Open Access Article
Open Access ArticleFacile synthesis of silver alkynide cluster and coordination polymers using picolinic acid as a co-ligand†
Arvind Kumar
Gupta
 and
Andreas
Orthaber
and
Andreas
Orthaber

Synthetic Molecular Chemistry, Department of Chemistry – Ångström, Uppsala University, Box 523, 75120 Uppsala, Sweden. E-mail: andreas.orthaber@kemi.uu.se
First published on 14th October 2019
Abstract
We describe five 1D-coordination polymers and two discrete silver clusters consisting of alkynides and picolinic carboxylates as co-ligands. In some cases, DMSO or EtOH further solvated the structural motifs. Utilising the sterically demanding tri-isopropylsilyl acetylene afforded a tridecanuclear cluster that possessed an unprecedented core with a silver center surrounded by six octahedrally arranged silver atoms.
Introduction
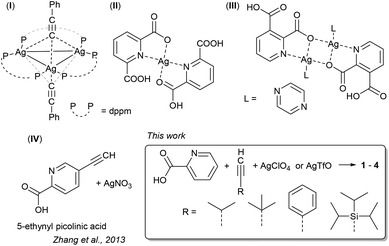
Alkynides are very versatile ligands giving multinuclear coinage metal coordination compounds.1 In particular for silver, alkynides can ligate one or more silver centres in various combinations of end-on (η1) or side-on (η2) fashion. Alkynides bridging up to four and in extremely rare cases even five silver centres have been previously reported, with μ3-η1η1η1 and μ3-η2η1η1 being the most prominent coordination modes.2 Although slightly weaker than those of gold clusters, metallophilic interactions in silver assemblies, i.e. argentophilic interactions,3 are a fundamental force for crystal engineering.4 In analogy to aurophilic interactions, a typical criterion used in the current literature for argentophilic interactions is a sub van der Waals contact, i.e. below 3.44 Å, with many examples of substantial argentophilic interactions found around Ag–Ag distances of 3.0 Å.5 Importantly, in most cases, no higher numbers of partners can be achieved, also due to the omni-directionality of these interactions.Bridging mono-and multidentate ligands play an important role in forming these assemblies. For example, the μ3-η1η1η1 and μ3-η2η1η1 trinuclear structural motifs are found in highly symmetric alkynide-only larger clusters,6 but also in co-ligand stabilized smaller clusters. For example, bidentate phosphane ligands stabilize the cationic [Ag3(CCR)nL3](3−n) motif having one (for R = (CMe2OH), n = 1, L = dppm, bis(diphenylphosphino)methane)7 or two (for R = Ph, n = 2, L = dppm, 1)8 alkynide ligands. Variation of silver salts in the cluster synthesis can also have a great impact on which structures are formed, in particular when coordinating counterions, e.g. oxo-anions are used. Their ability to act as (bridging) ligands or being encapsulated in the direct coordination sphere of clusters and coordination polymers has led to interesting structural motifs and host–guest cage compounds.9–11
Phosphane-based co-ligands are often used to steer the formation of larger cluster motifs,12 while the use of harder donor co-ligands such as N- or O-based Lewis bases is significantly less explored.13 Only very few silver coordination compounds using picolinic acid (and related pyridine carboxylic acid derivatives) have been reported to date, however recent examples clearly illustrate the possibilities offered by this co-ligand class. Various groups have reported the coordination of two ortho-pyridine carboxylates as the O,N-bidentate ligand towards one silver centre in the form of homoleptic coordination compounds14–17 or as a heteroleptic motif with one pyridine carboxylate and two monodentate ligands, e.g. PPh3.18 On the other hand, pyridine dicarboxylates have led to an assembly of the homoleptic structural motif into 2D-layered structures via additional hydrogen bonding (2),19 while in other cases carboxylate oxygen atoms also engage in bridging coordination modes yielding interesting Ag2O2-cyclic motifs (3).20,21 To the best of our knowledge, there is only one example in which alkynide coordination is combined with a pyridine carboxylate motif. Zhang and co-workers reported the reaction of 5-ethynyl nicotinic acid and 5-ethynyl-picolinic acid with silver salts. The combination of the pyridine carboxylate and the various alkynide coordination modes led to fascinating high nuclearity coordination compounds displaying argentophilic interactions. What is noteworthy is that in these clusters there is the tridentate (N,O,O′) binding of the pyridine carboxylates (picolinic and nicotinic acids), which is assisted by various alkynide coordination modes.22
In this report, we elaborate on how the interplay of picolinic acid as an N,O,O′-tridentate ligand and various alkynyl ligands leads to the formation of different coordination polymers and discrete tridecanuclear clusters. Different metal salts and synthesis conditions lead to commonly observed silver motifs, which arrange into a variety of structural motifs. The multidenticity of picolinic acid, various bridging alkynide coordination modes and argentophilic interactions give rise to a complex coordination chemistry with prominent coordination motifs found in both 1D-polymeric and discrete systems.
Results and discussion
This study reveals the formation of five different coordination compounds obtained by using different acetylene ligands (–CC–R, where R = Si(i-Pr3) subsequently abbreviated as TIPS, t-Bu, i-Pr, Ph) in the presence of silver salts (AgClO4 or AgTfO), picolinic acid and triethylamine. Variation of the steric demand as well as the cations impacts how the silver centres assemble and which coordination compounds are formed upon crystallisation (Scheme 1).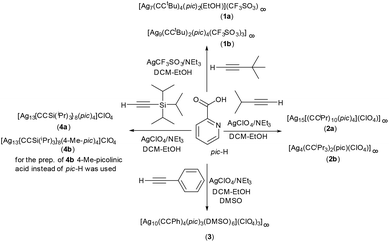 | ||
| Scheme 1 Synthesis of coordination polymers 1–3 and discrete cluster 4. Experimental details are summarized in the ESI.† | ||
In order to solubilize the reactants and intermediates during the product formation, a binary (or ternary) solvent mixture is used consisting of dichloro methane (DCM) and ethanol (EtOH). As discussed later, for compound 3, dimethylsulfoxide (DMSO) was added to facilitate the solubilisation of intermediates during the cluster formation (Fig. 1).
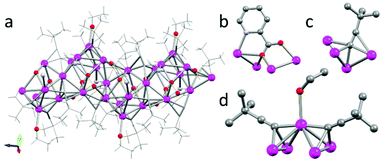
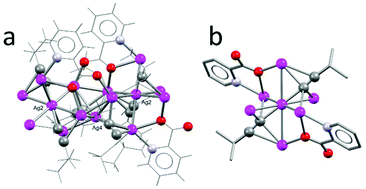
Firstly, we performed the reaction using t-Bu acetylene, picolinic acid, and silver triflate, with triethylamine acting as a base in a DCM/EtOH solvent mixture. Initially we isolated the single crystal of a coordination polymer (1a, [Ag7(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(t-Bu)4(pic)2(EtOH)](CF3SO3), Fig. 2). The reaction mixture was filtered and evaporation of the solvents gave small colorless blocks suitable for SC-XRD.‡ The unit cell contains seven silver atoms, coordinated by one ethanol, four t-BuCC−, and two picolinic carboxylate ligands in the asymmetric unit (Fdd2, no. 43, Z = 16). The metal core of 1a displays the unusual μ4-η2η1η1η1 coordination mode of acetylide ligands (Fig. 2c). The Ag–carbon distance in the almost linear arrangement is the shortest (2.153(17) Å) of the three η1-coordinations (2.286(19) and 2.37(2) Å), while the η2 coordination mode is rather long (2.33(2) and 2.63(2) Å). Besides this unusual coordination, the very common “pyramidal” arrangement of silver atoms with two acetylides coordinating in μ3-η1η1η1 is realized. Interestingly, the apical silver atom is further coordinated by an ethanol (EtOH) solvent molecule (Fig. 2d). Lastly, the picolinic acid bridges four silver centres acting as a tridentate ligand in the μ4-1:κ1N,1-2-3:κ3O,4:κ1O′ coordination mode (Fig. 2b). These three ligand environments form a rather complex 1D-coordination polymer stretching along the crystallographic [001]-direction.
C(t-Bu)4(pic)2(EtOH)](CF3SO3), Fig. 2). The reaction mixture was filtered and evaporation of the solvents gave small colorless blocks suitable for SC-XRD.‡ The unit cell contains seven silver atoms, coordinated by one ethanol, four t-BuCC−, and two picolinic carboxylate ligands in the asymmetric unit (Fdd2, no. 43, Z = 16). The metal core of 1a displays the unusual μ4-η2η1η1η1 coordination mode of acetylide ligands (Fig. 2c). The Ag–carbon distance in the almost linear arrangement is the shortest (2.153(17) Å) of the three η1-coordinations (2.286(19) and 2.37(2) Å), while the η2 coordination mode is rather long (2.33(2) and 2.63(2) Å). Besides this unusual coordination, the very common “pyramidal” arrangement of silver atoms with two acetylides coordinating in μ3-η1η1η1 is realized. Interestingly, the apical silver atom is further coordinated by an ethanol (EtOH) solvent molecule (Fig. 2d). Lastly, the picolinic acid bridges four silver centres acting as a tridentate ligand in the μ4-1:κ1N,1-2-3:κ3O,4:κ1O′ coordination mode (Fig. 2b). These three ligand environments form a rather complex 1D-coordination polymer stretching along the crystallographic [001]-direction.
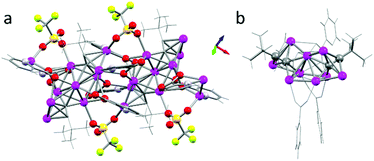
Powder XRD (PXRD) of the bulk material however suggested that the major product of this reaction differs from the isolated single crystals (see ESI Fig. S8†). Indeed for more than one week after initial crystallisation, differently shaped crystals of another coordination polymer 1b were obtained (Fig. 3).‡ The unit cell contains nine silver centers, two coordinating triflate counter ions, two t-butyl acetylides and four picolinate ligands (C2/c, no. 15, Z = 4). The core of this structure consists of five silver atoms arranged in a distorted pyramid. Two sides of the pyramid are capped by a silver dimer unit via a μ5-η1η1η1η2η2 acetylide coordination forming the nonanuclear building block of this coordination polymer. The other two sides of the pyramid are coordinated by two picolinic acids in a complex μ5-1:κ1N,1-2-3:κ3O,4-5:κ2O′ fashion. The basal plane of the central pyramid is coordinated by the carboxylates of the remaining two picolinic ligands, and the nitrogen atom and one oxygen atom link to a neighbouring unit (μ3-1:κ1O,2-3:κ2O′,3:κ1N). Furthermore, one triflate acts as a bridging ligand between the nonanuclear silver units, which are not connected via short Ag–Ag distances. The 1D polymer stretches along the crystallographic c-axis (see ESI Fig. S13 and S14†).
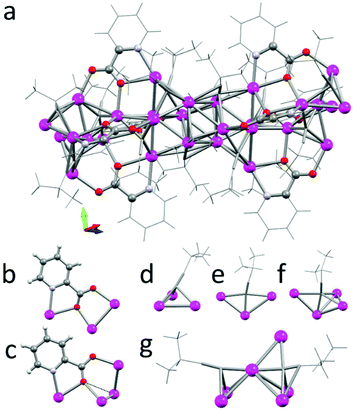
Changing to silver perchlorate and subtle variation of the steric demand of the acetylide ligand, i.e. changing to an i-propyl acetylene, gives a completely different reactivity. We were able to identify a set of 1D-coordination polymers (2a, Fig. 4a and 2bFig. 5) amongst other unidentified products during the reaction of AgClO4, i-PrCC-H, NEt3, in DMC/EtOH. Crystals suitable for SC-XRD for both species are obtained by slow evaporation from DCM/EtOH.‡ The 15 silver atoms in the asymmetric unit of 2a are coordinated by four picolinic acids and ten acetylide ligands. While two of the picolinic acids are in a μ3-1:κ1N,1-2:κ2O,3:κ1O′ mode (Fig. 4b), the other two bridge four silver centres in the μ4-1:κ1N,1-2-3:κ3O,4:κ1O′ coordination mode (Fig. 4c). The acetylide ligands mainly have a three-centre bridging environment (Fig. 4d and e), while some exhibit a four-centre coordination mode (Fig. 4f). In the latter, three silver atoms and the acetylene (C6![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C7) form an almost co-planar arrowhead with Ag1 as the tip, while the fourth silver atom (Ag2) locates above, forming an almost perpendicular plane together with C6
C7) form an almost co-planar arrowhead with Ag1 as the tip, while the fourth silver atom (Ag2) locates above, forming an almost perpendicular plane together with C6![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C7 and Ag1 (angle between both planes 97.34(1)°). Interestingly, a remarkably similar pyramid shaped motif of polymers 1a (Fig. 2d) and 1b (Fig. 3b) is again identified within this structure, in this case also embedded in the framework (similar to 1b) and not terminated by a solvent molecule as observed in compound 1a (Fig. 4g). The almost linear C7
C7 and Ag1 (angle between both planes 97.34(1)°). Interestingly, a remarkably similar pyramid shaped motif of polymers 1a (Fig. 2d) and 1b (Fig. 3b) is again identified within this structure, in this case also embedded in the framework (similar to 1b) and not terminated by a solvent molecule as observed in compound 1a (Fig. 4g). The almost linear C7![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C6–Ag–C1
C6–Ag–C1![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C2 fragment (C–Ag–C = 170.290(1)°) combines the coordination modes shown in Fig. 4d and f. Similarly, the acetylenic fragment containing C26
C2 fragment (C–Ag–C = 170.290(1)°) combines the coordination modes shown in Fig. 4d and f. Similarly, the acetylenic fragment containing C26![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C27 is embedded in such a motif with almost identical structural parameters. The various coordination modes of the two ligands lead to a complex pattern of silver atoms which form a continuous band via short Ag–Ag distances in the range of 2.8 to 3.2 Å.
C27 is embedded in such a motif with almost identical structural parameters. The various coordination modes of the two ligands lead to a complex pattern of silver atoms which form a continuous band via short Ag–Ag distances in the range of 2.8 to 3.2 Å.
On the other hand, SC-XRD of the crystals of 2b show a different stoichiometry with four silver centers, one picolinate, two acetylide ligands and one coordinated perchlorate counter ion in the asymmetric unit ([Ag4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CiPr3)2(pic)(ClO4)]∞). The symmetry generated 1D-coordination polymer reveals two interesting features: there are two distinct (almost) linearly arranged CC–Ag–CC units (around Ag2 and Ag4) very similar to the motif found in 2a (Fig. 4g), albeit incorporated into two different silver environments. While for 2b the silver atom Ag2 is quite regularly surrounded by six silver atoms, the six closest silver atoms around Ag4 are highly distorted due to the additional bridging coordination mode of the three perchlorate ions (Fig. 5a). The observed arrangement of the six nearest silver atoms around Ag2 is quite remarkable (Fig. 5b). These are octahedrally arranged around a silver center. This motif is stabilized by a μ3-1:κ1N,1-2:κ2O,3:κ1O′ bridging picolinate coordination mode and the μ4-η1η1η1η2 bridging acetylide ligands (vide infra coordination of 4). This silver packing motif alternates with the irregular environment around Ag4 in the 1D polymer along the crystallographic [101] direction (see Fig. S15 in the ESI†).
CiPr3)2(pic)(ClO4)]∞). The symmetry generated 1D-coordination polymer reveals two interesting features: there are two distinct (almost) linearly arranged CC–Ag–CC units (around Ag2 and Ag4) very similar to the motif found in 2a (Fig. 4g), albeit incorporated into two different silver environments. While for 2b the silver atom Ag2 is quite regularly surrounded by six silver atoms, the six closest silver atoms around Ag4 are highly distorted due to the additional bridging coordination mode of the three perchlorate ions (Fig. 5a). The observed arrangement of the six nearest silver atoms around Ag2 is quite remarkable (Fig. 5b). These are octahedrally arranged around a silver center. This motif is stabilized by a μ3-1:κ1N,1-2:κ2O,3:κ1O′ bridging picolinate coordination mode and the μ4-η1η1η1η2 bridging acetylide ligands (vide infra coordination of 4). This silver packing motif alternates with the irregular environment around Ag4 in the 1D polymer along the crystallographic [101] direction (see Fig. S15 in the ESI†).
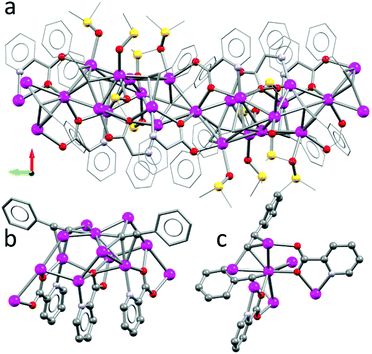
Reacting the simple aromatic acetylide ligand, i.e. phenyl acetylene, with silver perchlorate changed the initial solution behaviour of the reaction. Instead of a transparent solution, immediate formation of a precipitate was observed. In order to solubilize the material, a few drops of dimethyl sulfoxide (DMSO) were added and the reaction mixture was stirred for four hours. Crystallisation from DCM/EtOH/DMSO afforded single crystals of 3 suitable for X-ray diffraction. The structure was solved in the orthorhombic space group P212121 (no. 19, Z = 4).‡ This coordination polymer incorporated five DMSO solvent molecules as coordinating ligands. Besides these, the asymmetric unit contains ten silver atoms, three picolinic carboxylates, four phenyl acetylides, and three non-coordinating perchlorates. The ligand shell of the coordination polymer forms regimes of higher polarity (i.e. where the DMSO ligands and perchlorates are grouped) and areas dominated by π-interactions between the pyridyl fragments (containing N1, N2 and N3) (Fig. 6a). The centroid and shift distances between the rings are 3.592, 3.565 Å and 1.109, 1.356 Å, respectively. The picolinic carboxylates act as tridentate ligands aligning eight silver centres, in which the two outermost show the μ3-1:κ1N,2:κ1O,3:κ1O′ and μ3-1′′:κ1N,2′′:κ1O,3′′:κ1O′, while the sandwiched picolinic carboxylate links them in a μ4-1′:κ1N,3-3′′:κ3O,3′:κ1O′ coordination mode (Fig. 6b). The acetylide ligands are linked in either μ3- or μ4-coordination modes as described earlier. Two arrays of the three picolinic acids are related by symmetry via an almost linear Ag3 fragment (Fig. 6c and Fig. S16 in the ESI†).
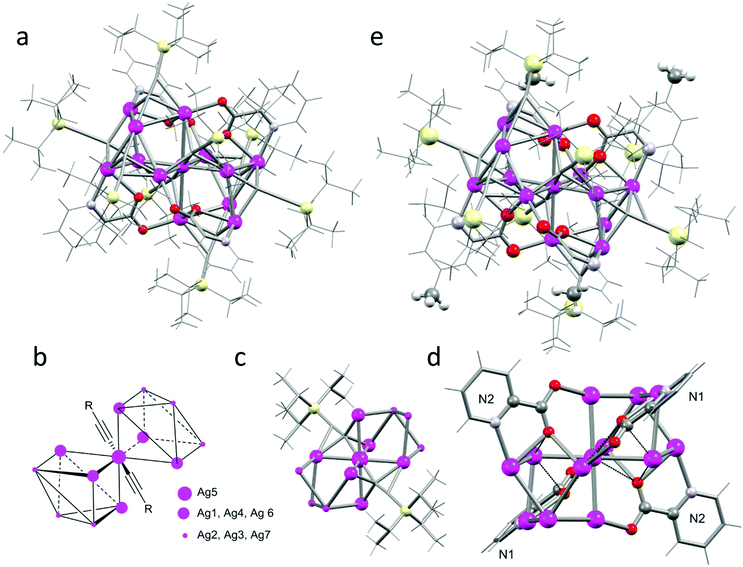
Ultimately, increasing the steric bulk of the ligands by using tri-i-propylsilyl acetylene (TIPS acetylene) affords a discrete silver cluster of thirteen metal centres (4a, Fig. 7a), which crystallises as colourless blocks in the monoclinic space group C2/c (no. 15, Z = 4).‡ The core of the cluster consists of a central silver atom (Ag5), which is surrounded by six silver atoms forming an octahedral arrangement around Ag5. Furthermore, two acetylide ligands are coordinated to Ag5 in a μ4 bridging mode (Fig. 7b). This structural motif is remarkably similar to the Ag2 environment in cluster 2b, however the smaller i-Pr acetylene ligands allows the formation of a coordination polymer, while the increased steric demand of the TIPS acetylene ligands leads to the formation of discrete clusters.
The first layer of six silver atoms forms an almost octahedral environment with Ag5–Ag distances of 2.8980(4), 2.9297(4), 3.147(5) Å, and Ag–Ag5–Ag angles close to right angles: 85.69(1), 93.13(1), and 92.25(1)°. The inner acetylide ligands exhibit the rarer μ4-η2η1η1η1 coordination mode with a short Ag5–C distance (2.112(4) Å) and slightly longer distances to the first layer of silver atoms (2.28687(5) & 2.44570(4) Å for the η2; and 2.37383(4) & 2.51499(4) Å, for the η1 coordination modes, Fig. 7d). This is similar to the previously observed μ4-coordination modes. The silver cluster is expanded by two Ag3 units located in two opposite octants. The Ag3 units are linked to the central octahedral motif with three acetylide ligands coordinating trigonal faces (two in μ3-η2η1η1 and one in a μ3-η1η1η1) as well as four picolinic acids acting as the μ3-1:κ1N,1-2:κ2O,3:κ1O′ tridentate bridging ligand (Fig. 7c). Related, octahedral motifs without a central silver atom are commonly found in silver clusters (e.g. in [Ag6(CCR)4L2])6 and in expanded motifs as the outer shell of a cubane core (see for example the [X⊂Ag14(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)12]+ series, where X = Cl, Br, I).23,24 However such an octahedral cluster with a central silver atom is to the best of our knowledge the first Ag(I) cluster that features such a structural motif. Surprised by these findings we wanted to investigate if minor changes in the picolinate ligand would alter its coordination behaviour. We identified from the solid-state X-ray analysis that substitution at the 4-position of picolinic acid, i.e. 4-methylpyridine-2-carboxylic acid, should have no impact on the coordination and packing of the discrete cluster. Indeed, this ligand affords an almost identical motif 4b, which crystallizes as colourless blocks in the monoclinic space group P
CtBu)12]+ series, where X = Cl, Br, I).23,24 However such an octahedral cluster with a central silver atom is to the best of our knowledge the first Ag(I) cluster that features such a structural motif. Surprised by these findings we wanted to investigate if minor changes in the picolinate ligand would alter its coordination behaviour. We identified from the solid-state X-ray analysis that substitution at the 4-position of picolinic acid, i.e. 4-methylpyridine-2-carboxylic acid, should have no impact on the coordination and packing of the discrete cluster. Indeed, this ligand affords an almost identical motif 4b, which crystallizes as colourless blocks in the monoclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2, Z = 2) as two halves of the tridecanuclear cluster in the asymmetric unit. One of the discrete clusters shows a slight disorder around the central silver atom, which is modelled as a position disorder with 1/2 occupancy. In the non-disordered moiety, we see silver distances of 2.910(1), 3.028(1) and 3.050(1) Å from the central to the first shell of six silver atoms, and angles in the range of 88.43(3)° to 91.76(3)° closely matching the metric parameters observed in cluster 4a. As expected, the methyl groups do not disturb the coordination of the picolinate moieties during the formation of the tridecanuclear assembly (Fig. 7e).
(no. 2, Z = 2) as two halves of the tridecanuclear cluster in the asymmetric unit. One of the discrete clusters shows a slight disorder around the central silver atom, which is modelled as a position disorder with 1/2 occupancy. In the non-disordered moiety, we see silver distances of 2.910(1), 3.028(1) and 3.050(1) Å from the central to the first shell of six silver atoms, and angles in the range of 88.43(3)° to 91.76(3)° closely matching the metric parameters observed in cluster 4a. As expected, the methyl groups do not disturb the coordination of the picolinate moieties during the formation of the tridecanuclear assembly (Fig. 7e).
Due to the discrete nature of these clusters, we performed solution studies using NMR spectroscopy (see also Fig. S1–S6 in the ESI†). Surprisingly, the clusters are sensitive towards chloroform solutions. Solution studies of 4a were performed in DCM-d2, while 4b could only be studied in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DCM-d2 and C6D6. For both compounds 4a and 4b, a broadening in the proton NMR of the signals for the picolinic carboxylate (8.41, 8.15, 7.80, and 7.43 ppm for 4a and 8.08, 7.83, 6.84, and 1.98 ppm for 4b) and multiple signals for the TIPS (1.19, 0.97, 0.83 ppm for 4a and multiple signals around 0.91 ppm for 4b) environments are observed. This is also seen in 13C-NMR spectra, where two (4a) sets of signals for the i-Pr-group are observed; the quaternary acetylide signals are however not detected, presumably due to Ag–C couplings. The observed broadening either indicates a dynamic behaviour in solution, represents the different environments seen in the solid-state structure or originates from the large size of the cluster. In order to probe the persistence of the cluster in solution, we performed further 1H-NMR DOSY and 1D-NOE measurements. Selective irradiation of the 2-H (8.18 ppm) and 4-H (8.48 ppm) of the picolinic acid in cluster 4a indicates close proximity to the i-Pr groups with a chemical shift of 0.97 ppm, while both protons 3-H and 4-H show no NOE correlation. These findings are in agreement with the spatial arrangement of the i-Pr groups with respect to the picolinic fragment in the solid-state structure (see also Fig. S7 in the ESI†). DOSY measurements have previously been used to confirm stability as well as the dissociation behaviour of various silver coordination compounds in solution.25–27 Measurements of 4a using mesitylene as an internal reference give similar diffusion rates for the picolinate and the acetylide ligands (D = 12.12 × 10−9 m2 s−1), suggesting that both ligands are part of the same entity (Fig. 8).§ The calculated hydrodynamic radius (rh) of 12.1 Å is slightly larger than expected from the solid-state structure, in which the longest axis in the approximately prolate spheroid structure of 4a is approx. 19.5 Å and the shorter axis falls between 15–17 Å. Re-crystallization from the NMR solutions again affords single crystals of 4a, supporting its solution stability. Similarly, 1H-DOSY measurements of 4b using the residual solvent peaks as internal standards give a diffusion coefficient of D = 12.13 × 10−9 m2 s−1 (rh = 12.6 Å), which is, as expected, slightly larger than that for 4a, but is within the error of the measurements. Comparing these values with the dimensions of the cluster also indicates significant interaction of the solvent molecules with the cluster, leading effectively to a larger hydrodynamic radius. It must be stated that these findings do not completely rule out a dynamic situation in solution, but can only confirm that the entity observed in solution is of similar size compared to the original clusters 4a and 4b, and complete fragmentation and aggregation (dimers or oligomers) can be excluded. Although not directly comparable, the ESI-MS analysis shows significant fragments that involve the loss of acetylide ligands, and/or the addition of chloride (from the solvent) and/or silver ions.¶
1 mixture of DCM-d2 and C6D6. For both compounds 4a and 4b, a broadening in the proton NMR of the signals for the picolinic carboxylate (8.41, 8.15, 7.80, and 7.43 ppm for 4a and 8.08, 7.83, 6.84, and 1.98 ppm for 4b) and multiple signals for the TIPS (1.19, 0.97, 0.83 ppm for 4a and multiple signals around 0.91 ppm for 4b) environments are observed. This is also seen in 13C-NMR spectra, where two (4a) sets of signals for the i-Pr-group are observed; the quaternary acetylide signals are however not detected, presumably due to Ag–C couplings. The observed broadening either indicates a dynamic behaviour in solution, represents the different environments seen in the solid-state structure or originates from the large size of the cluster. In order to probe the persistence of the cluster in solution, we performed further 1H-NMR DOSY and 1D-NOE measurements. Selective irradiation of the 2-H (8.18 ppm) and 4-H (8.48 ppm) of the picolinic acid in cluster 4a indicates close proximity to the i-Pr groups with a chemical shift of 0.97 ppm, while both protons 3-H and 4-H show no NOE correlation. These findings are in agreement with the spatial arrangement of the i-Pr groups with respect to the picolinic fragment in the solid-state structure (see also Fig. S7 in the ESI†). DOSY measurements have previously been used to confirm stability as well as the dissociation behaviour of various silver coordination compounds in solution.25–27 Measurements of 4a using mesitylene as an internal reference give similar diffusion rates for the picolinate and the acetylide ligands (D = 12.12 × 10−9 m2 s−1), suggesting that both ligands are part of the same entity (Fig. 8).§ The calculated hydrodynamic radius (rh) of 12.1 Å is slightly larger than expected from the solid-state structure, in which the longest axis in the approximately prolate spheroid structure of 4a is approx. 19.5 Å and the shorter axis falls between 15–17 Å. Re-crystallization from the NMR solutions again affords single crystals of 4a, supporting its solution stability. Similarly, 1H-DOSY measurements of 4b using the residual solvent peaks as internal standards give a diffusion coefficient of D = 12.13 × 10−9 m2 s−1 (rh = 12.6 Å), which is, as expected, slightly larger than that for 4a, but is within the error of the measurements. Comparing these values with the dimensions of the cluster also indicates significant interaction of the solvent molecules with the cluster, leading effectively to a larger hydrodynamic radius. It must be stated that these findings do not completely rule out a dynamic situation in solution, but can only confirm that the entity observed in solution is of similar size compared to the original clusters 4a and 4b, and complete fragmentation and aggregation (dimers or oligomers) can be excluded. Although not directly comparable, the ESI-MS analysis shows significant fragments that involve the loss of acetylide ligands, and/or the addition of chloride (from the solvent) and/or silver ions.¶
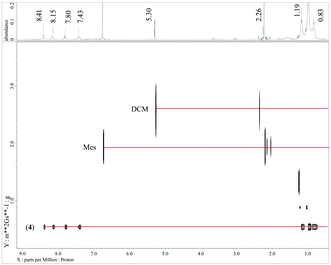 | ||
| Fig. 8 NMR studies of cluster 4 in DCM-d2. Top: 1H-NMR spectrum of 4 and mesitylene. 1H-DOSY analysis with mesitylene as the internal standard. | ||
Conclusions
In this manuscript we describe the synthesis and solid state structures of silver(I) coordination compounds stabilized by picolinic carboxylates and acetylide ligands. We illustrate how subtle variations of the silver salt and the steric demand of the acetylene ligand lead to the formation of complex 1D-coordination polymers or afford discrete cluster, when a sterically demanding TIPS acetylene is used. The obtained clusters show a very regular octahedral core of six silver atoms grouped around a silver centre, which is further expanded by two Ag3 units. The integrity of the discrete cluster in solution has been corroborated by solution NMR studies (1D proton and carbon as well as 2D-DOSY). The different ligand binding modes reoccur in this series of coordination compounds illustrating how subtle ligand, metal salt, and solvent variations can lead to a variety of silver coordination compounds. Notably, the combination of the cluster-directing acetylide ligands and the multidenticity of picolinic acid, together with the notably short Ag–Ag distances indicative of argentophilic interactions, gives rise to the diverse coordination chemistry reported herein.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the EU-COST action on Smart Inorganic Polymers (CM1302, SIPs), the Swedish Research Council (Vetenskapsrådet), Byggmästare-Olle-Engkvist Foundation.Notes and references
- A. K. Gupta and A. Orthaber, Chem. – Eur. J., 2018, 24, 7536–7559 CrossRef CAS PubMed.
- R. Nast, Coord. Chem. Rev., 1982, 47, 89–124 CrossRef CAS.
- P. Pyykkö, Chem. Rev., 1997, 97, 597–636 CrossRef PubMed.
- M. J. Katz, K. Sakai and D. B. Leznoff, Chem. Soc. Rev., 2008, 37, 1884–1895 RSC.
- H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed.
- Q.-H. Wei, L.-Y. Zhang, L.-X. Shi and Z.-N. Chen, Inorg. Chem. Commun., 2004, 7, 286–288 CrossRef CAS.
- R. Aroz, F. Mohr, E. Cerrada, E. R. T. Tiekink and M. Laguna, Polyhedron, 2006, 25, 3066–3070 CrossRef CAS.
- C.-F. Wang, S.-M. Peng, C.-K. Chan and C.-M. Che, Polyhedron, 1996, 15, 1853–1858 CrossRef CAS.
- S.-D. Bian, H.-B. Wu and Q.-M. Wang, Angew. Chem., Int. Ed., 2009, 48, 5363–5365 CrossRef CAS PubMed.
- S.-D. Bian, J.-H. Jia and Q.-M. Wang, J. Am. Chem. Soc., 2009, 131, 3422–3423 CrossRef CAS.
- K.-G. Liu, S.-K. Chen, Y.-M. Lin and Q.-M. Wang, Chem. Commun., 2015, 51, 9896–9898 RSC.
- A. Belyaev, T. M. Dau, J. Jänis, E. V. Grachova, S. P. Tunik and I. O. Koshevoy, Organometallics, 2016, 35, 3763–3774 CrossRef CAS.
- A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155–192 CrossRef CAS.
- J. P. Deloume, R. Faure and H. Loiseleur, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 2709–2712 CrossRef.
- Y. Wang, M. Odoko and N. Okabe, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, m1178–m1180 CrossRef CAS.
- M. G. B. Drew, R. W. Matthews and R. A. Walton, J. Chem. Soc. A, 1971, 2959–2962, 10.1039/J19710002959.
- H. Lee, E. J. Kim, J. Ahn, T. H. Noh and O.-S. Jung, J. Mol. Struct., 2012, 1010, 111–115 CrossRef CAS.
- R. A. Haque, S. W. Ng and M. R. Razali, J. Coord. Chem., 2015, 68, 1317–1331 CrossRef.
- P.-Y. Cheng, C.-Y. Chen and H. M. Lee, Inorg. Chim. Acta, 2009, 362, 1840–1846 CrossRef CAS.
- S. H. Alisir, B. Sariboga, Y. Topcu and S.-Y. Yang, J. Inorg. Organomet. Polym. Mater., 2013, 23, 1061–1067 CrossRef CAS.
- M. M. Amini, O. Sadeghi and S. W. Ng, J. Inorg. Organomet. Polym. Mater., 2013, 23, 826–830 CrossRef CAS.
- Z. Zhang, B. Li, X. Meng, X. Yin and T. Zhang, Dalton Trans., 2013, 42, 4306–4312 RSC.
- D. Rais, J. Yau, D. M. P. Mingos, R. Vilar, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2001, 40, 3464–3467 CrossRef CAS PubMed.
- D. Rais, D. M. P. Mingos, R. Vilar, A. J. P. White and D. J. Williams, J. Organomet. Chem., 2002, 652, 87–93 CrossRef CAS.
- K. J. Kilpin, M. L. Gower, S. G. Telfer, G. B. Jameson and J. D. Crowley, Inorg. Chem., 2011, 50, 1123–1134 CrossRef CAS.
- J. Fielden, D.-l. Long, A. M. Z. Slawin, P. Kögerler and L. Cronin, Inorg. Chem., 2007, 46, 9090–9097 CrossRef CAS PubMed.
- S. Bestgen, O. Fuhr, B. Breitung, V. S. Kiran Chakravadhanula, G. Guthausen, F. Hennrich, W. Yu, M. M. Kappes, P. W. Roesky and D. Fenske, Chem. Sci., 2017, 8, 2235–2240 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental data and synthetic procedures. CCDC 1902309–1902312 and 1953779–1953781. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/C9DT03697K |
‡ 1a (CCDC 1902312†): C39H50F3N2O8SAg7 (M = 1518.96 g mol−1): orthorhombic, space group Fdd2 (no. 43), a = 38.814(6) Å, b = 53.264(8) Å, c = 9.9806(15) Å, V = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634(5) Å3, Z = 16, T = 150.15 K, μ(MoKα) = 2.697 mm−1, Dcalc = 1.956 g cm−3, 41 634(5) Å3, Z = 16, T = 150.15 K, μ(MoKα) = 2.697 mm−1, Dcalc = 1.956 g cm−3, 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 reflections measured (3.058° ≤ 2Θ ≤ 50.492°), 8717 unique (Rint = 0.0880, Rsigma = 0.0717) which were used in all calculations. The final R1 was 0.0645 (I > 2σ(I)) and wR2 was 0.1321 (all data). 137 reflections measured (3.058° ≤ 2Θ ≤ 50.492°), 8717 unique (Rint = 0.0880, Rsigma = 0.0717) which were used in all calculations. The final R1 was 0.0645 (I > 2σ(I)) and wR2 was 0.1321 (all data).1b (CCDC 1953779†): C38H34Ag9F6N4O14S2 (M = 1919.64 g mol−1): monoclinic, space group C2/c (no. 15), a = 22.973(8) Å, b = 19.990(9) Å, c = 15.639(5) Å, β = 129.995(10)°, V = 5502(4) Å3, Z = 4, T = 150.15 K, μ(MoKα) = 3.286 mm−1, Dcalc = 2.317 g cm−3, 41 2a: (CCDC 1902311†): C74H86Ag15ClN4O12 (M = 2876.96 g mol−1): triclinic, space group P 2b: (CCDC 1953780†): C16H18Ag4ClNO6 (M = 787.24 g mol−1): monoclinic, space group C2/c (no. 15), a = 16.465(6) Å, b = 24.276(9) Å, c = 13.433(5) Å, β = 126.986(6)°, V = 4289(3) Å3, Z = 8, T = 150.15 K, μ(MoKα) = 3.755 mm−1, Dcalc = 2.438 g cm−3, 27 3 (CCDC 1902310†): C62H68Ag10Cl3N3O24S6 (M = 2616.60 g mol−1): orthorhombic, space group P212121 (no. 19), a = 16.489(3) Å, b = 18.767(4) Å, c = 25.470(5) Å, V = 7882(3) Å3, Z = 4, T = 123.15 K, μ(MoKα) = 2.762 mm−1, Dcalc = 2.205 g cm−3, 96 4a (CCDC 1902309†): C112H184N4O12Si8ClAg13 (M = 3441.10 g mol−1): monoclinic, space group C2/c (no. 15), a = 29.1488(6) Å, b = 16.3089(4) Å, c = 28.5179(6) Å, β = 91.7990(10)°, V = 13550.3(5) Å3, Z = 4, T = 150.15 K, μ(MoKα) = 1.975 mm−1, Dcalc = 1.687 g cm−3, 56 4b (CCDC 1953781†): C116H192Ag13ClN4O12Si8 (M = 3497.20 g mol−1): triclinic, space group P |
| § The DOSY analysis points towards similar diffusion of both the acetylide and picolinate ligands, indicating their presence in one discrete entity in solution on an NMR time scale. Based on the calculated size (rh) we can exclude small components and larger assemblies, however an equilibrium of multiple interchanging species cannot be ruled out completely. |
¶ General procedure for the synthesis of compound4b. A solution of (iPr)3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (50 mg, 0.27 mmol) in CH3CN/EtOH was treated with AgClO4 (92 mg, 0.44 mmol) and a few drops of triethylamine (Et3N) were added. To the resulting solution, 4-methylpyridine-2-carboxycylic acid (19 mg, 0.14 mmol) was added and stirred for 2 h. The reaction mixture was filtered through Celite pad and the solution was kept for crystallization. Colourless crystals were obtained after one week by slow evaporation. Yield 80% (96 mg). CH (50 mg, 0.27 mmol) in CH3CN/EtOH was treated with AgClO4 (92 mg, 0.44 mmol) and a few drops of triethylamine (Et3N) were added. To the resulting solution, 4-methylpyridine-2-carboxycylic acid (19 mg, 0.14 mmol) was added and stirred for 2 h. The reaction mixture was filtered through Celite pad and the solution was kept for crystallization. Colourless crystals were obtained after one week by slow evaporation. Yield 80% (96 mg). |
| This journal is © The Royal Society of Chemistry 2019 |