Aluminum containing molecular bowls and pyridinophanes: use of pyridine modules to access different molecular topologies†
Abstract
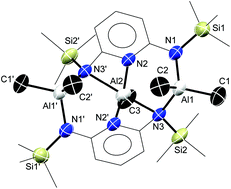
Molecular topologies varying from simple complexes to pyridinophanes (neutral and cationic) and to bicyclic pyridinophane containing organoaluminum (Al–Me) species were synthesized by varying the relative stoichiometry of bis(trimethylsilyl)-N,N′-2,6-diaminopyridine (bap) and the reactive partner (AlMe3). The ultimate goal of these reactions was to systematically design cyclic structures containing group 13 elements. To highlight the reaction potential of these shapes, the bowl-shaped pyridinophane was reacted with the Lewis acid, B(C6F5)3, to generate a stable cationic derivative. An unprecedented bicyclic pyridinophane, [2,6-(Me3SiN)2C5H3N]3Al2, was obtained from the reaction of bap with AlH3·NMe2Et. The formation of [2,6-(Me3SiN)2C5H3N]3Al2 is in contrast to the known reaction between BH3·SMe2 and bap that afforded the syn-tetraazadibora[3.3](2,6)pyridinophane. Quantum chemical calculations have been performed to rationalize the preference for the formation of B-pyridinophane and Al-bicyclic pyridinophane and can be attributed to the nature of B–N and Al–N bonds.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...