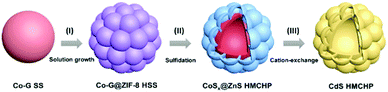
Fabrication of CdS hierarchical multi-cavity hollow particles for efficient visible light CO2 reduction†
Peng
Zhang‡
 ,
Sibo
Wang‡
,
Sibo
Wang‡
 ,
Bu Yuan
Guan
,
Bu Yuan
Guan
 and
Xiong Wen (David)
Lou
and
Xiong Wen (David)
Lou
 *
*
School of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459, Singapore. E-mail: xwlou@ntu.edu.sg; davidlou88@gmail.com; Web: http://www.ntu.edu.sg/home/xwlou/
First published on 7th November 2018
Abstract
Designing advanced structures for semiconductor photocatalysts is an effective approach to enhance their performance. However, it is not easy to fabricate functional photocatalytic materials with complex nano-architectures. Here we have developed a sequential solution growth, sulfidation and cation-exchange strategy to fabricate CdS hierarchical multi-cavity hollow particles (HMCHPs). This strategy starts with the growth of Zn-based zeolitic imidazolate framework (ZIF-8) onto cobalt glycerate (Co-G) solid spheres. Sulfidation of the obtained Co-G@ZIF-8 composite particles leads to the formation of CoSx@ZnS HMCHPs, which are converted into CdS HMCHPs via a cation-exchange reaction. Owing to the favourable properties of the well-defined hierarchical hollow structure, the CdS HMCHPs exhibit enhanced activity for photocatalytic CO2 reduction compared with other CdS photocatalysts with solid and common hollow structures. The performance of CdS HMCHPs can be further promoted by loading of Au to reach a CO generation rate of 3758 μmol h−1 g−1 under visible light irradiation.
Broader contextPhotocatalytic CO2 reduction is one of the most promising solutions to alleviate the energy and environmental problems of modern society. This solar energy conversion process requires the utilization of highly efficient photocatalysts, among which visible-light-responsive CdS materials show outstanding properties. Therefore, designing advanced architectures for CdS is of great importance to improve the photocatalytic performance. This study demonstrates the rational design and synthesis of CdS hierarchical multi-cavity hollow particles, which exhibit high activity toward photocatalytic CO2 reduction under visible-light irradiation. The large surface area and pore size of the complex hollow structure provide abundant active sites and mass transfer channels to accelerate the reaction. Moreover, the enhanced performance is also ascribed to the promoted light absorption ability as a result of the light scattering in the multi-cavity architecture. Au nanoparticles, acting as electron traps to facilitate the transfer kinetics of charge carriers, are further used as a cocatalyst to show a great promotive effect on the activity of CdS hierarchical multi-cavity hollow particles. This work may inspire the design and fabrication of complex hollow-structured hybrid photocatalysts for solar-to-chemical energy conversion. |
The increasing energy demand of modern society encourages vast efforts for the development of renewable energy sources, among which flexible, universal and inexhaustible solar energy is one of the most promising candidates.1 Although solar energy is utilized widely through photovoltaic and photothermal approaches, the intermittent nature of insolation makes such applications unsuitable for continuous energy consumption.2,3 Therefore, conversion of solar energy into controllable chemical energy has drawn great research interest.4 Photocatalytic CO2 reduction with the generation of valuable chemicals (e.g., CO, CH4, HCOOH and CH3OH) is an attractive strategy for solar-to-chemical energy conversion.4 Owing to the high thermodynamic stability of the linear CO2 molecules, it is essential to design high-performance, cost-effective and robust photocatalysts to achieve efficient CO2 reduction for practical applications.
Semiconductor materials, especially metal oxides such as TiO2, WO3, CeO2, Bi2WO4 and ZnGe2O4, have been widely investigated as photocatalysts for CO2 reduction.5–10 Nevertheless, most of the metal oxides can only respond to ultraviolent light as a result of their large band gaps. Besides, the relatively poor charge conductivity of metal oxides would lead to the recombination of charge carriers. Thus, much more effort is devoted to investigating transition metal sulfide photocatalysts with outstanding chemical, electrical and optical properties.11,12 Among various sulfide materials, CdS shows good performance for photocatalytic CO2 reduction.13,14 It possesses a narrow bandgap of about 2.4 eV to absorb a large fraction of visible light.15 Moreover, the conduction band minimum of CdS is more negative than the potential for the reduction of CO2 to CO, indicating that CdS is kinetically favorable for the reaction.16 To enhance the photocatalytic activity of CdS, rational design of advanced structures for the material is an effective approach.
As one unique type of material architectures, hollow structures show distinct advantages for energy related applications.17–19 Specifically, hollow-structured photocatalysts with large surface area and abundant active sites have been demonstrated to exhibit superior performance toward photoreduction of CO2.20,21 The distance for the diffusion of photogenerated charge carriers is reduced because of the thin-shelled topologies in the hollow structures.22 Additionally, fabrication of complex hierarchical hollow structures for photocatalysts can further enhance their activity owing to the high utilization efficiency of solar irradiation by the scattering and reflection effects.23 But it is still very challenging to synthesize well-defined hierarchical hollow particles by conventional methods.24
Recently, some strategies involving metal–organic framework materials and polyol-based metal alkoxide precursors provide new opportunities for fabricating materials with complex hollow structures.25–27 Herein we have developed a multi-step approach combining solution growth, sulfidation and cation-exchange processes for the synthesis of novel CdS hierarchical multi-cavity hollow particles (HMCHPs). When evaluated as a photocatalyst for CO2 reduction, the obtained CdS HMCHPs exhibit enhanced activity compared with both CdS solid spheres (SSs) and hollow spheres (HSs). Additionally, loading a small amount of Au can further improve the performance of CdS HMCHPs.
The synthetic procedure of CdS HMCHPs is illustrated in Fig. 1. Cobalt glycerate (Co-G) SSs are first synthesized by a conventional hydrothermal method with some modifications (see ESI† for the experimental details).28 In the first step, solution growth of a zinc-based zeolitic imidazolate framework (ZIF-8)29 on the Co-G SSs leads to the formation of Co-G@ZIF-8 hierarchical solid spheres (HSSs), which are converted into CoSx@ZnS HMCHPs by a sulfidation reaction. Then, a cation-exchange strategy is performed to transform as-prepared CoSx@ZnS HMCHPs into CdS HMCHPs.
The X-ray diffraction (XRD) pattern shows that the as-synthesized Co-G sample is amorphous and contains Co, C and O as revealed by the energy-dispersive X-ray (EDX) spectrum (Fig. S1, ESI†). The field-emission scanning electron microscopy (FESEM) image shows that the Co-G spheres are quite uniform with a size of about 500 nm (Fig. 2a). The transmission electron microscopy (TEM) image demonstrates the solid nature of the Co-G spheres (Fig. 2b). The successful growth of ZIF-8 on Co-G SSs is confirmed by the XRD pattern which shows typical diffraction peaks of (002), (112), (022), (013) and (222) planes (Fig. S2a, ESI†). The EDX spectrum reveals the presence of both Co and Zn in the as-prepared Co-G@ZIF-8 sample (Fig. S2b, ESI†). FESEM observation (Fig. 2c) exhibits that quasi-spherical ZIF-8 particles are grown on the surface of Co-G SSs forming a raspberry-like morphology, which is further confirmed by TEM observation (Fig. 2d).
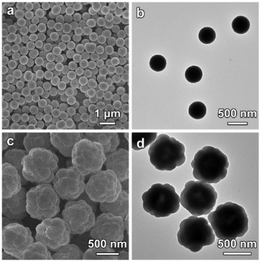 | ||
| Fig. 2 (a and c) FESEM images and (b and d) TEM images of (a and b) Co-G SSs and (c and d) Co-G@ZIF-8 HSSs. | ||
After the solvothermal sulfidation of the Co-G@ZIF-8 HSSs at 120 °C using thioacetamide as the sulfidation agent (step II in Fig. 1),19 the XRD pattern of the obtained sample shows typical diffraction peaks of (111), (220) and (311) planes, which indicates the conversion of ZIF-8 into ZnS (Fig. S3a, ESI†). The EDX spectrum (Fig. S3b, ESI†) reveals that the obtained sample possesses Co, Zn and S, confirming that Co-G is converted into amorphous CoSx. As shown in the FESEM image (Fig. 3a), the raspberry-like morphology is preserved after the sulfidation reaction. FESEM observation of a broken particle shows the presence of a big central chamber (Fig. 3b). Besides, the shell of the hollow particle is composed of many small cavities which are generated from the transformation of quasi-spherical ZIF-8 particles into sulfide materials (Fig. 3c). TEM observations further reveal that the as-prepared particles are constructed from a central spherical chamber covered by interconnected small cavities (Fig. 3d–f). These results confirm the successful conversion of Co-G@ZIF-8 HSSs into CoSx@ZnS HMCHPs through the sulfidation reaction.
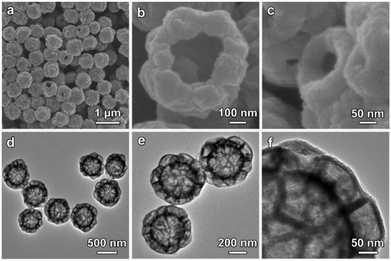
Subsequently, a hydrothermal cation-exchange reaction is conducted to convert the CoSx@ZnS HMCHPs into CdS HMCHPs (step III in Fig. 1). The XRD pattern of the sample shows typical diffraction peaks of (100), (002), (101) and (110) planes, which confirms the successful formation of CdS (Fig. S4a, ESI†). As revealed by the EDX spectrum, the obtained sample consists of Cd and S, which further proves that Co2+ and Zn2+ are successfully exchanged by Cd2+ during the reaction (Fig. S4b, ESI†). FESEM images (Fig. 4a and b) reveal that the obtained CdS particles preserve the raspberry-like morphology after the cation-exchange reaction. FESEM observation of one broken particle reveals both the large central cavity and the small hollow particles on the shell (Fig. 4c). This structure is further confirmed by TEM observation of the well-defined hierarchical multi-cavity hollow structure (Fig. 4d). The TEM image at a higher magnification reveals that the CdS HMCHPs are constructed from ultrafine nanoparticles (Fig. 4e), which are highly crystalline with a lattice spacing of 0.33 nm (corresponding to the (002) plane of CdS) (Fig. 4f). Moreover, the selected-area electron diffraction (SAED) pattern shows multiple rings, which further proves that the obtained sample is constructed from crystallized CdS nanoparticles (inset of Fig. 4f). To better reveal the advantages of the complex hollow structure, two other CdS photocatalysts in the forms of SSs and HSs are also synthesized as control samples. The CdS SSs synthesized by a hydrothermal approach (Fig. S5, ESI†) are uniform with a size of about 400 nm (Fig. S6, ESI†). The CdS HSs are synthesized by a similar sequential sulfidation and cation-exchange strategy using Co-G SSs as the precursor (Fig. S7–S11, ESI†).
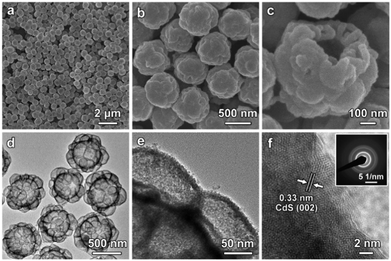 | ||
| Fig. 4 (a–c) FESEM images and (d and e) TEM images of CdS HMCHPs. (f) HRTEM image of a CdS HMCHP with the SAED pattern shown as the inset. | ||
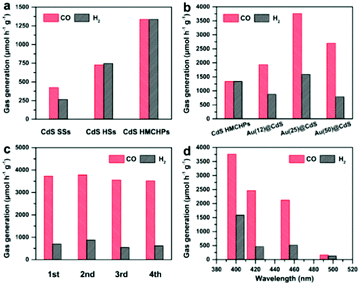
The photocatalytic CO2 reduction performance of the samples is evaluated in a typical tandem system operated in a mixture of H2O and acetonitrile together with Co(bpy)32+ (bpy = 2,2′-bipyridine) and triethanolamine (TEOA) as the cocatalyst and electron donor, respectively.30–33 To check the carbon source of CO, 13C-labelled isotope experiments are conducted using 13CO2 as the reactant. The results of gas chromatography-mass spectrometry (GC-MS) analysis of the produced CO show that only 13CO is generated (Fig. S12, ESI†). Such observations indicate that the produced CO originates from the CO2 reactant, rather than the organics in the system (e.g., acetonitrile and TEOA).34 Besides, no detectable liquid product (e.g., methanol or formic acid) is generated in the reaction system as determined by GC-MS, nuclear magnetic resonance (NMR), and high-performance liquid chromatography (HPLC) analyses.12,35 The CdS HSs show enhanced activity compared with the solid sample, while CdS HMCHPs exhibit the best performance with a CO generation rate of about 1337 μmol h−1 g−1 (Fig. 5a). The large surface areas of hollow structures should be responsible for the enhanced activity compared with CdS SSs (Fig. S13a, ESI†). The porous feature and relatively large pore size of CdS HMCHPs can effectively promote the adsorption of CO2 molecules and facilitate the mass transfer of reactants and products (Fig. S13b, ESI†). At the same time, the complex hollow structure might lead to promoted light absorption from the scattering effect, leading to further promoted performance (Fig. S14, ESI†).
In order to further improve the performance of CdS HMCHPs, a small amount of Au is loaded, which is a good catalyst for selective reduction of CO2 to CO.36,37 As determined by inductively coupled plasma optical emission spectrometry (ICP-OES), samples prepared with different amounts of the HAuCl4 precursor possess Au loadings of 0.12, 0.25 and 0.50 wt%, which are denoted as Au(12)@CdS, Au(25)@CdS and Au(50)@CdS, respectively. Compared with bare CdS HMCHPs, Au(12)@CdS shows slightly enhanced activity (Fig. 5b). Increasing the loading of Au to 0.25 wt% results in significant enhancement of the CO generation rate to 3758 μmol h−1 g−1, which is about 2.8 times higher than that of CdS HMCHPs. Nevertheless, a higher Au content of 0.50 wt% in the photocatalyst leads to the deterioration of the performance, possibly due to the fact that the excessive Au would aggregate and serve as the recombination centers for photoinduced charges. Loading of Au can also enhance the selectivity for the generation of CO. The CO selectivity of CdS HMCHPs is 50.0%, while Au(25)@CdS HMCHPs exhibit an enhanced CO selectivity of 70.3%, which is comparable to the reported CdS-based photocatalysts.16,38,39 Results of stability tests reveal that the Au(25)@CdS HMCHPs are stable without obvious deactivation in the course of four cycles (Fig. 5c). Wavelength-dependent CO2 reduction reactions are conducted with Au(25)@CdS HMCHPs. The trend of CO evolution matches well with the photoabsorption of the catalyst (Fig. 5d and Fig. S14, ESI†). This phenomenon indicates that the CO2 reduction reaction is induced by photoexcitation of the Au(25)@CdS HMCHPs. The apparent quantum yield (AQY) of Au(25)@CdS HMCHPs is determined to be 0.61% under monochromatic light irradiation with a wavelength of 420 nm. The photocatalytic CO2-to-CO conversion rate of Au(25)@CdS HMCHPs compares favorably to those of recently reported photocatalysts (Table S1, ESI†).12,16,30,32,33
To elucidate the high activity of Au(25)@CdS HMCHPs, different characterizations are conducted. As revealed by photoluminescence (PL) spectra, Au(25)@CdS exhibits distinct fluorescence quenching compared with CdS, indicating that Au might act as charge traps and prohibit the recombination of electrons and holes (Fig. S15, ESI†).40 On the other hand, Au(25)@CdS shows much enhanced transient photocurrent densities (Fig. S16, ESI†). This phenomenon shows that Au could promote the utilization efficiency of charge carriers and accelerate surface redox reactions. Furthermore, electrochemical impedance spectra (EIS) reveal that Au(25)@CdS manifests a smaller semicircle in the Nyquist plot, demonstrating a lower resistance for charge transfer and higher catalytic activity (Fig. S17, ESI†). According to these results, Au would effectively promote the separation and utilization of photogenerated charge carriers, leading to the enhanced performance of Au(25)@CdS HMCHPs.
Conclusions
In summary, novel CdS hierarchical multi-cavity hollow particles (HMCHPs) have been synthesized by a sequential solution growth, sulfidation and cation-exchange strategy. When evaluated as a photocatalyst for CO2 reduction, the CdS HMCHPs exhibit enhanced activity compared with both CdS solid spheres and hollow spheres. Moreover, the activity is further boosted greatly by loading a small amount of Au with a CO generation rate of 3758 μmol h−1 g−1 under visible light irradiation. The developed synthetic strategy may inspire further capability in constructing complex hollow-structured photocatalysts for efficient solar energy utilization. The promotive effect of Au to the activity of semiconductor photocatalysts would also encourage the development of novel material designs for photocatalytic CO2 reduction.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
X. W. Lou is grateful to the Ministry of Education of Singapore for the funding support through AcRF Tier 2 grant (MOE2017-T2-2-003(S); M4020386).Notes and references
- N. Armaroli and V. Balzani, Angew. Chem., Int. Ed., 2007, 46, 52 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS.
- N. S. Lewis, Science, 2016, 351, aad1920 CrossRef.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Angew. Chem., Int. Ed., 2013, 52, 7372 CrossRef CAS.
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637 CrossRef CAS.
- L. Liang, X. Li, Y. Sun, Y. Tan, X. Jiao, H. Ju, Z. Qi, J. Zhu and Y. Xie, Joule, 2018, 2, 1004 CrossRef CAS.
- P. Li, Y. Zhou, Z. Zhao, Q. Xu, X. Wang, M. Xiao and Z. Zou, J. Am. Chem. Soc., 2015, 137, 9547 CrossRef CAS.
- H. Cheng, B. Huang, Y. Liu, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 9729 RSC.
- Q. Liu, Y. Zhou, J. Kou, X. Chen, Z. Tian, J. Gao, S. Yan and Z. Zou, J. Am. Chem. Soc., 2010, 132, 14385 CrossRef CAS.
- X. Wang, G. Sun, N. Li and P. Chen, Chem. Soc. Rev., 2016, 45, 2239 RSC.
- S. Wang, B. Y. Guan, Y. Lu and X. W. Lou, J. Am. Chem. Soc., 2017, 139, 17305 CrossRef CAS.
- M. F. Kuehnel, K. L. Orchard, K. E. Dalle and E. Reisner, J. Am. Chem. Soc., 2017, 139, 7217 CrossRef CAS PubMed.
- S. Wang and X. Wang, Appl. Catal., B, 2015, 162, 494 CrossRef CAS.
- L. Cheng, Q. Xiang, Y. Liao and H. Zhang, Energy Environ. Sci., 2018, 11, 1362 RSC.
- G. Zhao, W. Zhou, Y. Sun, X. Wang, H. Liu, X. Meng, K. Chang and J. Ye, Appl. Catal., B, 2018, 226, 252 CrossRef CAS.
- Z. Dong, H. Ren, C. M. Hessel, J. Wang, R. Yu, Q. Jin, M. Yang, Z. Hu, Y. Chen, Z. Tang, H. Zhao and D. Wang, Adv. Mater., 2014, 26, 905 CrossRef CAS.
- L. Yu, H. B. Wu and X. W. Lou, Acc. Chem. Res., 2017, 50, 293 CrossRef CAS PubMed.
- P. Zhang, B. Y. Guan, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 7141 CrossRef CAS.
- S.-I. In, D. D. Vaughn and R. E. Schaak, Angew. Chem., Int. Ed., 2012, 51, 3915 CrossRef CAS.
- S. Wang, B. Y. Guan and X. W. Lou, Energy Environ. Sci., 2018, 11, 306 RSC.
- P. Zhang, B. Y. Guan, L. Yu and X. W. Lou, Chem, 2018, 4, 162 CAS.
- Z. Tong, D. Yang, Z. Li, Y. Nan, F. Ding, Y. Shen and Z. Jiang, ACS Nano, 2017, 11, 1103 CrossRef CAS PubMed.
- L. Yu, H. Hu, H. B. Wu and X. W. Lou, Adv. Mater., 2017, 29, 1604563 CrossRef PubMed.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2014, 136, 13925 CrossRef CAS PubMed.
- H. B. Wu and X. W. Lou, Sci. Adv., 2017, 3, eaap9252 CrossRef.
- H. Zhang, J. Nai, L. Yu and X. W. Lou, Joule, 2017, 1, 77 CrossRef CAS.
- L. Shen, L. Yu, H. B. Wu, X.-Y. Yu, X. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 CrossRef CAS PubMed.
- S. Wang, B. Y. Guan and X. W. Lou, J. Am. Chem. Soc., 2018, 140, 5037 CrossRef CAS PubMed.
- M. Zhou, S. Wang, P. Yang, C. Huang and X. Wang, ACS Catal., 2018, 8, 4928 CrossRef CAS.
- Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 11926 CrossRef CAS PubMed.
- C. Huang, C. Chen, M. Zhang, L. Lin, X. Ye, S. Lin, M. Antonietti and X. Wang, Nat. Commun., 2015, 6, 7698 CrossRef PubMed.
- S. Wang, W. Yao, J. Lin, Z. Ding and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 1034 CrossRef CAS PubMed.
- J. Zhou, W. Chen, C. Sun, L. Han, C. Qin, M. Chen, X. Wang, E. Wang and Z. Su, ACS Appl. Mater. Interfaces, 2017, 9, 11689 CrossRef CAS.
- W. Zhang, Y. Hu, L. Ma, G. Zhu, Y. Wang, X. Xue, R. Chen, S. Yang and Z. Jin, Adv. Sci., 2018, 5, 1700275 CrossRef.
- W. Zhu, R. Michalsky, Ö. Metin, H. Lv, S. Guo, C. J. Wright, X. Sun, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2013, 135, 16833 CrossRef CAS PubMed.
- M. Jiang, Y. Gao, Z. Wang and Z. Ding, Appl. Catal., B, 2016, 198, 180 CrossRef CAS.
- Z. Zhu, J. Qin, M. Jiang, Z. Ding and Y. Hou, Appl. Surf. Sci., 2017, 391, 572 CrossRef CAS.
- A. Demortière, R. D. Schaller, T. Li, S. Chattopadhyay, G. Krylova, T. Shibata, P. C. dos Santos Claro, C. E. Rowland, J. T. Miller, R. Cook, B. Lee and E. V. Shevchenko, J. Am. Chem. Soc., 2014, 136, 2342 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, XRD patterns, EDX spectra, FESEM images, TEM images, GC-MS analysis, BET surface areas, UV-vis absorption spectra, Nyquist plots, PL spectra, and comparison of CO2 photoreduction performance. See DOI: 10.1039/c8ee02538j |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |