Effect of the Si/Al ratio in Ga/mesoporous HZSM-5 on the production of benzene, toluene, and xylene via coaromatization of methane and propane†
Abstract
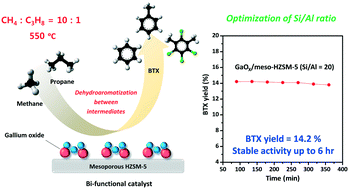
In this work, a series of GaOy supported on mesoporous HZSM-5 (GaOy/meso-XHZSM-5; Si/Al (X), X = 10, 20, 30, and 40) catalysts with different Si/Al molar ratios were prepared for use in the coaromatization of methane and propane. The coaromatization of methane and propane was conducted at 550 °C with a molar ratio of 10 : 1. Gallium oxide (2 wt%) supported on mesoporous HZSM-5 with a Si/Al molar ratio of 20 showed the highest catalytic performance, with an initial BTX yield of 14.2% and a final BTX yield of 13.8% after 6 h of reaction. The catalytic performances of the catalysts showed volcano-shaped trends depending on the Si/Al molar ratio. All of the catalysts showed a slight decrease in activity with time, although the degree of deactivation increased with increasing Si/Al ratio. The poisoning of acid sites by coke deposition is suggested to be the main reason for deactivation. The effects of the Si/Al molar ratio on the physicochemical properties and catalytic performance of the catalysts were investigated. As the molar ratio of Si/Al increased, the BET surface area and pore volume of the catalysts increased, while their total acidity decreased. Hence, it is concluded that both the acid properties and textural properties of the catalysts play a crucial role in determining the catalytic performance in the coaromatization of methane and propane. In summary, GaOy/meso-20HZSM-5 with optimum physicochemical properties demonstrated the best catalytic performance in the coaromatization of methane and propane.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...