Nitrite reductase activity of heme and copper bound Aβ peptides†
Abstract
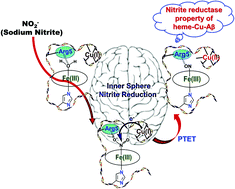
A significant abundance of copper (Cu) and iron in amyloid β (Aβ) plaques, and several heme related metabolic disorders are directly correlated with Alzheimer's disease (AD), and these together with co-localization of Aβ plaques with heme rich deposits in the brains of AD sufferers indicates a possible association of the said metals with the disease. Recently, the Aβ peptides have been found to bind heme and Cu individually as well as simultaneously. Another significant finding relevant to this is the lower levels of nitrite and nitrate found in the brains of patients suffering from AD. In this study, a combination of absorption and electron paramagnetic resonance spectroscopy and kinetic assays have been used to study the interaction of nitrite with the metal bound Aβ complexes. The data indicate that heme(III)-Cu(I)-Aβ, heme(II)-Cu(I)-Aβ, heme(II)-Aβ and Cu(I)-Aβ can reduce nitrite to nitric oxide (NO), an important biological messenger also related to AD, and thus behave as nitrite reductases. However these complexes reduce nitrite at different rates with heme(III)-Cu(I)-Aβ being the fastest following an inner sphere electron transfer mechanism. The rest of the metal-Aβ adducts follow an outer sphere electron transfer mechanism during nitrite reduction. Protonation from the Arg5 residue triggering the N–O bond heterolysis in heme(III) bound nitrite with a simultaneous electron transfer from the Cu(I) center to produce NO is the rate determining step, indicating a proton transfer followed by electron transfer (PTET) mechanism.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...