Open Access Article
Open Access ArticleBromoanisoles and methoxylated bromodiphenyl ethers in macroalgae from Nordic coastal regions†
Terry F.
Bidleman
 *a,
Agneta
Andersson
*a,
Agneta
Andersson
 bc,
Sonia
Brugel
bc,
Sonia
Brugel
 bc,
Lars
Ericson
bc,
Lars
Ericson
 b,
Peter
Haglund
b,
Peter
Haglund
 a,
Darya
Kupryianchyk
a,
Darya
Kupryianchyk
 a,
Danny C. P.
Lau
a,
Danny C. P.
Lau
 b,
Per
Liljelind
b,
Per
Liljelind
 a,
Lisa
Lundin
a,
Lisa
Lundin
 a,
Anders
Tysklind
a,
Anders
Tysklind
 d and
Mats
Tysklind
d and
Mats
Tysklind
 a
a
aDepartment of Chemistry, Umeå University (UmU), SE-901 87 Umeå, Sweden. E-mail: terry.bidleman@umu.se
bDepartment of Ecology & Environmental Science, UmU, SE-901 87 Umeå, Sweden
cUmeå Marine Sciences Centre, SE-905 71 Hörnefors, Sweden
dKosterhavet National Park, Länsstyrelsen I Västra Götaland, SE-452 05 Sydkoster, Sweden
First published on 10th April 2019
Abstract
Marine macroalgae are used worldwide for human consumption, animal feed, cosmetics and agriculture. In addition to beneficial nutrients, macroalgae contain halogenated natural products (HNPs), some of which have toxic properties similar to those of well-known anthropogenic contaminants. Sixteen species of red, green and brown macroalgae were collected in 2017–2018 from coastal waters of the northern Baltic Sea, Sweden Atlantic and Norway Atlantic, and analyzed for bromoanisoles (BAs) and methoxylated bromodiphenyl ethers (MeO-BDEs). Target compounds were quantified by gas chromatography-low resolution mass spectrometry (GC-LRMS), with qualitative confirmation in selected species by GC-high resolution mass spectrometry (GC-HRMS). Quantified compounds were 2,4-diBA, 2,4,6-triBA, 2′-MeO-BDE68, 6-MeO-BDE47, and two tribromo-MeO-BDEs and one tetrabromo-MeO-BDE with unknown bromine substituent positions. Semiquantitative results for pentabromo-MeO-BDEs were also obtained for a few species by GC-HRMS. Three extraction methods were compared; soaking in methanol, soaking in methanol–dichloromethane, and blending with mixed solvents. Extraction yields of BAs did not differ significantly (p > 0.05) with the three methods and the two soaking methods gave equivalent yields of MeO-BDEs. Extraction efficiencies of MeO-BDEs were significantly lower using the blend method (p < 0.05). For reasons of simplicity and efficiency, the soaking methods are preferred. Concentrations varied by orders of magnitude among species: ∑2BAs 57 to 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and ∑5MeO-BDEs < 10 to 476 pg g−1 wet weight (ww). Macroalgae standing out with ∑2BAs >1000 pg g−1 ww were Ascophyllum nodosum, Ceramium tenuicorne, Ceramium virgatum, Fucus radicans, Fucus serratus, Fucus vesiculosus, Saccharina latissima, Laminaria digitata, and Acrosiphonia/Spongomorpha sp. Species A. nodosum, C. tenuicorne, Chara virgata, F. radicans and F. vesiculosus (Sweden Atlantic only) had ∑5MeO-BDEs >100 pg g−1 ww. Profiles of individual compounds showed distinct differences among species and locations.
700 and ∑5MeO-BDEs < 10 to 476 pg g−1 wet weight (ww). Macroalgae standing out with ∑2BAs >1000 pg g−1 ww were Ascophyllum nodosum, Ceramium tenuicorne, Ceramium virgatum, Fucus radicans, Fucus serratus, Fucus vesiculosus, Saccharina latissima, Laminaria digitata, and Acrosiphonia/Spongomorpha sp. Species A. nodosum, C. tenuicorne, Chara virgata, F. radicans and F. vesiculosus (Sweden Atlantic only) had ∑5MeO-BDEs >100 pg g−1 ww. Profiles of individual compounds showed distinct differences among species and locations.
Environmental significanceMarine macroalgae (“seaweeds”) are used worldwide for human consumption and animal feed. In addition to beneficial nutrients, macroalgae contain brominated phenolic compounds, some of which have toxic properties similar to those of well-known anthropogenic contaminants. Knowledge of the bromophenolic content of macroalgae is needed to understand environmental pathways, including human exposure through consumption as well as bioaccumulation by grazers and transfer through the aquatic food web. Here we report bromoanisoles and methoxylated bromodiphenyl ethers in 16 species of macroalgae from the northern Baltic and Atlantic coasts of Sweden and Norway. This is the largest survey of these compounds in macroalgae from the Nordic region and the first for the Atlantic coasts. |
Introduction
Macroalgae (“seaweeds”, “sea vegetables”) are used worldwide for human consumption (>80% in 2016), animal feed, cosmetics and agriculture. The global market is large and growing, $11.1 U.S. billion in 2016 and projected to reach $22.1 U.S. billion by 2024.1 Macroalgae are a rich source of many nutrients, including vitamins, minerals, fiber, phytochemical antioxidants (fucoxanthin, polyphenols) and omega-3s.2–5 Several health benefits are attributed to consumption of whole macroalgae and they exert a preservative effect when incorporated into foods, due to their antimicrobial activity.3 On the other hand, caution for over-consumption has been advised due to the presence of accumulated heavy metals and other pollutants2,3 and to excessive exposure to iodine, linked to hypothyroidism.3Many halogenated natural products (HNPs) are found in marine macroalgae. Bromophenolic compounds are a prominent subset of HNPs, comprising bromophenols (BPs) and their transformation products bromoanisoles (BAs), hydroxylated and methoxylated bromodiphenyl ethers (OH-BDEs, MeO-BDEs) and polybrominated dibenzo-p-dioxins (PBDDs).6–12 BPs add characteristic and desirable flavors to seafood,13,14 and due to their high food quality macroalgae have been used to supplement the feed of aquacultured fish15,16 and farm animals.4 Several brominated polyphenols have antioxidant, antimicrobial, anticancer, antidiabetic and antithrombotic activities.4,17 Bromophenolic compounds enter the human diet through macroalgae consumption and from compounds bioaccumulated in seafood, and profiles of MeO-BDE congeners in human serum reflect dietary exposure.18 Toxic properties associated with bromophenolic compounds include disruption of hormone synthesis or activity (OH-BDEs and MeO-BDEs)19 and oxidative phosphorylation (OH-BDEs),20 and binding to the aryl hydrocarbon (Ah) receptor (PBDDs).21,22
Bromophenolic compounds have been identified in several macroalgae species from the Baltic Sea: Dictyosiphon foeniculaceus, Ceramium tenuicorne, Polysiphonia fucoides and Pilayella littoralis, as well as in cyanobacteria Nodularia spumigena and Aphanizomenon flos-aquae,8–11 but quantitative data have only been reported for C. tenuicorne,11,23–25D. foeniculaceus8 and N. spumigena.8 Brominated polyphenols were isolated from the red alga Vertebrata lanosa collected on the coast of Norway.26 Several simple BPs were identified in macroalgae species from the families Ceramiaceae, Delesseriaceae, Bonnemaisoniaceae, Rhodophyllaceae, Corallinaceae and Rhodomelaceae, collected on the Swedish west coast.27 One of these compounds, lanosol (2,3-dibromo-4,5-dihydroxybenzyl alcohol) was also identified in seawater. To our knowledge, this is the only report of bromophenolic compounds in macroalgae from the west coast of Sweden.
Macroalgae and cyanobacteria are important sources of bromophenolic compounds to the Baltic ecosystem and they are transferred through the food web from macroalgae to invertebrate grazers to fish.24 Several studies have reported bromophenolic compounds in Baltic mussels, which filter-feed on cyanobacteria8,10,21,28–30 and transferred from mussels to seaducks.28 Macroalgae from the Nordic coastal region are being promoted as human food, with published recipes utilizing local species.31 Consideration should be given to the bromophenolic compounds and other HNPs present in macroalgae used for human consumption, to place exposure in perspective with anthropogenic compounds.
In 2017–2018, we collected >30 species of macroalgae from the Bothnian Sea (northern Baltic), Sweden Atlantic coast (Skagerrak) and Norway Atlantic coast. Objectives are to establish analytical methods and determine the variability of bromophenolic compounds among species and locations, as a prelude to estimating the role of macroalgae in supplying bromophenolic compounds to Nordic estuaries. Here we report concentrations of some neutral bromophenolic compounds (BAs and MeO-BDEs) in an initial survey of 16 species. A major task was to compare methods of extraction for these compounds, since different methods have been applied in previous studies. Compounds with free phenolic groups (BPs, OH-BDEs) were not included at this stage because of the additional steps required for their isolation and derivatization.
Materials and methods
Macroalgae sampling
Macroalgae were collected from coastal sites in the Bothnian Sea (northern Baltic), Skagerrak (Sweden Atlantic) and Norway Atlantic. Amounts collected exceeded 20 g wet weight (ww) in most cases, except when limited by availability (Chara virgata 3 g, Ceramium tenuicorne 10 g ww). After species identification, the macrophytes were rinsed with deionized water, blot dried with laboratory tissues, and frozen at −20 °C until analysis. A subset of 16 macroalgae species (18 specimens, some species from two locations), including one stonewort, was selected for the initial survey of BAs and MeO-BDEs. Collection details for these are given in Table 1 and locations are shown in Fig. 1. Species were chosen to represent different taxonomic groups (red, green, brown algae), locations, morphologies and anticipated content of BAs and MeO-BDEs.| Abbreviation | Group | Speciesc | Latitude N | Longitude E | Collection date | pg g−1 ww | |
|---|---|---|---|---|---|---|---|
| ∑2BAs | ∑5MeO-BDEs | ||||||
| a ∑2,4-DiBA and 2,4,6-TriBA. b ∑2′-MeO-BDE68, 6-MeO-BDE47, one tetrabromo-MeO-BDE and two tribromo-MeO-BDEs with unknown bromine substituent positions. c Nomenclature follows Algae Base (www.algaebase.org). d Stonewort. | |||||||
| Bothnian Sea | |||||||
| Cet | Red alga | Ceramium tenuicorne | 60.769 | 17.349 | 2017-08-24 | 3360 | 199 |
| Chv | Green algad | Chara virgata | 63.414 | 19.491 | 2017-08-26 | 57 | 103 |
| Clg | Green alga | Cladophora glomerata | 63.461 | 19.805 | 2017-07-08 | 591 | 56 |
| Dif | Brown alga | Dictyosiphon foeniculaceus | 63.462 | 19.803 | 2017-07-08 | 324 | 61 |
| Fur | Brown alga | Fucus radicans | 60.806 | 17.356 | 2017-08-24 | 6690 | 476 |
| Stt | Brown alga | Stictyosiphon tortilis | 60.791 | 17.381 | 2017-08-24 | 976 | 56 |
| Uli | Green alga | Ulva intestinalis | 63.461 | 19.805 | 2017-07-08 | 726 | 45 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Skagerrak | |||||||
| Asn | Brown alga | Ascophyllum nodosum | 58.868 | 11.059 | 2017-10-06 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
396 |
| Cev | Red alga | Ceramium virgatum | 58.868 | 11.059 | 2017-10-06 | 1180 | 30 |
| Ful | Red alga | Furcellaria lumbricalis | 58.869 | 11.143 | 2017-10-06 | 854 | <10 |
| Fus | Brown alga | Fucus serratus | 58.868 | 11.059 | 2017-10-06 | 1160 | 85 |
| Fuv | Brown alga | Fucus vesiculosus | 58.868 | 11.059 | 2017-10-06 | 5570 | 253 |
| Rhc | Red alga | Rhodomela confervoides | 58.869 | 11.143 | 2017-10-06 | 437 | <10 |
| Sal | Brown alga | Saccharina latissima | 58.868 | 11.059 | 2017-10-06 | 1120 | <10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Coastal Norway | |||||||
| Ac/Sp | Green alga | Acrosiphonia/Spongomorpha sp. | 65.200 | 11.933 | 2018-05-13 | 1420 | <10 |
| Asn | Brown alga | Ascophyllum nodosum | 65.200 | 11.933 | 2018-05-13 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
34 |
| Fuv | Brown alga | Fucus vesiculosus | 65.200 | 11.933 | 2018-05-13 | 3970 | 52 |
| Lad | Brown alga | Laminaria digitata | 65.200 | 11.933 | 2018-05-13 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
<10 |
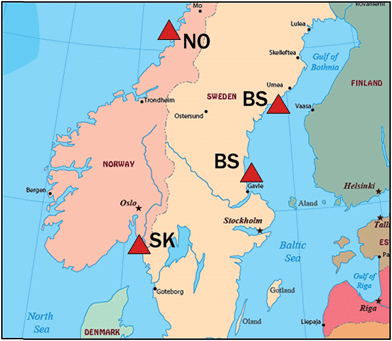 | ||
| Fig. 1 Locations for sampling macroalgae in the Bothnian Sea (BS), Skagerrak (SK) and coastal Norway (NO). See Table 1 for coordinates and sampling dates. | ||
Extraction and analytical methods
SOAK 1.7 Macroalgae pieces were placed in a glass jar with a polytetrafluoroethylene-lined cap and recovery surrogates were added: 15 ng 2,4,6-tribromoanisole-d5 (2,4,6-triBA-d5) and two PBDE congeners not found in commercial mixtures: 6.0 ng 3,3′,5-tribromodiphenyl ether (BDE-35) and 24 ng 2,3′,4,4′,6-pentabromodiphenyl ether (BDE-119) (Table 2). The macroalgae were covered with 15 mL methanol (MeOH) and allowed to stand in the refrigerator at 4 °C for one week.
| 2,4,6-TriBA-d5 | BDE-35 | BDE-119 | ||
|---|---|---|---|---|
| a 15 ng 2,4,6-triBA-d5, 6.0 ng BDE-35 and 24 ng BDE-119. b SOAK 1: methanol; SOAK 2: methanol–dichloromethane; BLEND: mixed solvents. c One-way ANOVA, post hoc Tukey HSD, p < 0.05. | ||||
| SOAK 1 | ||||
| Mean | 75.9 | 89.7 | 76.5 | |
| SD | 10.4 | 19.3 | 16.6 | |
| N | 18 | 17 | 17 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| SOAK 2 | ||||
| Mean | 85.1 | 103 | 98.4 | |
| SD | 10.7 | 13.3 | 14.0 | |
| N | 28 | 28 | 28 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| BLEND | ||||
| Mean | 81.4 | 109 | 103 | |
| SD | 9.4 | 10.3 | 8.9 | |
| N | 17 | 17 | 17 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Significance | ||||
| SOAK 1 | SOAK 2 | Y | Y | Y |
| SOAK 1 | BLEND | N | Y | Y |
| SOAK 2 | BLEND | N | N | N |
SOAK 2. As for SOAK 1, but using 10 mL MeOH + 5 mL dichloromethane (DCM).
Extracts from SOAK 1 or SOAK 2 were diluted with 60 mL deionized water (DIW), 3 mL saturated potassium chloride was added, and the mixture was partitioned three times with 20 mL DCM followed by 20 mL hexane. Combined organic layers were filtered through glass wool and concentrated by rotary evaporation and nitrogen blowdown into 5 mL hexane.
BLEND.32,33 Macroalgae pieces were placed in a thick-walled glass jar and the above surrogates were added. A stainless steel stick blender (Ultra Turrax T25, Janke & Kunkel GmbH, IKA Labortechnik, Staufen, Germany) was used to homogenize the macroalgae with 25 mL acetone + 10 mL hexane followed by two portions of 20 mL hexane + 3 mL diethyl ether, blending for one minute each time. The combined extracts were diluted with 60 mL DIW, 3 mL saturated potassium chloride was added and the mixture was partitioned. The organic layer was removed and the aqueous phase was extracted twice more with 20 mL hexane + 3 mL diethyl ether. Combined organic layers were filtered and concentrated as in the SOAK methods.
Of the 18 specimens, three were extracted in replicate (3–4) with each method, five were extracted once with each method and ten were extracted only with SOAK 2 (Tables 3, 4 and S1†).
| Sample | Methodc | 2,4-DiBA | 2,4,6-TriBA | triU1b | triU2b | 2′-68b | 6-47b | tetraU3b |
|---|---|---|---|---|---|---|---|---|
| a No entry indicates compound < LOD in more than one replicate. See Table S1. b Tri-U1, tri-U2 and tetraU3 = MeO-BDEs with unknown tribromo- or tetrabromo-substituent positions. 2′-28 = 2′-MeO-BDE68, 6-47 = 6-MeO-BDE47. c SOAK 1: methanol, SOAK 2: methanol–dichloromethane, BLEND: mixed solvents. d One-way ANOVA, post hoc Tukey HSD test. | ||||||||
| Cladophora glomerata | ||||||||
| Mean (n = 3–4) | SOAK 1 | 118 | 519 | 16.2 | 43.1 | |||
| s.d. | 10.6 | 27.9 | 0.6 | 10.1 | ||||
| RSD % | 9.0 | 5.4 | 3.5 | 23.3 | ||||
| Mean (n = 3–4) | SOAK 2 | 114 | 524 | 15.6 | 36.5 | |||
| s.d. | 14.2 | 79.5 | 2.6 | 5.9 | ||||
| RSD % | 12.4 | 15.2 | 16.5 | 16.2 | ||||
| Mean (n = 3–4) | BLEND | 90.1 | 403 | 11.6 | 34.3 | |||
| s.d. | 30.8 | 36.5 | 2.3 | 16.3 | ||||
| RSD % | 34.2 | 9.0 | 19.5 | 47.6 | ||||
| Significance (p < 0.05)d | ||||||||
| SOAK 1 | SOAK 2 | N | N | N | N | |||
| SOAK 1 | BLEND | N | Y | Y | N | |||
| SOAK 2 | BLEND | N | Y | N | N | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fucus radicans | ||||||||
| Mean (n = 3–4) | SOAK 1 | 829 | 6700 | 59.4 | 153 | 58.7 | 170 | 52.6 |
| s.d. | 88.7 | 828 | 10.1 | 32.0 | 3.1 | 15.3 | 20.9 | |
| RSD % | 10.7 | 12.3 | 17.0 | 20.9 | 5.3 | 9.0 | 39.7 | |
| Mean (n = 3–4) | SOAK 2 | 711 | 6400 | 49.7 | 131 | 66.8 | 160 | 50.2 |
| s.d. | 95.3 | 928 | 7.1 | 46.7 | 11.8 | 23.4 | 16.2 | |
| RSD % | 13.4 | 14.5 | 14.2 | 35.6 | 17.6 | 14.6 | 32.2 | |
| Mean (n = 3–4) | BLEND | 611 | 4830 | 25.0 | 89.6 | 40.7 | 89.8 | 32.5 |
| s.d. | 37.3 | 940 | 3.4 | 22.2 | 3.6 | 11.9 | 13.3 | |
| RSD % | 6.1 | 19.5 | 13.5 | 24.8 | 8.8 | 13.3 | 40.9 | |
| Significance (p < 0.05)d | ||||||||
| SOAK 1 | SOAK 2 | N | N | N | N | N | N | N |
| SOAK 1 | BLEND | Y | Y | Y | N | Y | Y | N |
| SOAK 2 | BLEND | N | N | Y | N | Y | Y | N |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fucus vesiculosus | ||||||||
| Mean (n = 4) | SOAK 1 | 967 | 5520 | 58.2 | 107 | 37.1 | 85.7 | |
| s.d. | 178 | 1720 | 12.7 | 21.0 | 6.8 | 32.1 | ||
| RSD % | 18.4 | 31.1 | 21.7 | 19.6 | 18.5 | 37.4 | ||
| Mean (n = 4) | SOAK 2 | 928 | 5260 | 58.3 | 84.7 | 25.3 | 49.3 | |
| s.d. | 287 | 1920 | 20.9 | 25.6 | 5.2 | 9.8 | ||
| RSD % | 30.9 | 36.5 | 35.8 | 30.2 | 20.7 | 19.9 | ||
| Mean (n = 4) | BLEND | 593 | 3440 | 26.1 | 46.8 | 14.6 | 24.8 | |
| s.d. | 123 | 923 | 6.3 | 10.0 | 3.3 | 7.2 | ||
| RSD % | 20.8 | 26.9 | 24.2 | 21.4 | 22.9 | 29.2 | ||
| Significance (p < 0.05)d | ||||||||
| SOAK 1 | SOAK 2 | N | N | N | N | Y | N | |
| SOAK 1 | BLEND | N | N | Y | Y | Y | Y | |
| SOAK 2 | BLEND | N | N | Y | N | Y | N | |
| Sample | Methodc | 2,4-DiBA | 2,4,6-TriBA | triU1 | triU2 | 2′-68 | 6-47 | tetraU3 |
|---|---|---|---|---|---|---|---|---|
| a tri-U1, tri-U2 and tetraU3 = MeO-BDEs with unknown tribromo- or tetrabromo-substituent positions. 2′-28 = 2′-MeO-BDE68, 6-47 = 6-MeO-BDE47. b Concentration data in Tables 3 and S2. No entry indicates compound < LOD. c SOAK 1: methanol, SOAK 2: methanol–dichloromethane, BLEND: mixed solvents. d Ratios calculated from mean concentrations in Table 3. e Ratio significantly different from 1.00, t-test at p < 0.05. | ||||||||
| Ascophyllum nodosum | ||||||||
| SOAK 1/SOAK 2 | 1.56 | 1.32 | 0.92 | 0.92 | 1.32 | |||
| BLEND/SOAK 2 | 0.91 | 0.91 | 0.47 | 0.55 | 0.76 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Cladophora glomerata | ||||||||
| SOAK 1/SOAK 2 | 1.03 | 0.99 | 1.04 | 1.18 | ||||
| BLEND/SOAK 2 | 0.79 | 0.77 | 0.67 | 0.94 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fucus radicans | ||||||||
| SOAK 1/SOAK 2 | 1.16 | 1.05 | 1.20 | 1.16 | 0.88 | 1.06 | 1.05 | |
| BLEND/SOAK 2 | 0.86 | 0.75 | 0.50 | 0.68 | 0.61 | 0.56 | 0.65 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fucus serratus | ||||||||
| SOAK 1/SOAK 2 | 0.49 | 0.61 | 1.04 | 1.32 | ||||
| BLEND/SOAK 2 | 0.89 | 1.30 | 0.83 | 0.60 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fucus vesiculosus | ||||||||
| SOAK 1/SOAK 2 | 1.04 | 1.05 | 1.00 | 1.26 | 1.46 | 1.74 | ||
| BLEND/SOAK 2 | 0.64 | 0.65 | 0.45 | 0.55 | 0.57 | 0.45 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Rhodomela confervoides | ||||||||
| SOAK 1/SOAK 2 | 1.34 | 0.92 | ||||||
| BLEND/SOAK 2 | 0.86 | 0.93 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Saccharina latissima | ||||||||
| SOAK 1/SOAK 2 | 0.73 | 0.58 | ||||||
| BLEND/SOAK 2 | 1.02 | 0.78 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Ulva intestinalis | ||||||||
| SOAK 1/SOAK 2 | 1.14 | 0.89 | 0.56 | |||||
| BLEND/SOAK 2 | 1.11 | 1.23 | 0.55 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Summary | ||||||||
| Mean BAs | SD BAs | Significancee | Mean MeO-BDEs | SD MeO-BDEs | Significancee | Mean BAs | SD BAs | |
| SOAK 1/SOAK 2 | 0.99 | 0.29 | N | 1.12 | 0.26 | N | 0.99 | 0.29 |
| BLEND/SOAK 2 | 0.90 | 0.19 | N | 0.61 | 0.13 | Y | 0.90 | 0.19 |
Internal standard (59 ng 2,2′,6,6′-tetrachlorobiphenyl, CB-54) was added to the macroalgae extracts, which were then vortexed for 1 min with 2 mL 99% sulfuric acid and placed in the refrigerator overnight to allow the phases to separate. Fucus serratus required a second sulfuric acid treatment. The upper hexane layer was transferred with hexane rinsings to a Pasteur pipet containing 0.5 g Florisil topped with 0.5 cm granular anhydrous sodium sulfate and the column was eluted with 12 mL 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) DCM–hexane. The eluate was concentrated into 100 to 200 μL isooctane. Blanks were run by carrying solvents through the extraction and cleanup procedures.
2 (v/v) DCM–hexane. The eluate was concentrated into 100 to 200 μL isooctane. Blanks were run by carrying solvents through the extraction and cleanup procedures.
Results and discussion
Compound identification and quality control
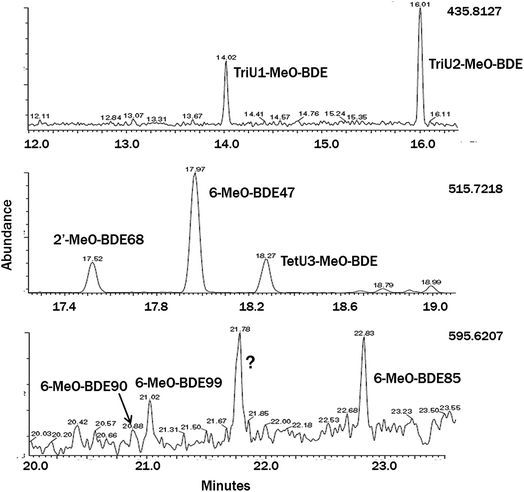
Only 2,4-diBA and 2,4,6-triBA were identified and quantified in the macroalgae by GC-LRMS, while 2,6-diBA and BAs with meta-substitutions were absent. The tetrabromo-compounds 2′-MeO-BDE68 and 6-MeO-BDE67 were identified using GC-LRMS and GC-HRMS by their retention times relative to authentic standards and ion ratios that fell within ±20% of those for standards, and quantified by GC-LRMS. Two tribromo-MeO-BDEs and one additional tetrabromo-MeO-BDE with unknown bromine substituent positions were also identified from their monitored ions by both LRMS and HRMS (Fig. 2). The unknown tribromo-MeO-BDEs were quantified by GC-LRMS versus the response factor (relative to CB-54) for a standard of 2′-MeO-BDE28 (which was not found in any of the macroalgae species) and the unknown tetrabromo-MeO-BDE was quantified by using the average of response factors for 2′-MeO-BDE68 and 6-MeO-BDE47. The two tribromo-MeO-BDEs appear to be the same ones previously identified in Baltic water and air samples.34 Pentabromo-MeO-BDEs showed poor response by GC-LRMS, but three compounds were identified in some selected macroalgae species by HRMS using authentic standards: 6-MeO-BDE85, 6-MeO-BDE90 and 6-MeO-BDE99; 2-MeO-BDE123 was also sought but not found. Due to poor performance of the CB54 internal standard in GC-HRMS runs, semiquantitative results for pentabromo-MeO-BDEs were estimated without use of an internal standard and assuming a 200 μL sample volume.Four blanks were run by carrying solvents through the extraction and cleanup procedures. No target peaks were found, and blank quantities were estimated by integrating baseline noise in the vicinity of expected peaks. Blanks (mean ± SD) by compound class were: BAs 6.3 ± 2.6 pg, tribromo-MeO-BDEs 5.7 ± 3.4 pg, tetrabromo-MeO-BDEs 22 ± 9 pg. Some BAs and MeO-BDEs were absent from all macroalgae; 2,6-diBA and 2′-MeO-BDE28, and other MeO-BDEs were absent in some of the species. “Blanks” were also assessed using a larger data set from algae extractions and quantifying baseline signal in the absence of a noticeable target peak. The overall limits of detection, LOD = (mean blank + 3 × SD)/sample weight, from both types of blanks were: BAs 15 pg g−1, tribromo-MeO-BDEs 10 pg g−1 and tetrabromo-MeO-BDEs 17 pg g−1, based on a 2.5 g (ww) sample.
Percent recoveries of 2,4,6-triBA-d5, BDE-35 and BDE-119 surrogate spikes varied among the three extraction methods, with means ranging from 76% to 109% and relative standard deviations (% RSD) ranging from 8.7% to 21.7% (Table 2). While mean recoveries of all surrogates using each method exceeded 75%, higher yields were generally obtained by SOAK 2 (MeOH–DCM) and BLEND methods than by SOAK 1 (MeOH). Differences in mean recoveries between SOAK 2 and SOAK 1 were significant for all three surrogates (single factor ANOVA, post hoc Tukey HSD, p < 0.05), between SOAK 1 and BLEND for the two BDEs and not significant between SOAK 2 and BLEND for any surrogate (p > 0.05). Concentrations of BAs and MeO-BDEs in macroalgae were corrected on a per-sample basis for surrogate yields (2,4,6-triBA-d5 recovery for BAs, average of BDE-35 and BDE-119 recoveries for MeO-BDEs).
Comparison of extraction methods and reproducibility
Replicate extractions (3 to 4) of C. glomerata and F. radicans from the Bothnian Sea and F. vesiculosus from Skagerrak were done using SOAK 1 (MeOH), SOAK 2 (MeOH–DCM) and BLEND (mixed solvents) methods. Analytical results are summarized in Table 3 and elaborated in Table S1.† Percent relative standard deviations (% RSD) for replicate analyses of a particular species with a specified method ranged from 5% to 36% for BAs and 4% to 48% for MeO-BDEs, with overall average % RSDs of 18% and 23%, respectively (Table 3). Sources of this variability are in the precision of the analytical method itself (the average % RSD of all surrogate recoveries was 13%, Table 2) and probably also in the reproducibility of removing water by blot drying with laboratory tissues. In most cases no significant differences in extraction yields were found using SOAK 1 or SOAK 2 methods (one-way ANOVA, post hoc Tukey HSD test, p > 0.05). Differences between the SOAK and BLEND methods were sometimes significant (p < 0.05) and lower with BLEND, especially for MeO-BDEs. The difference appears to be due to lower extraction efficiency of MeO-BDEs from the algal matrix, since surrogate recoveries with BLEND were high and not different from SOAK 2.Five other macroalgae species, U. intestinalis from the Bothnian Sea, and A. nodosum, F. serratus, R. confervoides and S. latissima from Skagerrak, were extracted once with each method. Here the extraction methods were compared by expressing yields relative to SOAK 2; i.e. SOAK 1/SOAK 2 and BLEND/SOAK 2. Results for these five macroalgae plus the three species used in methods replication (Table 3) are summarized in Table 4. Averaged over all eight species and compounds, SOAK 1/SOAK 2 yielded ratios of BAs (0.99 ± 0.29) and MeO-BDEs (1.12 ± 0.26) that were not significantly different from 1.00 (t-test, p > 0.05). BLEND/SOAK 2 ratios for BAs were slightly, but not significantly, lower (0.90 ± 0.19, p > 0.05), while BLEND/SOAK 2 ratios for MeO-BDEs were significantly lower (0.61 ± 0.13, p < 0.001). Thus, the extraction efficiency of BAs from these test species did not differ significantly by the three methods. Yields of MeO-BDEs were not significantly different by SOAK 1 and SOAK 2. The extraction efficiency for MeO-BDEs was significantly lower using BLEND, confirming results from replicate extractions of three species. For reasons of simplicity and efficiency, the soaking methods are preferred.
Six macroalgae species (A. nodosum, C. glomerata, F. radicans, F. serratus, F. vesiculosus and R. confervoides) that had been extracted by SOAK 1 or SOAK 2 received second week-long extractions with fresh solvents. Second extraction percentages were 6.6 ± 3.5% of first-extraction yields for BAs and 21 ± 19% for MeO-BDEs. The MeO-BDE percentages are likely inflated because second extraction quantities were often near or below the LOD (LOD/2 was entered).
Concentrations of BAs and MeO-BDEs in macroalgae
Table 1 gives concentrations of the ∑2BAs (2,4-diBA + 2,4,6-triBA) and ∑5MeO-BDEs (2′-MeO-BDE68, 6-MeO-BDE47, one tetrabromo-MeO-BDE and two tribromo-MeO-BDEs) in pg g−1 ww. For those macroalgae receiving extractions by different methods, yields by all three methods were averaged for BAs, while only SOAK 1 and SOAK 2 results were averaged for MeO-BDEs. Composition details are shown in Fig. 3 and Table S2.† BAs were found in all sixteen macroalgae species examined, while eleven species contained one or more tribromo- or tetrabromo-MeO-BDEs.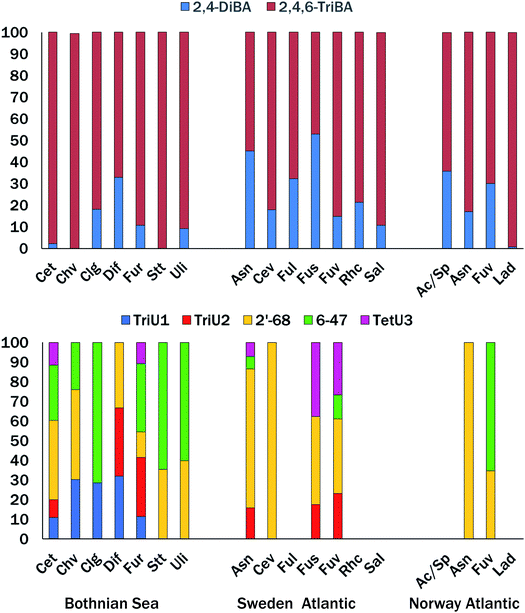 | ||
| Fig. 3 Percent contribution of BAs (top) tribromo- and tetrabromo-MeO-BDEs (bottom) to ∑2BAs and ∑5MeO-BDEs in Nordic macroalgae. See Table 1 for species abbreviations. No bar indicates all compounds < LOD. | ||
The bromophenolic content varied over several orders of magnitude; Σ2BAs 57 to 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 pg g−1 ww, and Σ5MeO-BDEs < 10 to 476 pg g−1 ww (Table 1). Macroalgae standing out with ∑2BAs >1000 pg g−1 ww were A. nodosum, C. tenuicorne, C. virgatum, F. radicans, F. serratus, F. vesiculosus, S. latissima, L. digitata and Acrosiphonia/Spongomorpha sp. The species A. nodosum, C. tenuicorne, C. virgata, F. radicans and F. vesiculosus (Skagerrak only) had ∑5MeO-BDEs >100 pg g−1 ww. The Bothnian Sea and Skagerrak species tended to have higher levels of ∑5MeO-BDEs, while the ∑5MeO-BDEs was low in all four species examined from coastal Norway. Considering all species and locations, there was no significant relationship between concentrations or logarithm transformed concentrations of ∑2BAs and ∑5MeO-BDEs (p > 0.05). The proportion of 2,4,6-triBA, expressed by the fraction 2,4,6-triBA/(2,4,6-triBA + 2,4-diBA), was significantly higher (p < 0.05) in Bothnian Sea macroalgae (0.883 ± 0.109), than in the combined set of Atlantic coast species (Skagerrak and Norway, 0.747 ± 0.154) (t-test of means, unequal variance).
700 pg g−1 ww, and Σ5MeO-BDEs < 10 to 476 pg g−1 ww (Table 1). Macroalgae standing out with ∑2BAs >1000 pg g−1 ww were A. nodosum, C. tenuicorne, C. virgatum, F. radicans, F. serratus, F. vesiculosus, S. latissima, L. digitata and Acrosiphonia/Spongomorpha sp. The species A. nodosum, C. tenuicorne, C. virgata, F. radicans and F. vesiculosus (Skagerrak only) had ∑5MeO-BDEs >100 pg g−1 ww. The Bothnian Sea and Skagerrak species tended to have higher levels of ∑5MeO-BDEs, while the ∑5MeO-BDEs was low in all four species examined from coastal Norway. Considering all species and locations, there was no significant relationship between concentrations or logarithm transformed concentrations of ∑2BAs and ∑5MeO-BDEs (p > 0.05). The proportion of 2,4,6-triBA, expressed by the fraction 2,4,6-triBA/(2,4,6-triBA + 2,4-diBA), was significantly higher (p < 0.05) in Bothnian Sea macroalgae (0.883 ± 0.109), than in the combined set of Atlantic coast species (Skagerrak and Norway, 0.747 ± 0.154) (t-test of means, unequal variance).
Nine species were checked for pentabromo-MeO-BDEs by HRMS (Table S3†). Due to poor performance of the CB54 internal standard in GC-HRMS runs, semiquantitative results were estimated without use of an internal standard and assuming a 200 μL sample volume. Only C. tenuicorne and F. radicans from the Bothnian Sea, and A. nodosum and F. vesiculosus from Skagerrak contained one or more of 6-MeO-BDE85, 6-MeO-BDE90 and 6-MeO-BDE99 at concentrations ranging from 1.3 to 18 pg g−1 ww.
Comparison with other macroalgae investigations
Relative to the plethora of reports on free BPs, OH-BDEs and polyphenols in macroalgae,4–13,17,23–27,36 the database for BAs and MeO-BDEs in marine algae is surprisingly small and summarized in Table 5. BAs and/or MeO-BDEs have been reported in C. tenuicorne from the Baltic Proper11,24 at pg g−1 ww concentrations of 2,4-diBA 4 to 70, 2,4,6-triBA 47 to 390, 2′-MeO-BDE68 3 to 100 and 6-MeO-BDE47 5 to 210. Reported average concentrations of these four compounds in D. foeniculaceus, also from the Baltic Proper, were 33, 230, 120 and 150 pg g−1, ww, respectively.8 These appear to be the only Baltic macroalgae species in which BAs and MeO-BDEs have been quantified. For comparison, our concentrations of 2,4-diBA, 2,4,6-triBA, 2′-MeO-BDE68 and 6-MeO-BDE47 were 71, 3290, 80 and 56 pg g−1, ww in C. tenuicorne, and 106, 217, 20 and < 17 pg g−1, ww in D. foeniculaceus (Table S2†).| Group | Genera | Location | 2,4-DiBA | 2,4,6-TriBA | 2′-MeO-BDE68 | 6-MeO-BDE47 | Reference |
|---|---|---|---|---|---|---|---|
| a Including stoneworts. b Ranges in a single collection period. c Ranges from June to August. d Peak concentrations, July. e East coast, near Sydney. f Stonewort. | |||||||
| Brown algae | 4 | Nordic | <15–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
217–48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18–280 | <17–165 | This study |
| Red algae | 3 | Nordic | 71–275 | 344–3290 | <17–80 | <17–56 | This study |
| Green algae | 3a | Nordic | <15–507 | 57–910 | <17–47 | <17–40 | This study |
| Brown algae | 3 | Philippines | <20–700 | 300–229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7 | |
| Red algae | 7 | Philippines | <20–1300 | 100–78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50–29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7 | |
| Green algae | 5a | Philippines | 100–2200 | <20–5900 | <20–1700 | 7 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Group | Species | ||||||
| Brown alga | Dictyosiphon foeniculaceus | Bothnian Sea | 106 | 217 | 20 | <17 | This study |
| Brown alga | Dictyosiphon foeniculaceus | Baltic Proper | 33 | 230 | 120 | 150 | 8 |
| Red alga | Ceramium tenuicorne | Bothnian Sea | 71 | 3290 | 80 | 56 | This study |
| Red alga | Ceramium tenuicorne | Baltic Properb | 75–100 | 160–210 | 11 | ||
| Red alga | Ceramium tenuicorne | Baltic Properc | 4–70 | 47–390 | 3–29 | 5–71 | 24 |
| Red alga | Ceramium tenuicorne | Baltic Properd | 85 | 40 | 23 | ||
| Red alga | Ceramium virgatum | Skagerrak | 210 | 970 | 30 | <17 | This study |
| Green alga | Chara virgata | Bothnian Sea | <15 | 57 | 47 | 25 | This study |
| Green alga | Chara sp.f | Philippines | 300 | <20 | 100 | 7 | |
| Green alga | Cladophora glomerata | Bothnian Sea | 107 | 484 | <17 | 40 | This study |
| Green alga | Cladophora sp. | Philippines | 1300 | <20 | <20 | 7 | |
| Red alga | Polysiphonia sphaerocarpa | Australiae | 100–300 | 200–700 | 36 | ||
Wide ranges of BAs and MeO-BDEs have been reported in 15 genera of red, green and brown macroalgae from Philippine waters, all collected in the same month: 2,4,6-triBA < 20 to 2200 pg g−1 ww, 2′-MeO-BDE68 < 20 to 229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg g−1 ww and 6-MeO-BDE47 < 20 to 27600 pg g−1 ww.7 Ranges of 2,4-diBA and 2,4,6-triBA in the red alga Polysiphonia sphaerocarpa from the littoral zone near Sydney, Australia were 100 to 300 and 200 to 700 pg g−1 ww.36 Concentrations of BAs in our set of macroalgae are generally higher than those found in other investigations (Table 5). While BAs were highest in Fucus spp. and A. nodosum, which have not been analyzed by others, we also found higher levels in C. tenuicorne than previously reported. On the other hand, our levels of BAs in the brown alga D. foeniculaceus were comparable to those found by Löfstrand et al.,8 while their concentrations of tetrabromo-MeO-BDEs were higher than ours. The semiquantitative estimate of 17 pg g−1 ww 6-MeO-BDE85 in C. tenuicorne (Table S3†) compares favorably with 35 pg g−1 ww reported in the same species.11
000 pg g−1 ww and 6-MeO-BDE47 < 20 to 27600 pg g−1 ww.7 Ranges of 2,4-diBA and 2,4,6-triBA in the red alga Polysiphonia sphaerocarpa from the littoral zone near Sydney, Australia were 100 to 300 and 200 to 700 pg g−1 ww.36 Concentrations of BAs in our set of macroalgae are generally higher than those found in other investigations (Table 5). While BAs were highest in Fucus spp. and A. nodosum, which have not been analyzed by others, we also found higher levels in C. tenuicorne than previously reported. On the other hand, our levels of BAs in the brown alga D. foeniculaceus were comparable to those found by Löfstrand et al.,8 while their concentrations of tetrabromo-MeO-BDEs were higher than ours. The semiquantitative estimate of 17 pg g−1 ww 6-MeO-BDE85 in C. tenuicorne (Table S3†) compares favorably with 35 pg g−1 ww reported in the same species.11
In addition to variation among macroalgae species,7 concentrations and proportions of bromophenolic compounds fluctuate with the season.6,11,23,24 The collection months of Nordic macroalgae specimens was July–August in the Bothnian Sea, October in Skagerrak and May in coastal Norway (Table 1), which may account for observed differences. Concentrations of 2,4-diBA and 2,4,6-triBA in C. tenuicorne, collected from the Baltic Proper between June and August, ranged from 4 to 70 and 47 to 390 pg g−1 ww, respectively, while ranges of 2′-MeO-BDE68 and 6-MeO-BDE47 were 3 to 29 and 5 to 71 pg g−1 ww (Table 5).24 Each of these bromophenolic compounds peaked in August. Ratios of compounds in C. tenuicorne varied over the spring-summer; 2,4,6-triBA/2,4-diBA from 5.5 to 17 and 6-MeO-BDE47/2′-MeO-BDE28 from 1.1 to 2.7.24 Ratios of precursor 2,4,6-triBP/2,4-diBP concentrations also showed monthly fluctuations.23 In comparison, 2,4,6-triBA/2,4-diBA was 46, while 6-MeO-BDE47/2′-MeO-BDE28 was 0.70 in our specimen of C. tenuicorne, collected in August. Stresses from salinity variations, light intensity and grazing by predators also cause changes in concentrations of 2,4,6-triBP.23 These factors hinder comparisons among species, locations and sampling times.
Both BAs and MeO-BDEs have been determined in macroalgae from Nordic ecosystems and the Philippines, with strikingly different results (Table 5). Even considering the spread of concentrations within each compound class and among species, the general trend is BAs > MeO-BDEs in Nordic ecosystems versus MeO-BDEs > BAs in the Philippines. Reasons are not apparent, and it would be interesting to conduct more comparisons between tropical and cold ecosystems.
Methods of extraction varied in the other studies. SOAK 1 as used here was employed to extract macroalgae from the Philippines.7 Variations of the BLEND method were used for Baltic specimens.8,11,24 BPs in macroalgae from Hong Kong6 and Australia12,36 were isolated by combined steam distillation-solvent extraction. Free bromophenols (BPs) were also determined in the studies listed in Table 5, with levels similar or higher than those of BAs.
The more polar BPs and OH-BDEs were not considered in this initial survey. These are also abundant in macroalgae.6–12,36 BPs and OH-BDEs are partially ionized at seawater pH,37,38 but are nevertheless subject to bioaccumulation.24,28,39 Diverse toxic effects of 2,4,6-triBP40 and OH-BDEs18–20 have been reported, and beneficial antimicrobial activity has been attributed to 6-MeO-BDE47.41 Many studies have demonstrated interconversion of BPs/BAs and OH-BDEs/MeO-BDEs through O-methylation/demethylation reactions,18,19,40 and it has been suggested that wildlife acquire OH-BDEs through O-demethylation of accumulated MeO-BDEs.42 Thus, it is important to include both free phenolic and O-methylated forms in subsequent surveys, along with additional HNPs that have been reported in marine macroalgae; e.g. PBDDs,8,9,11,21 BDEs substituted with multiple OH- or MeO-groups, hydroxylated and methoxylated bromobiphenyls7 and brominated methylpyrroles.43 The SOAK 1 (methanol) method has been used to extract compounds with free phenolic groups from macroalgae.7 Non-target screening by tandem GC-time of flight mass spectrometry (GCxGC-ToF-MS) is very effective for identifying HNPs.44 GCxGC-ToF-MS45–47 and advanced data processing techniques based on GC-MS48 and LC-MS49,50 methods have identified hundreds to thousands of natural and anthropogenic halogenated compounds in marine mammals and sediments but so far these have not been applied to identification of HNPs in macroalgae.
Conclusions
The data set for neutral bromophenolic compounds (BAs and MeO-BDEs) obtained in this survey is the largest for macroalgae in Nordic waters. A simple extraction procedure based on soaking the macroalgae in methanol (SOAK 1) or methanol-dichloromethane (SOAK 2) gave equivalent yields of both compound classes and was significantly more efficient for MeO-BDEs than blending with mixed solvents (BLEND) In retrospect, the sample sizes used in this survey are adequate for BAs, but somewhat low for MeO-BDEs, which were occasionally below or on the border of detection. Expanding the SOAK methods to accommodate larger samples is recommended.Concentrations of BAs and MeO-BDEs in macroalgae were highly variable, spanning orders of magnitude, and compound profiles differed according to the species. The concentrations and compound profiles observed here represent only single sampling events, and it is likely that a more extensive survey would reveal seasonal and spatial variability. Other considerations for future research are expanding the list of target compounds to include the more polar BPs and OH-BDEs, search for other HNPs by nontarget screening, and examining the link between macroalgae and bioaccumulation of bromophenolic compounds and other HNPs in Nordic coastal waters.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was supported by the Swedish strategic research environment EcoChange. Thanks to Prof. Lillemor Asplund, Stockholm University, for supplying analytical standards of MeO-BDEs.References
- GVR, Commercial Seaweed Market Size, Share & Trends Analysis Report By Product (Red, Brown, Green), By Form (Liquid, Powdered, Flakes), By Application (Animal Feed, Human Consumption), And Segment Forecasts, 2018–2024. Market research report, Grand View Research, San Francisco, CA, 2018, https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market, accessed January 22, 2019 Search PubMed.
- Berkeley Wellness, 2016, Newsletter, July 27, http://www.berkeleywellness.com/healthy-eating/food/article/6-things-know-about-seaweed, accessed January 22, 2019.
- I. Brownlee, A. Fairclough, A. Hall and J. Paxman, The potential health benefits of seaweed and seaweed extract, in Seaweed: ecology, nutrient composition and medicinal uses. Marine Biology: Earth Sciences in the 21st Century, ed. V. H. Pomin, Nova Science Publishers, Hauppauge, NY, 2000, pp. 119–136 Search PubMed.
- S. Holdt and S. Kraan, Bioactive compounds in seaweed: functional food applications and legislation, J. Appl. Phycol., 2011, 23, 543–597 CrossRef CAS.
- K. Li, X.-M. Li, J. B. Gloer and G.-G. Wang, Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga Rhodomela confervoides, J. Agric. Food Chem., 2011, 59, 9916–9921 CrossRef CAS PubMed.
- H. Y. Chung, W. C. J. Ma, P. O. Ang Jr, J.-S. Kim and F. Chen, Seasonal variations of bromophenols in brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegata) collected in Hong Kong, J. Agric. Food Chem., 2003, 51, 2619–2624 CrossRef CAS PubMed.
- K. Haraguchi, Y. Kotaki, J. R. Relox Jr, M. L. J. Romero and R. Terada, Monitoring of naturally produced brominated phenoxyphenols and phenoxyanisoles in aquatic plants from the Philippines, J. Agric. Food Chem., 2010, 58, 12385–12391 CrossRef CAS PubMed.
- K. Löfstrand, A. Malmvärn, P. Haglund, A. Bignert, Å. Bergman and L. Asplund, Brominated phenols, anisoles, and dioxins present in blue mussel from the Swedish coastline, Environ. Sci. Pollut. Res., 2010, 17, 1460–1468 CrossRef PubMed.
- A. Malmvärn, Brominated natural products at different trophic levels in the Baltic Sea, PhD thesis, Stockholm University, 2007.
- A. Malmvärn, G. Marsh, L. Kautsky, M. Athanasiadou, Å. Bergman and L. Asplund, Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea, Environ. Sci. Technol., 2005, 39, 2990–2997 CrossRef.
- A. Malmvärn, Y. Zebühr, L. Kautsky, Å. Bergman and L. Asplund, Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea, Chemosphere, 2008, 72, 910–916 CrossRef PubMed.
- F. B. Whitfield, F. Helidoniotis, K. J. Shaw and D. Svoronos, Distribution of bromophenols in species of algae from eastern Australia, J. Agric. Food Chem., 1999, 47, 2367–2373 CrossRef CAS PubMed.
- H. Y. Chung, W. C. J. Ma and J.-S. Kim, Seasonal distribution of bromophenols in Hong Kong seafood, J. Agric. Food Chem., 2003, 51, 6752–6760 CrossRef CAS PubMed.
- F. B. Whitfield, M. Drew, F. Helidoniotis and D. Svoronos, Distribution of bromophenols in species of marine polychaetes and bryozoans from eastern Australia and the role of such animals in the flavor of edible ocean fish and prawns (shrimp), J. Agric. Food Chem., 1999, 47, 4756–4762 CrossRef CAS PubMed.
- B. Jones, R. Smullen and A. G. Carton, Flavour enhancement of freshwater farmed barramundi (Lates calcarifer), through dietary enrichment with cultivated sea lettuce, Ulva ohnoi, Aquaculture, 2016, 454, 192–198 CrossRef CAS.
- L. M. P. Valente, M. Araújo, S. Batista, M. J. Peixoto, I. Sousa-Pinto, V. Brotas and L. M. Cunha, Carotenoid deposition, flesh quality and immunological response of Nile tilapia fed increasing levels of IMTA-cultivated Ulva spp, J. Appl. Phycol., 2016, 28, 691–701 CrossRef CAS.
- M. Liu, P. E. Hanser and X. Lin, Bromophenols in marine algae and their bioactivities, Mar. Drugs, 2011, 9, 1273–1292 CrossRef CAS PubMed.
- K. Haraguchi, Y. Ito, M. Takagi, Y. Fujii, K. H. Harada and A. Koizumi, Levels, profiles and dietary sources of hydroxylated PCBs and hydroxylated and methoxylated PBDEs in Japanese women serum samples, Environ. Int., 2016, 97, 155–162 CrossRef CAS PubMed.
- S. B. Wiseman, Y. Wan, H. Chang, X. Zhang, M. Hecker, P. D. Jones and J. P. Giesy, Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: environmental sources, metabolic relationships, and relative toxicities, Mar. Pollut. Bull., 2011, 63, 179–188 CrossRef CAS PubMed.
- J. Legradi, A.-K. Dahlberg, P. Cenijn, G. Marsh, L. Asplund, Å. Bergman and J. Legler, Disruption of oxidative phosphorylation (OXPHOS) by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) present in the marine environment, Environ. Sci. Technol., 2014, 48, 14703–14711 CrossRef CAS PubMed.
- P. Haglund, A. Malmvärn, S. Bergek, A. Bignert, L. Kautsky, T. Nakano, K. Wiberg and L. Asplund, Brominated dibenzo-p-dioxins: a new class of marine toxins? 2007, Environ. Sci. Technol., 2007, 41, 3069–3074 CrossRef CAS PubMed.
- M. van den Berg, M. S. Denison, L. S. Birnbaum, M. J. DeVito, H. Fiedler, J. Falandysz, M. Rose, D. Schrenk, S. Safe, C. Tohyama, A. Tritscher, M. Tysklind and R. E. Peterson, Review: PBDD/Fs and PBBs: inclusion in the toxicity equivalency factor concept for dioxin-like compounds, Toxicol. Sci., 2013, 133, 197–208 CrossRef CAS PubMed.
- E. Dahlgren, C. Enhus, D. Lindqvist, B. Eklund and L. Asplund, Induced production of brominated aromatic compounds in the alga Ceramium tenuicorne, Environ. Sci. Pollut. Res., 2015, 22, 18107–18114 CrossRef CAS PubMed.
- E. Dahlgren, D. Lindqvist, H. Dahlgren, L. Asplund and K. Lehtilä, Trophic transfer of naturally produced brominated aromatic compounds in a Baltic Sea food chain, Chemosphere, 2016, 144, 1597–1604 CrossRef CAS PubMed.
- D. Lindqvist, E. Dahlgren and L. Asplund, Biosynthesis of hydroxylated polybrominated diphenyl ethers and the correlation with photosynthetic pigments in the red alga Ceramium tenuicorne, Phytochemistry, 2017, 133, 51–58 CrossRef CAS PubMed.
- E. K. Olsen, E. Hansen, J. Isaksson and J. H. Andersen, Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa, Mar. Drugs, 2013, 11, 2769–2784 CrossRef PubMed.
- M. Pedersén, P. Saenger and L. Fries, Simple brominated phenols in red algae, Phytochemistry, 1974, 13, 2273–2279 CrossRef.
- A. K. M. Dahlberg, V. L. Chen, K. Larsson, Å. Bergman and L. Asplund, Hydroxylated and methoxylated polybrominated diphenyl ethers in long-tailed ducks (Clangula hyemalis) and their main food, Baltic blue mussels (Mytilus trossulus × Mytilus edulis), Chemosphere, 2016, 144, 1475–1483 CrossRef CAS PubMed.
- A. Malmvärn, Y. Zebühr, S. Jensen, L. Kautsky, E. Greyerz, T. Nakano and L. Asplund, Identification of polybrominated dibenzo-p-dioxins in blue mussels (Mytilus edulis) from the Baltic Sea, Environ. Sci. Technol., 2005, 39, 8235–8242 CrossRef.
- K. Löfstrand, X. Liu, D. Lindqvist, S. Jensen and L. Asplund, Seasonal variations of hydroxylated and methoxylated brominated diphenyl ethers in blue mussels from the Baltic Sea, Chemosphere, 2011, 84, 527–532 CrossRef PubMed.
- M. Bisther and F. Gröndahl, Laga Mat Med Alger, Gothenburg University, Seafarm, 2015, p. 16 (in Swedish). ISBN 978-91-7595-533-9, ISSN 1402-7615, https://cms.it.gu.se/infoglueDeliverWorking/digitalAssets/1686/1686060_laga-mat-med-alger_2015.pdf, accessed January 22, 2019 Search PubMed.
- S. Jensen, L. Häggberg, H. Jörundsdottir and G. Odham, A quantitative lipid extraction method for residue analysis of fish involving nonhalogenated solvents, J. Agric. Food Chem., 2003, 51, 5607–5611 CrossRef CAS PubMed.
- S. Jensen, L. Reutergårdh and B. Jansson, Analytical methods for measuring organochlorines and methyl mercury by gas chromatography, FAO Fisheries Tech. Paper, 1983, 212, 21–33 Search PubMed.
- T. F. Bidleman, K. Agosta, A. Andersson, P. Haglund, A. Hegmans, P. Liljelind, L. M. Jantunen, O. Nygren, J. Poole, M. Ripszam and M. Tysklind, Sea-air exchange of bromoanisoles and methoxylated bromodiphenyl ethers in the Northern Baltic, Mar. Pollut. Bull., 2016, 112, 58–64 CrossRef CAS PubMed.
- T. F. Bidleman, E. Brorström-Lundén, K. Hansson, H. Laudon, O. Nygren and M. Tysklind, Atmospheric transport and deposition of bromoanisoles along a temperate to arctic gradient, Environ. Sci. Technol., 2017, 51, 10974–10982 CrossRef CAS PubMed.
- C. Flodin and F. B. Whitfield, Brominated anisoles and cresols in the red alga Polysiphonia sphaerocarpa, Phytochemistry, 2000, 53, 77–80 CrossRef CAS PubMed.
- P. D. Howe, S. Dobson and H. M. Malcolm, 2,4,6-Tribromophenol and Other Simple Phenols. Concise International Chemical Assessment Document 66, World Health Organization, Geneva, 2005, ISBN 92-4-153066-9, ISSN 1020-6167, p. 47, http://www.who.int/ipcs/publications/cicad/cicad_66_web_version.pdf, accessed January 22, 2019 Search PubMed.
- S. Rayne and K. Forest, pKa values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs), J. Environ. Sci. Health A, 2010, 45, 1322–1346 CrossRef CAS PubMed.
- A. K. M. Dahlberg, A. Bignert, J. Legradi, J. Legler and L. Asplund, Anthropogenic and naturally produced brominated substances in Baltic herring (Clupea harengus membras) from two sites in the Baltic Sea, Chemosphere, 2016, 144, 2408–2414 CrossRef CAS PubMed.
- C. Koch and B. Sures, Environmental concentrations and toxicology of 2,4,6-tribromophenol, Environ. Pollut., 2018, 233, 706–713 CrossRef CAS PubMed.
- D. M. P. Shridhar, G. B. Mahajan, V. P. Kamat, C. G. Naik, R. R. Parab, N. R. Thakur and P. D. Mishra, Antibacterial activity of 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol from Dysidea granulosa, Mar. Drugs, 2009, 7, 264–271 CrossRef PubMed.
- Y. Wan, S. Wiseman, H. Chang, X. Zhang, P. D. Jones, M. Hecker, K. Kannan, S. Tanabe, J. Hu, M. W. Lam and J. P. Giesy, Origin of hydroxylated brominated diphenyl ethers: natural compounds or man-made flame retardants?, Environ. Sci. Technol., 2009, 43, 7536–7542 CrossRef CAS PubMed.
- S. Gaul, P. Bendig, O. Olbrich, N. Rosenfelder, P. Ruff, C. Gaus, J. F. Mueller and W. Vetter, Identification of the natural product 2,3,4,5-tetrabromo-1-methylpyrrole in Pacific biota, passive samplers and seagrass from Queensland, Australia, Mar. Pollut. Bull., 2011, 62, 2463–2468 CrossRef CAS PubMed.
- P. Haglund, K. Löfstrand, K. Siek and L. Asplund, Powerful GC-ToF-MS techniques for screening identification and quantification of halogenated natural products, in Advances in Mass Spectrometry, ed. Y. Wada, Mass Spectrometry Society of Japan, Tokyo, 2013, vol. 19, pp. 187–198, ISBN 978-4-902590-30-2 Search PubMed.
- M. B. Alonso, K. A. Maruya, N. G. Dodder, J. Lailson-Brito Jr, A. Azevedo, E. Santos-Neto, J. P. M. Torres, O. Malm and E. Hoh, Nontarget screening of halogenated organic compounds in bottlenose dolphins (Tursiops truncatus) from Rio de Janeiro, Brazil, Environ. Sci. Technol., 2017, 51, 1176–1185 CrossRef CAS PubMed.
- E. Hoh, N. G. Dodder, S. J. Lehotay, K. C. Pangallo, C. M. Reddy and K. A. Maruya, Nontargeted comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry method and software for inventorying persistent and bioaccumulative contaminants in marine environments, Environ. Sci. Technol., 2012, 46, 8001–8008 CrossRef CAS PubMed.
- N. J. Shaul, N. G. Dodder, L. I. Aluwihare, S. A. Mackintosh, K. A. Maruya, S. Chivers, K. Kerri Danil, D. W. Weller and E. Hoh, Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the Southern California Bight, Environ. Sci. Technol., 2015, 49, 1328–1338 CrossRef CAS PubMed.
- C. Hauler and W. Vetter, A non-targeted gas chromatography/electron capture negative ionization mass spectrometry selected ion monitoring screening method for polyhalogenated compounds in environmental samples, Rapid Commun. Mass Spectrom., 2015, 29, 619–628 CrossRef CAS PubMed.
- H. Peng, C. Chun, D. V. M. Saunders, J. Sun, S. Tang, G. Codling, M. Hecker, S. Wiseman, P. D. Jones, A. Li, K. J. Rockne and J. P. Giesy, Untargeted identification of organo-bromine compounds in lake sediments by ultrahigh-resolution mass spectrometry with the data-independent precursor isolation and characteristic fragment method, Anal. Chem., 2015, 87, 10237–10246 CrossRef CAS PubMed.
- H. Peng, C. Chen, J. Cantin, D. V. M. Saunders, J. Sun, T. Song, G. Codling, M. Hecker, S. Wiseman, P. D. Jones, A. Li, K. J. Rockne, N. C. Sturchio, M. Cai and J. P. Giesy, Untargeted screening and distribution of organo-iodine compounds in sediments from Lake Michigan and the Arctic Ocean, Environ. Sci. Technol., 2016, 50, 10097–10105 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00042a |
| This journal is © The Royal Society of Chemistry 2019 |