Stereospecific polymerization of conjugated dienes using neodymium alkylborohydride complexes†
Abstract
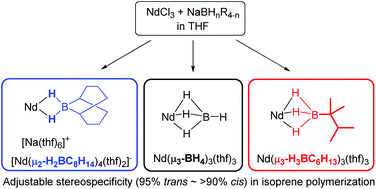
Two neodymium alkylborohydride complexes that differ with respect to the number of hydrides on the boron center were synthesized and characterized. The μ2-ligated complex exhibits Nd–B bond lengths that are approximately 10% longer than those in previously reported μ3-ligated borohydride complexes. For the μ3-alkylborohydride complex, the Nd–H–B stretching bands observed by IR spectroscopy suggested stronger borohydride coordination than that of [BH4]−, which is probably due to the electron donation of the alkyl groups. These newly prepared complexes successfully promoted trans-1,4 specific polymerization of conjugate dienes upon activation by Bu2Mg, whereas activation of these complexes by Bu2Mg/MMAO gave cis-1,4 regular polymer. The trans-1,4 defect of the polyisoprene obtained from the Nd μ2-alkylborohydride complex/Bu2Mg/MMAO system (<2%) was much lower than that obtained from previously reported μ3-BH4-ligated neodymium complex (11%). Moreover, μ2-alkylborohydride complex was a good initiator for the polymerization of MMA, probably because of its anionic nature.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...