible immobilization onto PEG-based emulsion-templated porous polymers by co-assembly of stimuli responsive polymers, Adv. Mater., 2009, 21, 55–59 CrossRef.
- Y. Chen, S. Han, X. Li, Z. Zhang and S. Ma, Why does enzyme not leach from metal-organic frameworks (MOFs)? Unveiling the interactions between an enzyme molecule and a MOF, Inorg. Chem., 2014, 53, 10006–10008 CrossRef CAS PubMed.
- C. Williams, A. Tolia, H. Chan, C. Takoudis and M. Weaver, Surface-enhanced raman spectroscopy as an in situ real-time probe of catalytic mechanisms at high gas pressures: The CO-NO reaction on platinum and palladium, J. Catal., 1996, 163, 63–76 CrossRef CAS.
- S. Kumar, A. Ojha and R. Singh, Synthesis and raman signature for the formation of CdO/MnO2 (core/shell) nanostructures, J. Raman Spectrosc., 2014, 45, 717–722 CrossRef CAS.
- S. Liu, Y. Liu, Q. Mu, F. Zhang, H. Li and Y. Wang, Synthesis, characterization and photoluminescent properties of rare-earth hydroxides and oxides nanorods by hydrothermal route, Appl. Phys. A: Mater. Sci. Process., 2013, 111, 1229–1240 CrossRef CAS.
- N. Bobbitt, M. Mendonca, A. Howarth, T. Islamoglu, J. Hupp, O. Farha and R. Snurr, Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents, Chem. Soc. Rev., 2017, 46, 3357 RSC.
- B. Ouay, S. Kitagawa and T. Uemura, Opening of an accessible microporosity in an otherwise nonporous metal-organic framework by polymeric guests, J. Am. Chem. Soc., 2017, 139, 7886–7892 CrossRef PubMed.
- T. Wang, L. Zhang, C. Li, W. Yang, T. Song, C. Tang, Y. Meng, S. Dai, H. Wang, L. Chai and J. Luo, Synthesis of core-shell magnetic Fe3O4@poly(m-Phenylenediamine) particles for chromium reduction and adsorption, Environ. Sci. Technol., 2015, 49, 5654–5662 CrossRef CAS PubMed.
- X. Luo, L. Ding and J. Luo, Adsorptive removal of Pb(II) ions from aqueous samples with amino-functionalization of metal-organic frameworks MIL-101(Cr), J. Chem. Eng. Data, 2015, 60, 1732–1743 CrossRef CAS.
- M. Dinker, T. Ajithkumar and P. Kulkarni,
L-Proline functionalized dicationic framework of bifunctional mesoporous organosilica for the simultaneous removal of lead and nitrate ions, ACS Sustainable Chem. Eng., 2017, 5, 4188–4196 CrossRef CAS.
- C. Ding, W. Cheng, Y. Sun and X. Wang, Novel fungus-Fe3O4 bio-nanocomposites as high performance adsorbents for the removal of radionuclides, J. Hazard. Mater., 2015, 295, 127–137 CrossRef CAS PubMed.
- T. Wen, Q. Fan, X. Tan, Y. Chen, C. Chen, A. Xu and X. Wang, A core-shell structure of polyaniline coated protonic titanate nanobelt composites for both Cr(VI) and humic acid removal, Polym. Chem., 2016, 7, 785–794 RSC.
- C. Zhu, A. Soldatov and A. Mathew, Advanced microscopy and spectroscopy reveal the adsorption and clustering of Cu(II) onto TEMPO-oxidized cellulose nanofibers, Nanoscale, 2017, 9, 7419–7428 RSC.
- J. Liang, X. Li, Z. Yu, G. Zeng, Y. Luo, L. Jiang, Z. Yang, Y. Qian and H. Wu, Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II), ACS Sustainable Chem. Eng., 2017, 5, 5049–5058 CrossRef CAS.
- H. Mei, X. Tan, L. Tan, Y. Meng, C. Chen, M. Fang and X. Wang, Retention of U(VI) by the formation of Fe precipitates from oxidation of Fe(II), ACS Earth Space Chem., 2018, 2, 968–976 CrossRef CAS.
- J. Wu, K. Chen, X. Tan, M. Fang, X. Hu, Z. Tang and X. Wang, Core-shell CMNP@PDAP nanocomposites for simultaneous removal of chromium and arsenic, Chem. Eng. J., 2018, 349, 481–490 CrossRef CAS.
- A. Khattak, Z. Ghazi, B. Liang, N. Khan, A. Iqbal, L. Li and Z. Tang, A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage, J. Mater. Chem. A, 2016, 4, 16312–16317 RSC.
- S. Zhang, J. Li, X. Wang, Y. Huang, M. Zeng and J. Xu, Rationally designed 1D Ag@AgVO3 nanowire/graphene/protonated g-C3N4 nanosheet heterojunctions for enhanced photocatalysis via electrostatic self-assembly and photochemical reduction methods, J. Mater. Chem. A, 2015, 3, 10119–10126 RSC.
- R. He, W. Li, D. Deng, W. Chen, H. Li, C. Wei and Y. Tang, Efficient removal of lead from highly acidic wastewater by periodic ion imprinted mesoporous SBA-15 organosilica combining metal coordination and co-condensation, J. Mater. Chem. A, 2015, 3, 9789–9798 RSC.
- H. Yan, Y. Lin, H. Wu, W. Zhang, Z. Sun, H. Cheng, W. Liu, C. Wang, J. Li, X. Huang, T. Yao, J. Yang, S. Wei and J. Lu, Bottom-up precise synthesis of stable platinum dimers on graphene, Nat. Commun., 2017, 8, 1070 CrossRef PubMed.
- Y. Ai, Y. Liu, W. Lan, J. Jin, J. Xing, Y. Zou, C. Zhao and X. Wang, The effect of pH on the U(VI) sorption on graphene oxide (GO): A theoretical study, Chem. Eng. J., 2018, 343, 460–466 CrossRef CAS.
- M. Batzill and U. Diebold, Surface studies of gas sensing metal oxides, Phys. Chem. Chem. Phys., 2007, 9, 2307–2318 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en01066h |
| ‡ Hongshan Zhu and Jinyun Yuan contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  *ab,
Wenhua
Zhang
*ab,
Wenhua
Zhang
 c,
Ming
Fang
c,
Ming
Fang
 *a and
Xiangke
Wang
*a and
Xiangke
Wang
 a
a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) between the nitrogenous groups of Tb-MOFs and Pb2+ is the primary adsorption mechanism. Furthermore, density functional theory (DFT) calculations confirm that the most favorable adsorption configuration varies with the reaction conditions and deprotonated functional groups tend to bond with Pb2+ at high pH. These results suggest that Tb-MOFs could be used as a promising adsorbent for the removal of Pb2+ ions from an aqueous environment.
N⋯Pb) between the nitrogenous groups of Tb-MOFs and Pb2+ is the primary adsorption mechanism. Furthermore, density functional theory (DFT) calculations confirm that the most favorable adsorption configuration varies with the reaction conditions and deprotonated functional groups tend to bond with Pb2+ at high pH. These results suggest that Tb-MOFs could be used as a promising adsorbent for the removal of Pb2+ ions from an aqueous environment.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) formed with stable adsorption configurations contribute greatly to the adsorption. These findings illustrate that Tb-MOFs can be regarded as promising adsorbents for the removal of Pb2+ from aqueous solutions in environmental cleanup.
N⋯Pb) formed with stable adsorption configurations contribute greatly to the adsorption. These findings illustrate that Tb-MOFs can be regarded as promising adsorbents for the removal of Pb2+ from aqueous solutions in environmental cleanup.
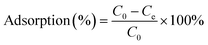
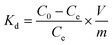
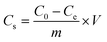
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups) were fixed and the other O atoms of the carboxylate (COO−) were saturated by H atoms.14 Considering the possible deprotonation of N atoms in Tb-MOFs, both deprotonated and undeprotonated TATAB models were used in calculations. All possible initial structures of Pb2+ binding on various sites of MOF were considered and optimized to local minima.4
O groups) were fixed and the other O atoms of the carboxylate (COO−) were saturated by H atoms.14 Considering the possible deprotonation of N atoms in Tb-MOFs, both deprotonated and undeprotonated TATAB models were used in calculations. All possible initial structures of Pb2+ binding on various sites of MOF were considered and optimized to local minima.4

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –NH stretching vibrations,21,27–29 respectively, and indicate the abundance of nitrogenous groups (such as amino and imine) on the surface of Tb-MOFs. The nitrogen-containing functional groups can offer plenty of active sites and thus contribute to the excellent adsorption performance of Tb-MOFs. The other absorption bands at 795 (C–H stretching), 1180 (C–O stretching), 1600 (aromatic ring stretching), 1660 (C
N and –NH stretching vibrations,21,27–29 respectively, and indicate the abundance of nitrogenous groups (such as amino and imine) on the surface of Tb-MOFs. The nitrogen-containing functional groups can offer plenty of active sites and thus contribute to the excellent adsorption performance of Tb-MOFs. The other absorption bands at 795 (C–H stretching), 1180 (C–O stretching), 1600 (aromatic ring stretching), 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), and 2930 cm−1 (C–H stretching) suggested that the integrated structure of TATAB still remains unchanged even after the formation of Tb-carboxylate chains.30–33
C stretching), and 2930 cm−1 (C–H stretching) suggested that the integrated structure of TATAB still remains unchanged even after the formation of Tb-carboxylate chains.30–33
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode of the benzene ring in the TATAB linker.34,35 The peak at 1414 cm−1 is associated with the vibration of triazine and the peak at 977 cm−1 is assigned to the C–N stretching mode.35 The strong broad peak at 665 cm−1 is attributed to the stretching vibration of Tb–O,36,37 suggesting high affinity between the Tb and O atoms. The peak at 422 cm−1 is attributed to the Tb–O stretching mode.38 The peaks at 579, 736, 1155 and 1306 cm−1 are attributed to the C–H stretching mode of benzene ring and N–H stretching mode of triazine.29 The obtained Raman spectra is further evidence that the functional groups of TATAB were still maintained in the Tb-MOF structures.
C stretching mode of the benzene ring in the TATAB linker.34,35 The peak at 1414 cm−1 is associated with the vibration of triazine and the peak at 977 cm−1 is assigned to the C–N stretching mode.35 The strong broad peak at 665 cm−1 is attributed to the stretching vibration of Tb–O,36,37 suggesting high affinity between the Tb and O atoms. The peak at 422 cm−1 is attributed to the Tb–O stretching mode.38 The peaks at 579, 736, 1155 and 1306 cm−1 are attributed to the C–H stretching mode of benzene ring and N–H stretching mode of triazine.29 The obtained Raman spectra is further evidence that the functional groups of TATAB were still maintained in the Tb-MOF structures.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd for Tb-MOFs are above 4.0 mL g−1, which indicates good affinity of Tb-MOFs for Pb2+ ions.15,21 Therefore, the Tb-MOFs can be considered as an efficient adsorbent for Pb2+ removal from large volumes of wastewater.
Kd for Tb-MOFs are above 4.0 mL g−1, which indicates good affinity of Tb-MOFs for Pb2+ ions.15,21 Therefore, the Tb-MOFs can be considered as an efficient adsorbent for Pb2+ removal from large volumes of wastewater.

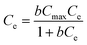

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0versus 1/T (b).
K0versus 1/T (b).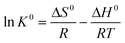
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 was be obtained by plotting ln
K0 was be obtained by plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kdversus Ce and extrapolating the curve to 0. The thermodynamic parameters can reveal the mechanism of Pb2+ ion adsorption onto Tb-MOFs (Fig. 6b and Table S2†). The increase in positive ΔH0 with increase in temperature suggests an endothermic adsorption process. A likely explanation for the positive value of ΔH0 is that the Pb2+ cations dissolve well in water, while the hydration sheath of Pb2+ cations may hinder its adsorption on the Tb-MOFs surface. However, with the increase in temperature, the enhanced molecular motion may reduce the thickness of the hydration sheath. The potential barrier between Pb2+ ions and Tb-MOFs is reduced, thus enhancing the adsorption of Pb2+ ions on the Tb-MOFs. ΔG0 becomes more negative with the increase in temperature, which implies that the adsorption process is spontaneous and higher temperature is beneficial to enhance the adsorption performance. Furthermore, the positive value for ΔS0 reveals the random increase in the amount of Pb2+ adsorbed on the Tb-MOFs at the solid/solution interface and the high affinity between the Tb-MOFs and Pb2+.23
Kdversus Ce and extrapolating the curve to 0. The thermodynamic parameters can reveal the mechanism of Pb2+ ion adsorption onto Tb-MOFs (Fig. 6b and Table S2†). The increase in positive ΔH0 with increase in temperature suggests an endothermic adsorption process. A likely explanation for the positive value of ΔH0 is that the Pb2+ cations dissolve well in water, while the hydration sheath of Pb2+ cations may hinder its adsorption on the Tb-MOFs surface. However, with the increase in temperature, the enhanced molecular motion may reduce the thickness of the hydration sheath. The potential barrier between Pb2+ ions and Tb-MOFs is reduced, thus enhancing the adsorption of Pb2+ ions on the Tb-MOFs. ΔG0 becomes more negative with the increase in temperature, which implies that the adsorption process is spontaneous and higher temperature is beneficial to enhance the adsorption performance. Furthermore, the positive value for ΔS0 reveals the random increase in the amount of Pb2+ adsorbed on the Tb-MOFs at the solid/solution interface and the high affinity between the Tb-MOFs and Pb2+.23
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and benzene ring,43–46 respectively, which are consistent with the FT-IR and Raman analyses. In the high-resolution O 1s spectrum of Tb-MOFs, the two peaks at 532.1 and 531.4 eV are attributed to Tb–O and C–O,47,48 respectively (Fig. 7c). Interestingly, after Pb2+ ions adsorbed on the Tb-MOFs, the deconvoluted O1s spectrum of Tb-MOF–Pb shows almost unchanged Tb–O and C–O peaks (Fig. 7e), indicating that the contribution of surface oxygenic functional groups in the ion removal process may have been overlooked. This phenomenon can be attributed to the saturated coordination of Tb atoms and the hydrophobic benzene hindering the heavy metal ions in the liquid from the surface oxygenic functional groups of the Tb-MOFs.14 The N 1s band (Fig. 7d) can be divided into three peaks of N species, namely, 400.4, 399.7, and 398.7 eV, corresponding to C–N, N–H, and C
N and benzene ring,43–46 respectively, which are consistent with the FT-IR and Raman analyses. In the high-resolution O 1s spectrum of Tb-MOFs, the two peaks at 532.1 and 531.4 eV are attributed to Tb–O and C–O,47,48 respectively (Fig. 7c). Interestingly, after Pb2+ ions adsorbed on the Tb-MOFs, the deconvoluted O1s spectrum of Tb-MOF–Pb shows almost unchanged Tb–O and C–O peaks (Fig. 7e), indicating that the contribution of surface oxygenic functional groups in the ion removal process may have been overlooked. This phenomenon can be attributed to the saturated coordination of Tb atoms and the hydrophobic benzene hindering the heavy metal ions in the liquid from the surface oxygenic functional groups of the Tb-MOFs.14 The N 1s band (Fig. 7d) can be divided into three peaks of N species, namely, 400.4, 399.7, and 398.7 eV, corresponding to C–N, N–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N.49–51 It is clearly seen that the binding energies of C
N.49–51 It is clearly seen that the binding energies of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N, and N–H increase after Pb2+ ions adsorbed on Tb-MOFs. This is because the shared bond between the N atom and Pb2+ ions can decrease the electron cloud density on the N atom, and hence increase the binding energy of the nitrogenous groups.52 Moreover, two new peaks at 399.3 and 398.3 eV can be probably attributed to amine (–N–) and imine (–N
N, C–N, and N–H increase after Pb2+ ions adsorbed on Tb-MOFs. This is because the shared bond between the N atom and Pb2+ ions can decrease the electron cloud density on the N atom, and hence increase the binding energy of the nitrogenous groups.52 Moreover, two new peaks at 399.3 and 398.3 eV can be probably attributed to amine (–N–) and imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) from the Pb–N– and Pb–N
) from the Pb–N– and Pb–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) bonding modes. Thus, the nitrogenous groups could act as active sites for coordination with Pb2+ ions. These results are consistent with the fact that the adsorption of Pb2+ ions on the Tb-MOFs is mainly ascribed to the complexation with nitrogenous groups via the inner-sphere mechanism (Pb–N– and Pb–N
bonding modes. Thus, the nitrogenous groups could act as active sites for coordination with Pb2+ ions. These results are consistent with the fact that the adsorption of Pb2+ ions on the Tb-MOFs is mainly ascribed to the complexation with nitrogenous groups via the inner-sphere mechanism (Pb–N– and Pb–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ).
).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). As binding energy Eb can indicate the possible interaction mechanism between the heavy metal ions and adsorbents, it was calculated as follows:54
). As binding energy Eb can indicate the possible interaction mechanism between the heavy metal ions and adsorbents, it was calculated as follows:54![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯Pb) between the nitrogenous groups and Pb2+. The adsorption configurations optimized by DFT suggest that the Tb-MOFs structure offers adequate space to adsorb Pb2+ ions and the deprotonated functional groups bond easily with Pb2+, forming stable adsorption complexes even at high pH. The adsorption mechanism of Tb-MOFs suggests its great potential in the removal of Pb2+ from contaminated water.
N⋯Pb) between the nitrogenous groups and Pb2+. The adsorption configurations optimized by DFT suggest that the Tb-MOFs structure offers adequate space to adsorb Pb2+ ions and the deprotonated functional groups bond easily with Pb2+, forming stable adsorption complexes even at high pH. The adsorption mechanism of Tb-MOFs suggests its great potential in the removal of Pb2+ from contaminated water.



