Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A novel polymer containing phosphorus–nitrogen ligands for stabilization of palladium nanoparticles: an efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water
M.
Gholinejad
*a,
F.
Hamed
a and
P.
Biji
b
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), P. O. Box 45195-1159, Gavazang, Zanjan 45137-6731, Iran. E-mail: gholinejad@iasbs.ac.ir
bNanotech Research, Innovation and Incubation Center, PSG Institute of Advanced Studies, Coimbatore-641 004, India
First published on 6th March 2019
Abstract
Correction for ‘A novel polymer containing phosphorus–nitrogen ligands for stabilization of palladium nanoparticles: an efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water’ by M. Gholinejad, et al., Dalton Trans., 2015, 44, 14293–14303.
The authors regret that the second step in Scheme 1 of the above mentioned paper was incorrect, this has been corrected below and the characterization supporting the identity of compound 2 is presented herein. This correction does not affect the discussion or conclusions of the original article.
The 31P NMR spectrum of product 2 showed a chemical shift for 31P at 36.7 ppm (Fig. 1).
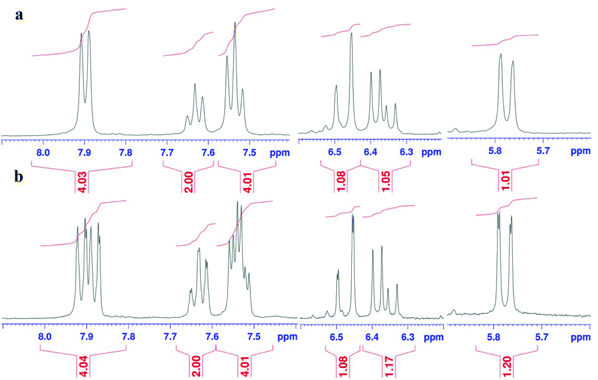
The 1H NMR spectrum of compound 2 showed a single peak at 9.64 which is related to a phenolic proton (Fig. 1a). Adding a drop of D2O to the sample, resulted in removing the phenolic hydrogen signal at 9.6 ppm and confirmed that the compound has a phenolic proton (Fig. 2b). The presence of the hydroxyl group was also confirmed by FTIR showing a related peak at 3347 cm−1 (Fig. 3).
 | ||
| Fig. 2 1H NMR of 2-((1-((diphenylphosphino)oxy)allylidene)amino)phenol in CDCl3 and in the presence of D2O. | ||
The 1H NMR spectrum of the product shows three multiple signals at (5.7–5.8), (6.3–6.6), (7.5–7.7), and (7.8–8.0) ppm that may be coupled with the 31P nucleus. To investigate this issue, we compared the original 1H NMR spectrum with its 1H{31P} spectrum. Results indicated removal of the small splitting caused by the phosphorous group in the above mentioned area (Fig. 4).
Procedure for the preparation of polymer 3.
Acryloyl chloride (1 mmol, 0.08 mL) was added to a flask containing a solution of 2-aminophenol (1 mmol, 0.11 g) in dry THF (5 mL) at 0 °C under an argon atmosphere. Then, Et3N (1 mmol, 0.14 mL) was added dropwise at 0 °C and the mixture was stirred for 2 h at room temperature. Afterwards, the reaction mixture was filtered to separate the triethylammonium chloride salt produced. To the resulting solution, chlorodiphenylphosphine (1.1 mmol, 0.2 g) and Et3N (1 mmol, 0.14 mL) was added and the reaction was stirred for 24 h at 50 °C. Then, solvent was evaporated and the desired product was purified by plate chromatography. For polymerization of the obtained monomer, the phosphinite monomer (2) (5 mmol, 1.7 g) was dissolved in t-BuOH (5 mL) and AIBN (0.008 g, 0.5% w/w) was added to the mixture under argon protection. The mixture was stirred for 24 h at 70 °C under an argon atmosphere. Then, t-BuOH was evaporated and the obtained pale yellow solid was washed with H2O (10 mL) and hexane (30 mL) and dried in an oven for 24 h at 80 °C.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |