Co3O4-nanoparticle-entrapped nitrogen and boron codoped mesoporous carbon as an efficient electrocatalyst for hydrogen evolution†
Abstract
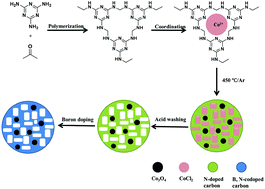
Co3O4-nanoparticle-entrapped nitrogen and boron codoped mesoporous carbon was synthesized via the molten salt method. Melamine formaldehyde resin (MF resin) was used as the nitrogen and carbon precursor, and boric acid was utilized as the boron precursor. Furthermore, cobalt chloride was used as the cobalt precursor and the template for the formation of mesopores, which could also be removed and partly recovered by acid washing. The characterization results revealed that the as-obtained samples possessed mesoporous structures, with high cobalt, boron, and nitrogen content values. For the sample of Co0.65B0.3NC800, the atomic content values of Co, N, and B are 2.3%, 8.87%, and 8.67%, respectively. Moreover, the carbonation temperature and the amount of salt template could both affect the mesoporous structures of the final samples and then affect the electrocatalytic activities for the hydrogen evolution reaction (HER). When the carbonation temperature was 800 °C, the sample of Co0.65B0.3NC800 showed superior performance for the HER under basic conditions, with high current density, low overpotential, and good stability.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...