Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of enantiomerically pure alcohols and amines via biocatalytic deracemisation methods
Musa M.
Musa
 *a,
Frank
Hollmann
*a,
Frank
Hollmann
 *b and
Francesco G.
Mutti
*b and
Francesco G.
Mutti
 *c
*c
aDepartment of Chemistry, King Fahd University of Petroleum and Minerals, Dhahran 31261, Kingdom of Saudi Arabia. E-mail: musam@kfupm.edu.sa
bDepartment of Biotechnology, Delft University of Technology, van der Maasweg 9, 2629HZ Delft, The Netherlands. E-mail: f.hollmann@tudelft.nl
cVan't Hoff Institute for Molecular Sciences, HIMS-Biocat, University of Amsterdam, Science Park 904, 1098 XH, Amsterdam, The Netherlands. E-mail: f.mutti@uva.nl
First published on 3rd September 2019
Abstract
Deracemisation via chemo-enzymatic or multi-enzymatic approaches is the optimum substitute for kinetic resolution, which suffers from the limitation of a theoretical maximum 50% yield albeit high enantiomeric excess is attainable. This review covers the recent progress in various deracemisation approaches applied to the synthesis of enantiomerically pure alcohols and amines, such as (1) dynamic kinetic resolution, (2) cyclic deracemisation, (3) linear deracemisation (including stereoinversion) and (4) enantioconvergent methods.
Introduction
Chiral alcohols1,2 and amines3–7 are pivotal building blocks for the manufacturing of chemical products such as agrochemicals, flavours, fragrances and pharmaceutical. This predominance of chiral alcohol and amine moieties has stimulated the development of a multitude of synthetic and physicochemical approaches including chromatographic separation of racemates on high-performance porous chiral stationary phases,8 enantioselective resolution using resolving agents9 and stereoselective synthesis from ketones and imines.10–15Due to their inherent homochirality, enzymes play an important role as catalysts in the synthesis of optically active alcohols and amines.16,17 Historically, hydrolase-catalysed kinetic resolution (KR, Scheme 1) reactions were the first ones to be explored.18 Despite the recently reduced interest in the academic world on these enzymes (especially because of the theoretical maximal yield of 50%), hydrolase-catalysed KR reactions are still largely applied on industrial scale production of such optically active compounds (Scheme 1).19,20
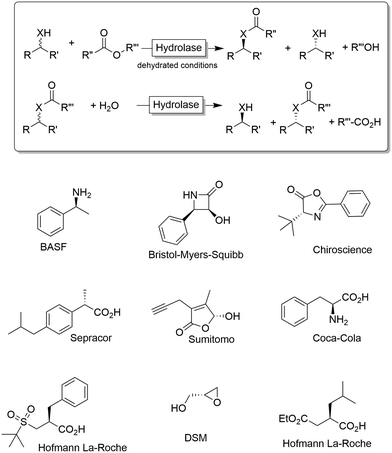 | ||
| Scheme 1 Kinetic resolution of alcohols (X = O) or amines (X = NH) using hydrolases. Under non-aqueous conditions, the synthetic direction (esterification, amidation) is preferred while under aqueous conditions the hydrolytic direction is preferred. Below a selection of industrial products obtained via kinetic resolution (KR) or dynamic (in this case, spontaneous) kinetic resolution (DKR) is shown.19,20 | ||
In an ideal KR reaction, only one enantiomer of a racemic substrate mixture is converted by an enantioselective catalyst. That results in the complete conversion one enantiomer into a product that can be separated physicochemically from the starting material. Principally, this transformation can occur either through retention of the chiral information (e.g., via (de)acylation of the heteroatom), or by destroying it (e.g., via oxidation of the α-chiral alcohol or amine moiety to a prochiral carbonyl or imine, respectively). In the former case, at least theoretically, the other enantiomer (product) can also be recovered, whereas the achiral product is generally discarded in the latter case. Hence, the maximal yield of 50% makes KR reactions unattractive from an environmental point of view as a significant amount of starting material per definition is waste.
In recent years, enantioselective reactions yielding enantiomerically pure alcohols and amines from their prochiral carbonyl and imine starting materials, respectively, have gained significant attention. Particularly, alcohol dehydrogenases (for the synthesis of optically active alcohols) as well as transaminases and imine reductases (for the synthesis of optically active amines) are well-established today. Recent advances in this area have been deliberated in some excellent recent review articles and therefore will not be further discussed here.7,15,21–28
Nevertheless, often, racemic alcohols and amines are more readily available than their corresponding carbonyl and imine compounds, respectively; therefore, starting from racemic alcohols or amines often represents the more straightforward and/or more cost-efficient approach as compared to the stereoselective reductions/reductive aminations mentioned above. To overcome the intrinsic disadvantage of KR reactions, a range of deracemisation protocols have been developed in the past years, which can be subdivided into different categories such as (1) dynamic kinetic resolution, (2) cyclic deracemisation, (3) stereoinversion and (4) enantioconvergent methods (Scheme 2).29–36
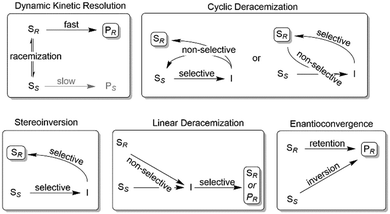 | ||
| Scheme 2 Common deracemisation methods. S: chiral starting material such as an amine or alcohol, P: product, I: prochiral intermediate such as a ketone or an imine. | ||
In the following sections, recent progress in each deracemisation approach is outlined and critically discussed.
Dynamic kinetic resolution (DKR) reactions
Dynamic kinetic resolution (DKR) protocols couple an in situ racemisation of the starting alcohol or amine substrate with an enantioselective (enzymatic) follow-up reaction, thus yielding a non-racemisable product (ideally in optically pure form, Scheme 3).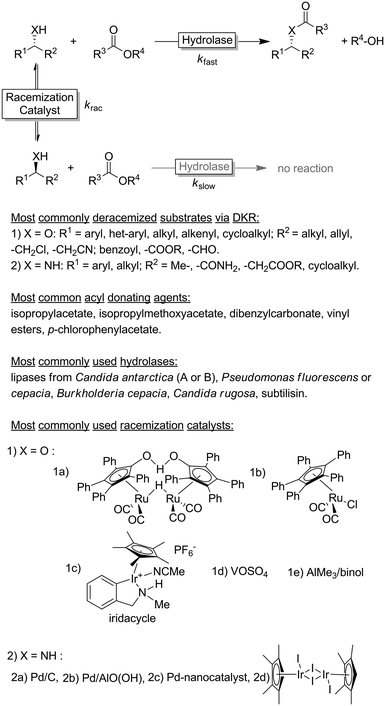 | ||
| Scheme 3 Generalised scheme of a dynamic kinetic resolution reaction involving hydrolases as KR-catalyst under non-aqueous conditions. | ||
The selection criteria for a successful DKR were formulated by Verho and Bäckvall:37 (1) the KR reaction must be sufficiently enantioselective (i.e., kfast/kslow ≥ 20); (2) both racemisation catalyst and hydrolase must be compatible (i.e., exhibiting comparable operational windows in terms of solvent, temperature etc. and do not mutually inactivate each other); (3) the rate of the racemisation step must be at least 10-fold higher than the rate of the enzymatic acylation step for the slow reacting enantiomer (i.e., krac /kslow > 10); and (4) the racemisation catalyst must not react with the final KR product.
Since the pioneering work by the groups of Williams38 and Bäckvall39,40 on the DKR of racemic alcohols, the field has grown significantly during the past two decades, reaching a high level of maturity. Some excellent recent reviews covered the blooming field of DKR.37,41–43 The DKR of racemic alcohols is classically performed using transfer hydrogenation-type ruthenium catalysts.37 The most famous racemisation catalyst is the dimeric Ru-Shvo catalysts, whose utilisation requires relatively high temperatures and the requisite of activated aryl esters as acyl donating agents in the enzymatic step. In fact, the need for high temperature in the racemisation step restricts the possible DKR reactions to a combination of Ru-Shvo catalyst with only highly thermostable hydrolases (mainly (R)-selective).44 On the other hand, the advantage of the classical Ru-Shvo catalyst is its versatility as a wide range of structurally diverse functionalised alcohols could be deracemised through the combination with hydrolases possessing different substrate scope.37,41,42 However, most of the research in this field has been devoted to the development of room temperature-active racemisation catalysts.45,46 Although a “universally” applicable racemisation catalyst remains elusive, improved generations of transfer-hydrogenation Ru-catalysts are available37 along with Ir-based catalysts, the latter being particularly suitable for the DKR of halohydrins.47 As alternatives to transfer hydrogenation-type racemisation catalysts, other catalysts operating via dehydrative mechanisms such as AlMe3, VOSO4 have been implemented in DKR.37 These catalysts are not only cheaper than Ru-, Ir- or Pd-catalysts but also enable the DKR of tertiary alcohols, which is mechanistically impossible via hydrogen transfer catalysis. However, only starting materials that form stable carbocations are converted.
The DKR of amines is a significantly more challenging process because the substrate racemization catalysed by Ru-based catalysts requires temperatures above 100 °C.37,41–43 Under these conditions, the amine substrate can act as a coordinating ligand to the metal centre of the racemization catalyst, thus reducing the rate or even interrupting the DKR process. Furthermore, the in situ formed imine intermediate can react with the starting amine yielding various by-products through aminal intermediates. As these types of side-reactions are favoured only above a certain temperature, most of the research was and still is focused on developing racemisation catalysts active at temperatures of 50 °C or below. A number of catalytic systems based on Pd/BaSO4, Pd/CaCO3, Pd/AlO(OH), Pd nanoparticles, Ir, etc. proved to be promising.43
Incompatibility of enzymes and transition metals is well noted; accordingly, the development of transition metal-independent in situ racemisation methods is a current focus of research. Racemases, for example, are attractive catalysts for the racemisation step in DKR due to the expected intrinsic compatibility with the enzymatic KR-catalysts. In an early contribution, Faber and coworkers reported a sequential deracemisation of mandelic acid through the combination of a lipase-catalysed KR followed by a racemase step to convert the enantiomerically pure starting alcohol back into the racemate.48 Both steps had to be performed separately because the lipase-catalysed acylation was efficient in a non-aqueous medium only, whereas the racemase-step could only be performed in an aqueous medium. Nevertheless, over four consecutive cycles of KR and racemisation an overall yield of 80% of enantiomerically pure (S)-O-acetyl mandelic acid was obtained.
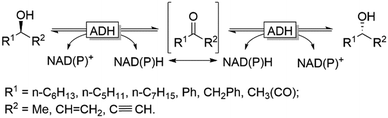
Naturally, racemases that act on alcohols and amines are rather scarce, which becomes obvious considering the homochirality of e.g. amino acids and carbohydrates throughout all kingdoms of life. An alternative approach for the in situ racemisation explored in recent years is to exploit enzymes with non-exclusive enantioselectivity in reversible transformations. Alcohol dehydrogenases (ADHs), for example, have been demonstrated as efficient racemisation catalysts. In the absence of a cofactor regeneration system, ADHs catalyse the reversible equilibrium between alcohols and the corresponding prochiral carbonyl compounds. Provided that the enantioselectivity of a given ADH is not exclusive, the thermodynamically favoured (entropy-driven) racemisation will take place (Scheme 4).
This approach was pioneered by Kroutil and coworkers, who demonstrated that even highly enantiospecific ADHs such as the ADH from Lactobacillus kefir (LkADH) or Rhodococcus ruber (ADH-A) are not perfectly enantiospecific and will, in the absence of a cofactor regeneration system, mediate the racemisation of a broad range of enantiomerically pure alcohols.49 On the other hand, the use of highly enantioselective ADHs is disfavoured from a kinetic perspective as, for instance, the racemisation of ca. 20 mM of (R)- or (S)-configured 2-octanol (one of the optimal substrates) requires about 30 h using elevated concentrations of the ADH.
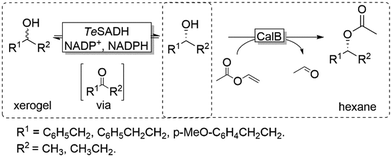
Coupling the above-described racemisation reaction to a hydrolase-catalysed KR, however, is not straightforward in aqueous media due to the favoured hydrolysis of the desired enantiomerically pure ester product. To circumvent this limitation, Musa and coworkers established a procedure utilizing xerogel-immobilised secondary ADH from Thermoanaerobacter ethanolicus (TeSADH, EC 1.1.1.2, more specifically its W110A variant)50,51 for the in situ racemisation of various alcohols under water-deficient reaction conditions.52 Under such semi-arid conditions, the lipase-catalysed KR reaction occurred efficiently, thus obtaining a bi-enzymatic DKR (Scheme 5).
Using a teabag-variation of the above-described bi-enzymatic DKR system (Scheme 5),53 Kroutil and coworkers also developed this approach by implementing a variant of TeSADH (i.e., containing the following mutations: W110A I86A C295A).50,53 These two reports provided a proof-of-concept demonstration for a bi-enzymatic DKR of alcohols. However, the productivity of this DKR was moderate because of diffusion limitation of the alcohol substrates, which was probably due to the complexity of the system requiring two liquid phases (organic and aqueous buffer) as well as tea-bag compartmentalisation of the lipase.
Overall, DKR reactions still represent a flourishing area of research. Currently, the transition-metal-catalysed racemisation approaches prevail but some purely enzymatic approaches are reaching maturity and preparative applicability.
Cyclic deracemisation approaches
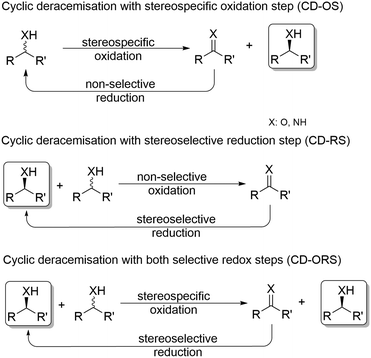
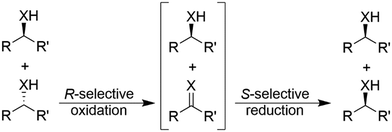
In general, cyclic deracemisation (CD) approaches combine two redox steps in one-pot (Scheme 6).In the majority of cases reported, a highly enantioselective or enantiospecific reaction step (commonly catalysed by an enzyme) is combined with a concurrent non-stereoselective step (often mediated by a chemical reagent). However, both oxidation and reduction steps can be, at least partly, stereoselective. Notably, if both steps are completely stereoselective, a single cycle can lead already to full deracemisation. This particular case will be treated separately in the section deracemisation via stereoinversion, whereas in this section the focus will be on truly “cyclic” processes. The most common type of cyclic deracemisation reactions comprises a stereospecific oxidation step (essentially representing a KR)—thus leaving the desired alcohol or amine enantiomer untouched—while transforming the other enantiomer into the prochiral intermediate (ketone or imine). The intermediate is then chemically reduced back into the racemic starting material by a non-stereoselective reagent or catalyst. Iterations of this reaction sequence will eventually yield the enantiomerically pure starting material. If the oxidation biocatalyst is fairly stereospecific, this “cyclic deracemisation via stereospecific oxidative step” (CD-OS) becomes a rather efficient deracemisation method. Indeed, if the enzymatic oxidation step is enantiospecific, already after eight theoretical rounds the enantiomeric access of the starting material will exceed 99.5%.
Cyclic deracemisation reactions with a stereoselective reduction step (CD-RS)
Cyclic deracemisation reactions can comprise a stereoselective reduction reaction along with a non-selective oxidation step (i.e., CD-RS). One obvious disadvantage of this CD approach is that, during the non-selective oxidation step, the desired enantiomer is also converted thereby lowering the overall efficiency of this approach by futile redox cycles. A proof of principle of CD-RS was provided for the partial deracemisation of chlorohydrins by combining an Ir-organocatalyst with an ADH (Scheme 7),54 the former of which was previously implemented in DKR for the racemisation step of chlorohydrins (Scheme 3).47 In this deracemisation strategy—as no ADH was known to be able to oxidize chlorohydrins to α-chloroketone intermediates—the authors envisaged to exploit an ‘outer-sphere’ oxidation mechanism catalysed by the iridacycle catalyst at the expense of a sacrificial oxidant (2–10 equiv.). Although ‘outer-sphere’ oxidation occurred, the iridacycle catalyst still performed its ‘inner-sphere’ racemisation thus partly eroding the enantiomeric excess obtained after each run of the chemo-enzymatic cascade. Therefore, the one-pot, concurrent and orthogonal cascade yielded only modest results in terms of optical purity of the final chlorohydrin. Theoretically, the system could lead to enantiomerically pure chlorohydrins by applying a redox metal-catalyst that operates solely through an ‘outer-sphere’ mechanism and by fixing the number of deracemisation cycles through the equivalents of the sacrificial oxidant.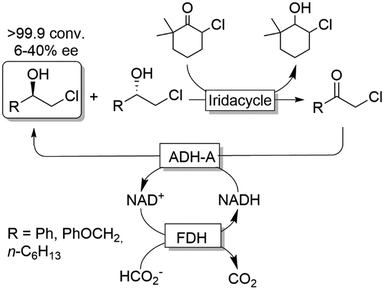 | ||
| Scheme 7 Enantioselective reductive cyclic deracemisation (CD-RS) of chlorohydrins using a concurrent iridium-catalysed non-stereoselective oxidation and ADH-A-catalysed stereoselective reduction. Structure of iridacycle is shown in Scheme 3. | ||
Cyclic deracemisation reactions with a stereospecific oxidation step (CD-OS)
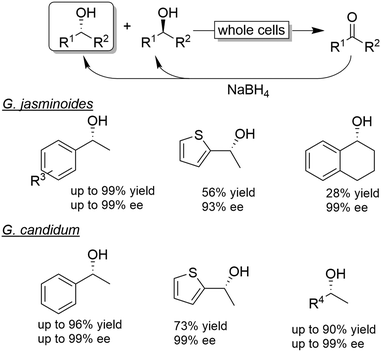 | ||
| Scheme 8 Examples for CD-OS of alcohols using Gardenia jasminoides or Geotricium candidum as KR catalysts coupled to non-selective reduction of the intermediate ketone by NaBH4. | ||
The chemo-enzymatic deracemisation using Gardenia jasminoides immobilised cells in calcium alginate with NaBH4 enabled the production of the (R)-configured alcohols in enantioenriched form.55 For instance, a hundred of milligrams scale CD-OS reaction starting from racemic 1-phenylethanol yielded the (R)-configured enantiomer in 84% isolated yield and 97% ee. However, the process of enantioenrichment required three cycles for a total of seven days. In another study, Geotrichum candidum cells were applied in combination with NaBH4 as a reducing agent in ionic liquids.56 The enantiomeric excess of the final product was generally elevated, albeit variable levels of ketone intermediate accumulation were observed (0–32%).
Enantioselective oxidation of secondary alcohols is also feasible using engineered variants of galactose oxidase.57
These publications highlight how CD-OS can be a useful strategy for deracemisation of racemic alcohols along with the more established DKR. However, only a limited number of studies have been carried out until to date on CD-OS of alcohols.
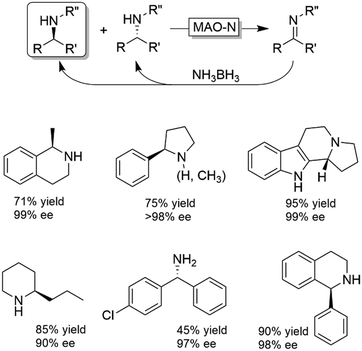
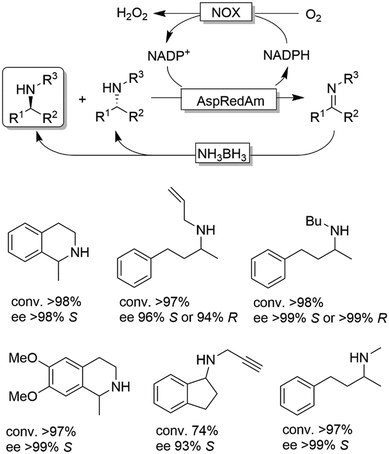
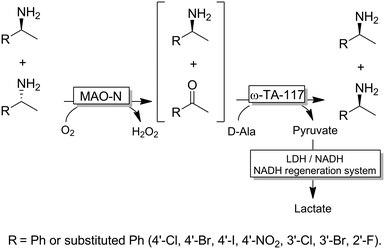
Probably the most widespread enzyme used in CD-OS of amines is the monoamine oxidase from Aspergillus niger (MAO-N, EC 1.4.3.4) and its manifold mutants.58,59 While the wild-type enzyme clearly preferred primary amines, Turner and coworkers have engineered an impressive range of enantioselective variants that exhibit high activities towards pharmaceutically relevant secondary and tertiary amines (e.g., (R)-nicotine, crispine A), thus affording excellent yields and often perfect enantiomeric excess.58 The same group also introduced ammonia borane (NH3BH3) as a more versatile reducing agent for the concurrent use with oxidases (Scheme 9).59 Today, the combination of MAO-N or its variants and NH3BH3 has become a standard protocol for the deracemisation of amines into enantiomerically pure amines.60–64 A selection of enantiomerically pure amines obtainable via the MAO-N-NH3BH3 strategy is shown in Scheme 9.
However, monoamine oxidases (naturally occurring or engineered) display mainly (S)-selectivity towards their substrates, thereby limiting the scope of obtainable enantiomer products. Asano, Turner and their coworkers independently filled this gap by reporting on (R)-selective amine oxidases engineered from either a 6-hydroxy-D-nicotine oxidase (6-HDNO)65 or a (R)-selective amino acid oxidase from porcine kidney.66
An interesting alternative to NH3BH3 as a stoichiometric reductant has been proposed by Lloyd and coworkers.67 Whole cells of recombinant E. coli overexpressing MAO-N and presenting Pd-nanoparticles at the surface were used in this approach. Unfortunately, both reaction steps (MAO-N-catalysed KR and nano-Pd-catalysed non-stereoselective hydrogenation of the imine intermediate) had to be performed sequentially due to the formation of undesired by-products in the concurrent mode.
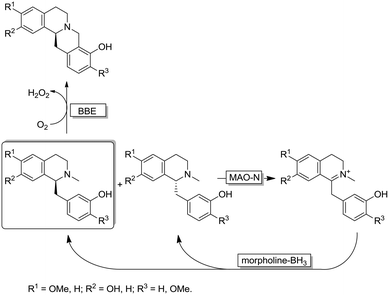
Interestingly, the MAO-N/BH3 system was implemented in a deracemisation by simultaneous bio-oxidative kinetic resolution and stereoinversion. (Scheme 10).68 In this study, racemic 1-benzyl-1,2,3,4-tetrahydroisoquinolines were subjected to cyclic deracemisation by MAO-N/morpholine·BH3, while the formed (S)-configured tetrahydroisoquinolines were concurrently cyclised by a berberine bridge enzyme (BBE) to yield berberine in elevated yields (79–97%) and enantiomeric excess (>97%). It is noteworthy that DKR of 1-benzyl-1,2,3,4-tetrahydroisoquinolines by a combination of a Shvo's Ru-catalyst with BBE is inapplicable because the metal catalyst also racemises the final cyclized product.68
CD-OS of amines has also been carried out using dehydrogenases rather than oxidases. In fact, a simple KR of primary amines can be accomplished by an amine dehydrogenase (AmDH) in combination with water or H2O2-forming NAD(P)H oxidase as exemplified by Yun and coworkers.69 Therefore, this oxidative deamination can represent the KR part of the CD-OS reaction, thereby overall mimicking the MAO-N system. Very recently, Turner and coworkers reported a CD-OS through the application of a sub-family of imine reductases (called reductive aminases, RedAms) in the KR step of secondary amines (Scheme 11) combined with a reduction step performed by NH3BH3 as for the MAO system.70 It remains to be shown whether this (more complex system) will be able to compete with the very efficient MAO-N system. At the moment, the reported substrate scope is limited to the structures depicted in Scheme 11.
Linear deracemisation including stereoinversion reactions
Stereoinversion reactions resemble the above-described cyclic deracemisation (CD) strategies in the sense that the undesired enantiomer in a first step is oxidised to the prochiral ketone or imine functionality in a theoretically enantioselective manner. Then, the intermediate is reduced stereoselectively to the desired enantiomer (Scheme 12). Hence, as previously observed, stereoinversion-based deracemisations are more efficient compared to CD as only one cycle is necessary. Practically, enzymes exhibiting strict enantiocomplementarity, which are relatively accessible nowadays,71 are necessary for stereoinversion reactions.As suggested by the name, stereoinversion methods can also be used to invert the stereochemistry at a given (enantiomerically pure) alcohol or amine (i.e., in this way emulating the outcome of a Mitsunobu-type reaction).
Finally, the same approach can be used for either conversion of racemic alcohols into enantiomerically pure amines or conversion of racemic amines into enantiomerically pure alcohols. These cases will also be discussed in this section.
Deracemisation of alcohols via stereoinversion
The example shown in Scheme 13 yields a broad range of enantiomerically pure (S)-configured alcohols. By choosing another combination of enzymes (e.g. (S)-selective TbADH and (R)-selective ADH-PR2), (R)-configured alcohols could be produced as well. Instead of using isolated enzymes, a number of microbial stereoinversion systems as well as hybrid systems containing whole cells/isolated enzymes have also been reported in deracemisation via stereoinversion.73–81 Here, it should, however, be mentioned that due to the presence of various endogenous ADHs and alcohol oxidases (AOxs) in the microbial “black-box” systems, the real deracemisation pathway may differ case by case from the depiction of Scheme 13; consequently, identifying suitable enantiocomplementary systems can be challenging. Scheme 14 depicts a selection of enantiomerically pure alcohols obtained through enzymatic and microbial deracemisation via stereoinversion.
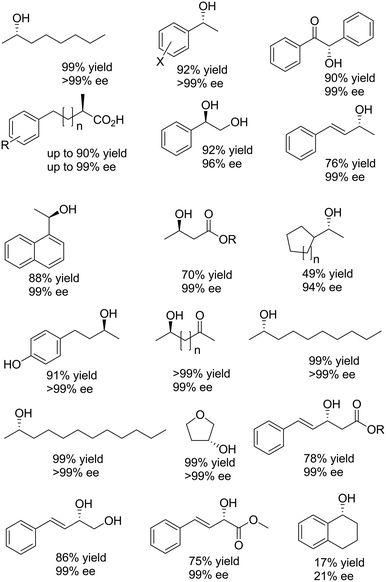 | ||
| Scheme 14 Selection of enantiomerically pure alcohols obtained via stereoinversion.73–81 | ||
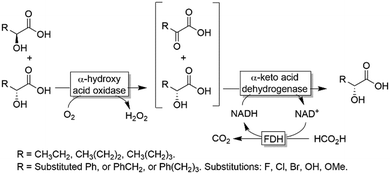 | ||
| Scheme 15 Deracemisation of α-hydroxy carboxylic acids by sequential aerobic oxidation followed by stereoselective reduction. | ||
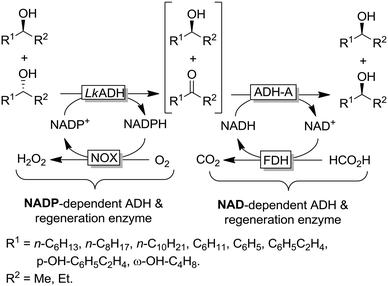
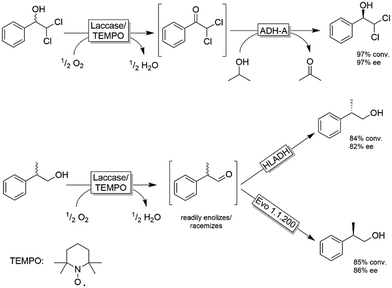
The extension of this system to substrates devoid of the α-carboxylic moiety was explored by Gotor, Gotor-Fernandez and coworkers (Scheme 16).84–86 They applied either TEMPO/I2 or the established laccase-mediator system (LMS) for the non-stereoselective oxidation of primary and secondary alcohols followed by a second stereoselective reduction step of the intermediate carbonyl group. In all these studies, the reduction was catalysed by an ADH, either as whole cell or lyophilized crude biocatalyst. Scheme 16 depicts selected examples for this concept. The TEMPO-I2/E. coli(ADH) chemo-enzymatic sequential system was applied for the deracemisation of 1-phenylethanol as well as selected aliphatic alcohols to yield enantiomerically pure alcohols (ee >99%) in nearly quantitative conversion.84 The use of stereocomplementary ADHs enables the isolation of either (R)- or (S)-configured alcohols. However, the overall deracemisation required 2 equiv. of I2 as a sacrificial oxidant along with 0.2 equiv. of TEMPO as the oxidation catalyst, whereas the enzymatic reduction step was driven to completion by using an excess of 2-propanol as a cosubstrate. The laccase/TEMPO system was successfully coupled to E. coli(ADH) cells in the deracemisation of aromatic α-dichloro alcohols as model substrates to afford the final product with variable levels of yields (62–95%) and elevated ee (97%).86 The advantage of the laccase/TEMPO compared to the TEMPO-I2 systems in the oxidation step is that the former consumes only dioxygen as oxidant.
In the case of deracemisation of 2-phenyl-1-propanols, the laccase/TEMPO oxidation generates an enolizable intermediate under the reaction conditions; thus, by using stereocomplementary ADHs, both enantiomers could be obtained in high yields (95% or higher) but moderate optical purities in most cases (6–94%).85 A variation of this method using 2-azaadamantane N-oxyl as oxidation catalyst and HOCl as a stoichiometric oxidant was reported recently.87
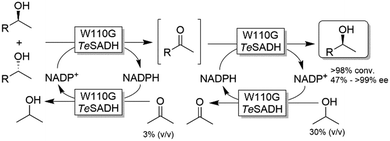
Musa and coworkers reported a single enzymatic two-step deracemisation approach of secondary alcohols using W110G TeSADH (Scheme 17).88 It relies on facilitating “selectivity mistakes” in the oxidation pathway by applying a precise concentration of acetone cosubstrate; this approach enables the depletion of both enantiomers of an alcohol racemate to afford the corresponding prochiral ketone, the latter of which is subsequently reduced—under stereoselective conditions and using the proper concentration of 2-propanol—to the corresponding (S)-configured alcohol. Thus, the overall reaction proceeds in one-pot removing the need to isolate the ketone intermediate. More recently, the same strategy was implemented in stereoinversion of (R)-configured alcohols (i.e., mimicking the actions of Mitsunobu reaction).89
Linear deracemisation of amines via stereoinversion
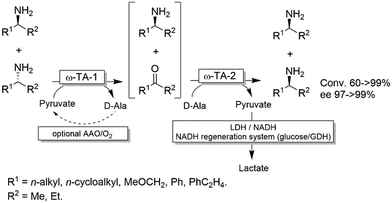
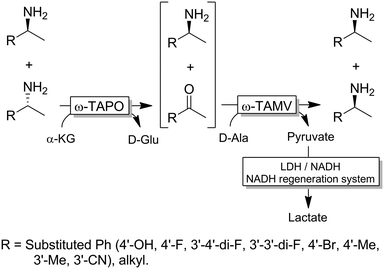
For stereoinversion of amines nowadays a broad range of enantiocomplementary ω-transaminases (ω-TAs) are available. The major disadvantage of these methods, however, is that no orthogonal systems are available today. Accordingly, ω-TA-based stereoinversions of amines can be performed in one-pot, albeit in two steps separated in time; thus, the general protocol often includes the inactivation of the first ω-TA used (i.e., employed in the KR step) to avoid re-formation of the undesired enantiomer in the second formal reductive amination step operated by the stereocomplementary enzyme (Scheme 18).90 In this initial work, a stoichiometric amount of pyruvate was consumed in the first KR step, whereas the second amination step required the addition of further five equivalents of alanine along with a bi-enzymatic pyruvate-removal network (i.e., NAD (cat.)/LDH/GDH/glucose (ca. 3 equiv.)) in order to shift the thermodynamic equilibrium towards amine formation. Notably, the atom-efficiency of the deracemisation was improved by performing the first KR step using a catalytic amount of pyruvate, which was recycled in situ by an amino acid oxidase (AAO) at the expense of dioxygen. The strategy was applied to the deracemisation of the drug Mexiletine in hundred milligram-scale (97% yield, ee >99% S or R).90 In all these studies, the first ω-TA used for KR was thermally deactivated before starting the second step. In a follow-up study, immobilised enzymes were employed thereby enabling a more convenient removal of the ω-TA used in the first step.91Yun and coworkers presented a possible solution to circumvent the bioorthogonality issue by introducing a new ω-TA from Polaromonas sp. (ω-TAPO) that is capable of accepting 2-ketoglutarate as a sacrificial cosubstrate (thus yielding glutamic acid as a by-product).92 In combination with one other ω-TA from a selected pool (e.g., from Mycobacterium vanbaalenii, ω-TAMV), which did not accept aspartic acid as an amine donor, oxidative deamination KR reaction could be performed simultaneously with the stereoselective formal reductive amination reaction (Scheme 19). This approach was improved further by using a (R)-selective ω-TA from Arthrobacter sp. (ARTA) a (S)-selective ω-TA from Ochrobactrum anthropic (OATA) and using pyruvate and isopropyl amine as cosubstrates.93 This approach has the advantage of eliminating the use of the expensive 2-ketoglutarate and D-alanine cosubstrates and having acetone as a coproduct, which can be removed under reduced pressure. Certainly, this approach will receive more attention in the future.
Besides ω-TAs, amine oxidases can be used for the first step (i.e., KR) of the stereoinversion cascade (Scheme 20).94 In this case, provided that the enzymes used are highly stereoselective, no cross-reactivities or futile cycles occur. However, the substrate scope of the reaction was limited to substituted 1-benzyl-ethylamines because of the intrinsic catalytic activity of MAO-N.
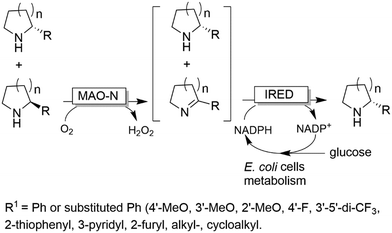
In a similar approach (albeit using amino acid oxidases), homoalanine was deracemised.95 Finally, it is worth mentioning that the stereoselective amination can also be accomplished either by dehydrogenases such as imine reductases (IRED),96 or artificial transfer hydrogenases97 rather than ω-TAs. The advantage of this system stems from the possibility to also deracemise secondary amines. In particular, the MAO-N/IRED system was implemented using resting E. coli cells overexpressing the enzymes separately; thus, the E. coli metabolism could be exploited for NADH regeneration (Scheme 21). In another work, para-bromo-L-phenylalanine was converted into the opposite enantiomer by performing a one-pot enantioselective oxidative deamination catalysed by an amino acid dehydrogenase (AADH) followed by a formal stereoselective reductive amination catalysed by a α-TA.98
Ward and coworkers reported a chemo-enzymatic stereoinversion (i.e., CD-ORS) of secondary amines (Scheme 22) by combining MAO-N with an artificial transfer hydrogenase (ATHase).97 The oxidative step is the classical MAO-N-based oxidation at the expense of dioxygen; the second step is a stereoselective imine reduction that is performed by an artificial enzymes in which an iridacycle organometallic-catalyst is embedded in a protein scaffold, thus providing the stereoselective environment. Notably, the reduction step required simple formate as sacrificial reductant. However, as the stereoselectivity of the ATHase is not always perfect, more than one cycle can be required to reach full deracemisation.
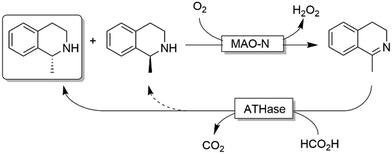 | ||
| Scheme 22 Chemo-enzymatic deracemisation of secondary amines combining MAO-N with an artificial Ir-based enzyme. | ||
Linear deracemisation of alcohols to optically active amines
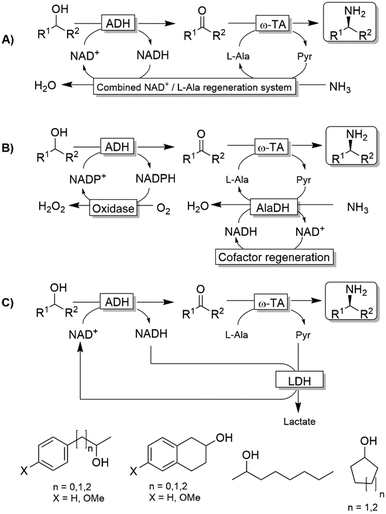
Kroutil and coworkers generated a multi-enzymatic network that combined the oxidation of alcohols to their corresponding carbonyl compounds—catalysed by an ADH—to a subsequent formal reductive amination of the intermediate, which is catalyzed by a ω-TA (Scheme 23a).99,100 The system was initially applied to the synthesis of non-chiral amines and diamines starting from primary alcohols and diols. This redox-neutral alcohol amination network comprises an L-alanine dehydrogenase (L-AlaDH)-catalysed simultaneous regeneration of both NADH and amine-donor (L-alanine); thus, it produces theoretically only water and consumes ammonia. However, besides the requirement for three enzymes (ADH, ω-TA and L-AlaDH) and two cofactors (NAD+ and pyridoxal 5′-phosphate (PLP)), five equivalents of L-alanine as an amine donor were required. In another independent study, Janssen and coworkers could run the same system for the amination of primary ether-alcohols (10 mM) using a catalytic amount of L-alanine as the amine donor (5 mol%); however, the maximum conversion into the amine was only 30%.101 These results indicated that an excess of sacrificial amine donor might be mandatory in order to achieve a significant amine product formation with this system. In this context, the deracemisation-amination of secondary alcohols (50 mM) by the ADH/ω-TA/ L-AlaDH system proved to be even more challenging (Scheme 23a).99 In fact, the amination led to moderate levels of amine formation (10–64%) along with a significant accumulation of ketone intermediates (up to 47%). The use of orthogonal oxidation (NADP) and amination (L-Ala/NAD) regeneration systems afforded a maximum conversion of 24% (Scheme 23b), whereas pyruvate removal coupled with NAD regeneration afforded a maximum of 64% conversion and 96% ee (Scheme 23c). Applying the system depicted in Scheme 23a, Skerra and coworkers observed an analogous trend in the enantioselective mono-amination of renewable isosorbide (200 mM concentration) that proceeded with 7% conversion, but excellent selectivity (d.e. <99%).102The deracemisation of alcohols to optically active amines is, in principle, possible by combination of an oxidative step catalysed by an alcohol oxidase (AOx) with a concurrent amination catalysed by a ω-TA. In reality, the apparent scarcity of AOxs that are able to oxidize racemic mixtures of secondary alcohols to their ketone intermediates, has limited this strategy to the amination of primary alcohols until to date.103,104 Gotor and coworkers overcame this limitation by applying the aforementioned laccase/TEMPO system in the oxidative step, in this case in combination with the ω-TA-catalysed amination (Scheme 24).105 Additionally, this latter system exploited the capability of a number of commercially available ω-TAs, to accept 2-propylamine as a sacrificial amine donor. The deracemisation-amination on 50 mM alcohol concentration yielded conversions from 67% to >99%, using 1.33 M of 2-propylamine to push the equilibrium into the desired direction. Notably, the final amine product was obtained in high enantiomeric purity (90 ≥ 99%).
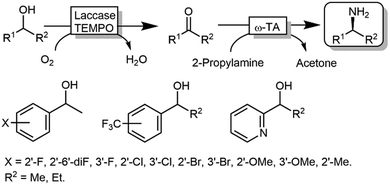 | ||
| Scheme 24 Deracemisation of alcohols to yield optically pure amines by combining laccase-TEMPO oxidation and reductive amination mediated by ω-TAs. | ||
ω-Transaminases bear the intrinsic disadvantage of relying on organic amines as N-donors for the reductive amination reaction. Therefore, issues of low atom efficiency and kinetic impediments such as product inhibition limit the applicability of these ω-TA-based cascades. More recently, however, amine dehydrogenases (AmDHs) and imine reductases (IREDs) dramatically expanded the scope of the formal biocatalytic aza-Mitsunobu reactions.106–111 If combined with an ADH-catalysed alcohol oxidations, AmDHs enable the direct use of NH3 as N-donor (Scheme 25). In this hydrogen-borrowing cascade, the hydride abstracted from the alcohol is re-introduced in the reductive amination step. In general, two ADHs with opposite stereopreferences have been commonly used together in the oxidation step,106 depending on the substrate of choice; conversions varied from 9% to 96% while enantiomeric excess was excellent (ee > 99%, R) in most of the cases. Attempts to employ a single ADH possessing low enantioselectivity in the oxidation step provided modest results until to date, since a significant amount of alcohol substrates was detected at the end of the reaction (as average ca. 30%, and up to 82%), besides ketone accumulation (5–15%).110 Unfortunately, the enantiomeric excess of the remaining alcohol substrate was not determined in this study, which complicates the rationalisation of the incomplete conversion to amine; however, it is likely that the incomplete alcohol oxidation in several cases stems from the retained enantioselectivity of the used ADH variant (W110A/G198D TeSADH, an NAD+-dependent TeSADH), which prevents the complete depletion of the both alcohol enantiomers. Thus, engineering or discovery of non-enantioselective ADHs that are applicable on a wide range of substrates still represents a major challenge in this area.
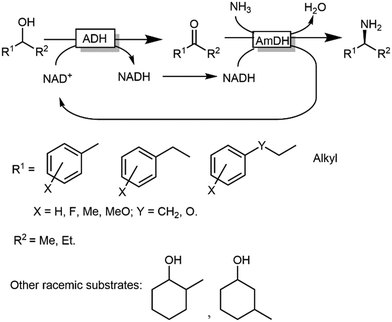 | ||
| Scheme 25 Hydrogen borrowing cascade for the transformation of alcohols into primary and secondary amines combining ADHs and AmDHs. | ||
Recently, Turner and coworkers extended the applicability of the racemic alcohol bioamination cascade to the generation of secondary amines by replacing the AmDH with an imine reductase, from the “reductive aminase” sub-family (Scheme 26).109
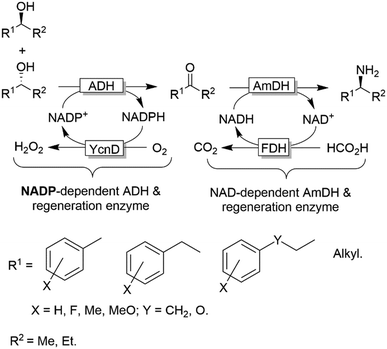
Notably, in all studies mentioned above, the conversion obtained for the amination of racemic alcohols into the optically active amines never exceeded the threshold value of 95–96% although a high concentration of ammonium/ammonia buffer was applied (ca. 2 M). In contrast, primary alcohols could be aminated quantitatively. Mutti and coworkers explained these findings with a more unfavourable thermodynamic equilibrium for the amination of secondary alcohols compared to primary alcohols.112 Therefore they proposed an alternative orthogonal network in which a single NADP-dependent ADH (i.e., I86A W110A TeSADH variant, among the least selective mutants of TeSADH) oxidises the secondary alcohol to ketone, whereas a NAD-dependent AmDH performs the stereoselective reductive amination (Scheme 27). Orthogonal cofactor regeneration systems were applied in a similar way as for the deracemisation of secondary alcohols.72 Indeed, conversions above 99% was achieved with excellent enantiomeric excess (>99% R).
Linear deracemisation of amines to optically active alcohols
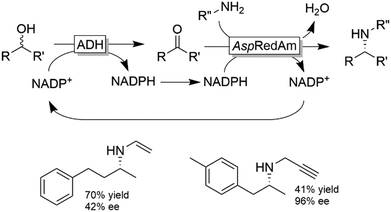
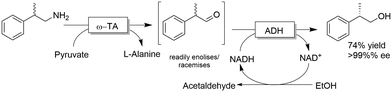
The deracemisation of amines to yield optically active alcohols has attracted less interest until to date. Nevertheless, in some cases, racemic amines can be more accessible from natural resources than their structurally related alcohols. In a recent study, Contente and Paradisi performed the deracemisation of (R,S)-2-phenylpropylamine to afford (S)-2-phenylpropanol in 74% isolated yield and enantiomerically pure form (Scheme 28).113 The first step of the deracemisation is a non-stereoselective formal oxidative deamination catalysed by a ω-TA, which consumes one equivalent of pyruvate. The racemic α-substituted aldehyde is then resolved in the second step since the applied alcohol dehydrogenase distinguishes between the two enantiomeric forms of the intermediate. As the unreacted aldehyde can also racemise spontaneously in solution, quantitative conversion into the final alcohol product can be achieved (i.e., DKR of the α-substituted aldehyde intermediate). Notably, the deracemisation sequence was carried out in a continuous flow system operating with an in-line recovery of products (including by-products) and a recirculation of the aqueous media that contains the cofactors. Thus, the ratio between cofactors (i.e., NAD, PLP) and substrates could be reduced from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (typical for batch reactions) to 1
100 (typical for batch reactions) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000.
2000.
Enantioconvergent deracemisation reactions
In enantioconvergent processes, the enantiomers of the starting material are transformed through inversion (undesired enantiomer) and retention (desired enantiomer) into an enantiomerically pure product (Scheme 2).114An interesting chemoenzymatic enantioconvergent method was reported by Matsumoto and coworkers (Scheme 29).115,116 Racemic acetate esters were first submitted to a lipase-mediated KR followed by a chemical Mitsunobu reaction of the resulting alcohol enantiomer yielding essentially enantiomerically pure (S)-2-acetoxy tosylates. The tedious removal of non-reacted PPh3 from the Mitsunobu step was circumvented by using insoluble, polymer-supported PPh3.
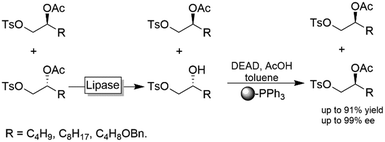 | ||
| Scheme 29 Chemoenzymatic enantioconvergent deracemisation of acetyl esters. Lipase PS from Burkholderia cepacia (EC 3.1.1.3), DEAD: diethyl azodicarboxylate. | ||
Another chemoenzymatic approach was suggested and implemented by Feringa and coworkers.117 α-Bromoamides were subjected to a KR catalysed by a haloalkane dehalogenase yielding the corresponding α-hydroxyamide through inversion of the configuration (i.e., the non-reacted starting material and the product were of the same configuration). After converting the alcohol into a leaving group by mesylation, both the unreacted bromide and the mesylated alcohol were subjected to SN2-type nucleophilic substitution by various nucleophiles including azide, benzylamine and phenol (Scheme 30).
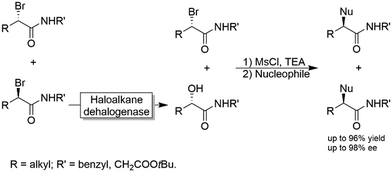 | ||
| Scheme 30 Chemo-enzymatic enantioconvergent synthesis of optically active α-substituted amides. Nucleophiles: Azide, benzylamine and phenol. | ||
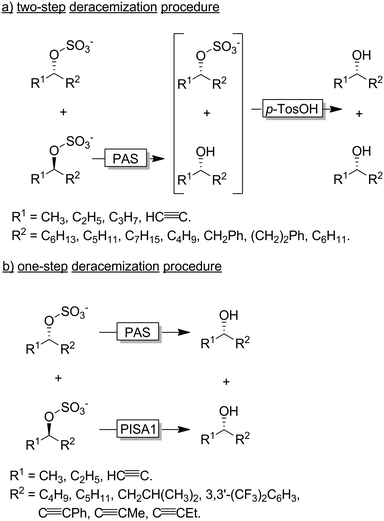
Faber and coworkers have established alkyl sulfatases as enantioselective catalysts for the preparation of enantiomerically pure alcohols from racemic sulfate esters.118–120 In a first set of experiments the authors used an inverting sulfatase (i.e., catalysing the nucleophilic substitution at the C-atom) from Pseudomonas aeruginosa (PAS) thereby mediating a KR and leading to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the homochiral alcohol and sulfonate ester (Scheme 31a). Acidic hydrolysis lead to the deracemised alcohol.119,121 More elegantly, the Faber group later reported on the discovery of an enantiocomplementary and retaining sulfatase (i.e., catalysing the nucleophilic substitution at the S-atom of the sulfonate group) from Rhodopirellula baltica (PISA1). A combination of both sulfatases enabled the one-step synthesis of various enantiopure alcohols from their sec-alkylsulfates (Scheme 31b).118
1 mixture of the homochiral alcohol and sulfonate ester (Scheme 31a). Acidic hydrolysis lead to the deracemised alcohol.119,121 More elegantly, the Faber group later reported on the discovery of an enantiocomplementary and retaining sulfatase (i.e., catalysing the nucleophilic substitution at the S-atom of the sulfonate group) from Rhodopirellula baltica (PISA1). A combination of both sulfatases enabled the one-step synthesis of various enantiopure alcohols from their sec-alkylsulfates (Scheme 31b).118
Like alkylsulfatases, epoxide hydrolases (EHs) can act on their substrates in Markovnikov- or anti-Markovnikov modes thereby leading to inversion or retention, respectively, of the absolute configuration. For example, as early as 1996, Furstoss and coworkers reported on two microbial strains (Aspergillus niger and Beauveria sulfurescens) exhibiting complementary EH activities resulting in the deracemisation of e.g. styrene oxide (Scheme 32).122 This enantioconvergence is due to a Markovnikov-attack catalysed by B. sulfurescens to (S)-styrene oxide leading to the (R)-diol combined with an anti-Markovnikov-attack by A. niger to the (R)-epoxide thereby retaining the absolute configuration.
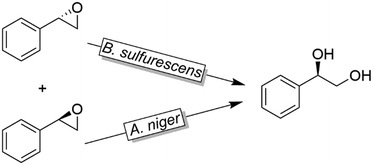 | ||
| Scheme 32 Deracemisation of styrene oxide using enantiocomplementary microbial strains (Aspergillus niger and Beauveria sulfurescens). | ||
Further examples with isolated enzymes have been reported ever since.123,124 An interesting alternative to the aforementioned bienzymatic EH-based deracemisation processes is to use EHs acting on both enantiomers of an oxirane albeit in Markovnikov and anti-Markovnikov selectivity. Next to the example by Furstoss and coworkers using the EH from Solanum tuberosum (StEH, Scheme 33),125 further EHs and their use in corresponding single enzymatic enantioconvergent reactions have been reported.126–130
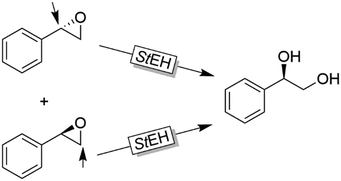 | ||
| Scheme 33 Deracemisation of styrene oxide using a single epoxide hydrolase. StEH: Epoxide hydrolase from Solanum tuberosum (EC 3.3.2.3). | ||
Conclusions
Deracemisation is a powerful approach for the synthesis of enantiopure alcohols and amines. This field has significantly advanced in the last few years, thus generating viable alternatives to the established kinetic resolution approaches.The chemoenzymatic DKR of non-activated alcohols has reached maturity. More recently, bienzymatic DKR are moving into the focus. The promise is more compatible catalysts and simpler reaction schemes. A shortcoming for DKR is that the majority of the reported examples yield the (R)-configured product because they principally rely on a resolution step catalysed by well-known lipases that exhibit (R)-stereoselectivity. A larger portfolio of (S)-selective hydrolases will certainly boost the scope of DKRs. Fewer examples are known on DKR of amines because of the harsher conditions required for the racemisation of amines. Moreover, a bienzymatic DKR of amines is not reported yet. Other deracemisation strategies have the advantage of producing enantiopure alcohols or amines from their racemates without the need for an extra hydrolytic step (e.g., de-acylation).
Chemoenzymatic cyclic deracemisation of amines by combining a stereospecific oxidation and a non-stereoselective reduction is an appealing approach. However, the same approach is not well-known for deracemisation of alcohols. Deracemisation via stereoinversion is another promising strategy for obtaining enantiopure alcohols and amines for the advantage of avoiding futile cycles that are encountered in cyclic deracemisation. The challenge in this strategy is to design two stereocomplementary redox reactions that are compatible and orthogonal. When the latter condition is not possible, the deracemisation can still be conducted in one pot, but in two steps (i.e., linear deracemisation). Chemoenzymatic and bienzymatic sequential deracemisation via non-stereoselective oxidation along with a stereoselective reduction has also been reported. More efforts should be devoted in designing orthogonal redox reactions that enable a one-pot, one-step deracemisation approaches.
Hydrogen borrowing approaches to produce enantiopure amines from their racemic alcohols have also been reported. Although resolved in few cases, the design of an orthogonal network remains the critical point in this strategy.
Most of the reported deracemisation strategies for alcohols rely on formation of carbonyl-containing intermediates, and thus makes the deracemisation of tertiary alcohols a challenging problem. Enzymatic or chemical racemisation methods for tertiary alcohols, which are also compatible with a simultaneous enzymatic resolution step, should be developed in order to enable the development of efficient enzymatic or chemo-enzymatic deracemisation approach for tertiary alcohols. Overall, another common difficulty encountered in enzyme-based deracemisation approaches of alcohols and amines is the relatively narrow substrate scopes of enzymes when compared with organometallic- or organo-catalysis. Recent advances in protein engineering including directed evolution and structure-guided enzyme design will allow for filling these gaps by creating new enzyme variants possessing the desired and wider substrate scope, and controlled enantioselectivity. The future will tell if the promise of more efficient deracemisation methods will be kept on industrial scale.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
M.M. gratefully acknowledges funding by Deanship of Scientific Research (DSR) at King Fahd University of Petroleum and Minerals (KFUPM), project number IN151032. F.H. gratefully acknowledges support by the European Research Council (ERC consolidator grant, No.648026) and the Netherlands Organisation for Scientific Research (VICI grant, No. 724.014.003). F.G.M. gratefully acknowledges support by the European Research Council (ERC starting grant, No. 638271) and the Netherlands Organisation for Scientific Research (Sector Plan for Physics and Chemistry).Notes and references
- K. Goldberg, K. Schroer, S. Lutz and A. Liese, Appl. Microbiol. Biotechnol., 2007, 76, 237–248 CrossRef CAS PubMed.
- W. Kroutil, H. Mang, K. Edegger and K. Faber, Curr. Opin. Chem. Biol., 2004, 8, 120–126 CrossRef CAS PubMed.
- A. Gomm and E. O'Reilly, Curr. Opin. Chem. Biol., 2018, 43, 106–112 CrossRef CAS PubMed.
- G. Grogan, Curr. Opin. Chem. Biol., 2018, 43, 15–22 CrossRef CAS PubMed.
- D. Ghislieri and N. J. Turner, Top. Catal., 2014, 57, 284–300 CrossRef CAS.
- M. D. Patil, G. Grogan, A. Bommarius and H. Yun, ACS Catal., 2018, 8, 10985–11015 CrossRef CAS.
- H. Kohls, F. Steffen-Munsberg and M. Höhne, Curr. Opin. Chem. Biol., 2014, 19, 180–192 CrossRef CAS PubMed.
- M. Catani, O. H. Ismail, F. Gasparrini, M. Antonelli, L. Pasti, N. Marchetti, S. Felletti and A. Cavazzini, Analyst, 2017, 142, 555–566 RSC.
- M. Kellogg, J. W. Nieuwenhuijzen, K. Pouwer, T. R. Vries, Q. B. Broxterman, R. F. P. Grimbergen, B. Kaptein, R. M. L. Crois, E. d. Wever, K. Zwaagstra and A. c. v. d. Laan, Synthesis, 2003, 1626–1638 CrossRef.
- T. C. Nugent, Chiral amine synthesis: Methods, Developments and Applications, Wiley-VCH, Weinheim, 2010 Search PubMed.
- C. Wang and J. Xiao, in Stereoselective Formation of Amines, ed. W. Li and X. Zhang, Springer-Verlag Berlin, Heidelberg, 2014, vol. 343, pp. 261–282 Search PubMed.
- M. Sharma, J. Mangas-Sanchez, N. J. Turner and G. Grogan, Adv. Synth. Catal., 2017, 359, 2011–2025 CrossRef CAS PubMed.
- Q.-A. Chen, Z.-S. Ye, Y. Duan and Y.-G. Zhou, Chem. Soc. Rev., 2013, 42, 497–511 RSC.
- B. T. Cho, Chem. Soc. Rev., 2009, 38, 443–452 RSC.
- I. Slabu, J. L. Galman, R. C. Lloyd and N. J. Turner, ACS Catal., 2017, 7, 8263–8284 CrossRef CAS.
- G. Torrelo, U. Hanefeld and F. Hollmann, Catal. Lett., 2015, 145, 309–345 CrossRef CAS.
- B. M. Nestl, S. C. Hammer, B. A. Nebel and B. Hauer, Angew. Chem., Int. Ed., 2014, 53, 3070–3095 CrossRef CAS PubMed.
- U. Bornscheuer and R. Kazlauskas, Hydrolases in Organic Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2006 Search PubMed.
- L. Hilterhaus and A. Liese, in White Biotechnology, ed. R. Ulber and D. Sell, Springer-Verlag Berlin, Berlin, 2007, vol. 105, pp. 133–173 Search PubMed.
- A. Liese, K. Seelbach and C. Wandrey, Industrial Biotransformations, Wiley-VCH, Weinheim, 2006 Search PubMed.
- M. D. Patil, G. Grogan, A. Bommarius and H. Yun, Catalysts, 2018, 8, 25 CrossRef.
- G. W. Huisman and S. J. Collier, Curr. Opin. Chem. Biol., 2013, 17, 284–292 CrossRef CAS PubMed.
- G. W. Huisman, J. Liang and A. Krebber, Curr. Opin. Chem. Biol., 2010, 14, 122–129 CrossRef CAS PubMed.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore and K. Robins, Nature, 2012, 485, 185–194 CrossRef CAS PubMed.
- S. A. Kelly, S. Pohle, S. Wharry, S. Mix, C. C. R. Allen, T. S. Moody and B. F. Gilmore, Chem. Rev., 2018, 118, 349–367 CrossRef CAS PubMed.
- J. H. Schrittwieser, S. Velikogne and W. Kroutil, Adv. Synth. Catal., 2015, 357, 1655–1685 CrossRef CAS.
- M. Fuchs, J. E. Farnberger and W. Kroutil, Eur. J. Org. Chem., 2015, 2015, 6965–6982 CrossRef CAS PubMed.
- F. Guo and P. Berglund, Green Chem., 2017, 19, 333–360 RSC.
- D.-R. Alba, L. Ivan and G. Vicente, Curr. Green Chem., 2015, 2, 192–211 CrossRef.
- M. Rachwalski, N. Vermue and F. P. J. T. Rutjes, Chem. Soc. Rev., 2013, 42, 9268–9282 RSC.
- J. Steinreiber, K. Faber and H. Griengl, Chem. – Eur. J., 2008, 14, 8060–8072 CrossRef CAS PubMed.
- A. Diaz-Rodriguez, I. Lavandera and V. Gotor, Curr. Green Chem., 2015, 2, 192–211 CrossRef CAS.
- G. A. Applegate and D. B. Berkowitz, Adv. Synth. Catal., 2015, 357, 1619–1632 CrossRef CAS PubMed.
- J. Cheng-jun and C. Gui-lin, Curr. Org. Chem., 2013, 17, 1225–1234 CrossRef.
- N. J. Turner, in Asymmetric Organic Synthesis with Enzymes, 2008, pp. 115–131, DOI:10.1002/9783527622481.ch5.
- D. Tessaro, G. Molla, L. Pollegioni and S. Servi, in Modern Biocatalysis, 2009, pp. 195–228, DOI:10.1002/9783527623839.ch13.
- O. Verho and J. E. Bäckvall, J. Am. Chem. Soc., 2015, 137, 3996–4009 CrossRef CAS PubMed.
- J. V. Allen and J. M. J. Williams, Tetrahedron Lett., 1996, 37, 1859–1862 CrossRef CAS.
- B. A. Persson, A. L. E. Larsson, M. Le Ray and J. E. Bäckvall, J. Am. Chem. Soc., 1999, 121, 1645–1650 CrossRef CAS.
- A. L. E. Larsson, B. A. Persson and J. E. Bäckvall, Angew. Chem., Int. Ed. Engl., 1997, 36, 1211–1212 CrossRef CAS.
- H. Pellissier, Tetrahedron, 2016, 72, 3133–3150 CrossRef CAS.
- O. Langvik, T. Saloranta, D. Y. Murzin and R. Leino, ChemCatChem, 2015, 7, 4004–4015 CrossRef CAS.
- Y. Kim, J. Park and M.-J. Kim, ChemCatChem, 2011, 3, 271–277 CrossRef CAS.
- B. L. Conley, M. K. Pennington-Boggio, E. Boz and T. J. Williams, Chem. Rev., 2010, 110, 2294–2312 CrossRef CAS PubMed.
- J. H. Choi, Y. K. Choi, Y. H. Kim, E. S. Park, E. J. Kim, M.-J. Kim and J. Park, J. Org. Chem., 2004, 69, 1972–1977 CrossRef CAS PubMed.
- J. H. Choi, Y. H. Kim, S. H. Nam, S. T. Shin, M.-J. Kim and J. Park, Angew. Chem., Int. Ed., 2002, 41, 2373–2376 CrossRef CAS PubMed.
- R. M. Haak, F. Berthiol, T. Jerphagnon, A. J. A. Gayet, C. Tarabiono, C. P. Postema, V. Ritleng, M. Pfeffer, D. B. Janssen, A. J. Minnaard, B. L. Feringa and J. G. de Vries, J. Am. Chem. Soc., 2008, 130, 13508–13509 CrossRef CAS PubMed.
- U. T. Strauss and K. Faber, Tetrahedron: Asymmetry, 1999, 10, 4079–4081 CrossRef CAS.
- C. C. Gruber, B. M. Nestl, J. Gross, P. Hildebrandt, U. T. Bornscheuer, K. Faber and W. Kroutil, Chem. – Eur. J., 2007, 13, 8271–8276 CrossRef CAS PubMed.
- M. M. Musa, J. M. Patel, C. M. Nealon, C. S. Kim, R. S. Phillips and I. Karume, J. Mol. Catal. B: Enzym., 2015, 115, 155–159 CrossRef CAS.
- M. M. Musa, K. I. Ziegelmann-Fjeld, C. Vieille and R. S. Phillips, Org. Biomol. Chem., 2008, 6, 887–892 RSC.
- I. Karume, M. M. Musa, O. Bsharat, M. Takahashi, S. M. Hamdan and B. El Ali, RSC Adv., 2016, 6, 96616–96622 RSC.
- J. Popłoński, T. Reiter and W. Kroutil, ChemCatChem, 2018, 10, 763–768 CrossRef.
- F. G. Mutti, A. Orthaber, J. H. Schrittwieser, J. G. d. Vries, R. Pietschnig and W. Kroutil, Chem. Commun., 2010, 46, 8046–8048 RSC.
- C. Magallanes-Noguera, M. M. Ferrari, M. Kurina-Sanz and A. A. Orden, J. Biotechnol., 2012, 160, 189–194 CrossRef CAS PubMed.
- T. Tanaka, N. Iwai, T. Matsuda and T. Kitazume, J. Mol. Catal. B: Enzym., 2009, 57, 317–320 CrossRef CAS.
- F. Escalettes and N. J. Turner, ChemBioChem, 2008, 9, 857–860 CrossRef CAS PubMed.
- N. J. Turner, Curr. Opin. Chem. Biol., 2010, 14, 115–121 CrossRef CAS PubMed.
- M. Alexeeva, A. Enright, M. J. Dawson, M. Mahmoudian and N. J. Turner, Angew. Chem., Int. Ed., 2002, 41, 3177–3179 CrossRef CAS PubMed.
- S. Herter, F. Medina, S. Wagschal, C. Benhaïm, F. Leipold and N. J. Turner, Bioorg. Med. Chem., 2018, 26, 1338–1346 CrossRef CAS PubMed.
- D. Ghislieri, A. P. Green, M. Pontini, S. C. Willies, I. Rowles, A. Frank, G. Grogan and N. J. Turner, J. Am. Chem. Soc., 2013, 135, 10863–10869 CrossRef CAS PubMed.
- I. Rowles, K. J. Malone, L. L. Etchells, S. C. Willies and N. J. Turner, ChemCatChem, 2012, 4, 1259–1261 CrossRef CAS.
- C. J. Dunsmore, R. Carr, T. Fleming and N. J. Turner, J. Am. Chem. Soc., 2006, 128, 2224–2225 CrossRef CAS PubMed.
- R. Carr, M. Alexeeva, M. J. Dawson, V. Gotor-Fernández, C. E. Humphrey and N. J. Turner, ChemBioChem, 2005, 6, 637–639 CrossRef CAS PubMed.
- R. S. Heath, M. Pontini, B. Bechi and N. J. Turner, ChemCatChem, 2014, 6, 996–1002 CrossRef CAS.
- K. Yasukawa, S. Nakano and Y. Asano, Angew. Chem., Int. Ed., 2014, 53, 4428–4431 CrossRef CAS PubMed.
- J. M. Foulkes, K. J. Malone, V. S. Coker, N. J. Turner and J. R. Lloyd, ACS Catal., 2011, 1, 1589–1594 CrossRef CAS.
- J. H. Schrittwieser, B. Groenendaal, V. Resch, D. Ghislieri, S. Wallner, E. M. Fischereder, E. Fuchs, B. Grischek, J. H. Sattler, P. Macheroux, N. J. Turner and W. Kroutil, Angew. Chem., Int. Ed., 2014, 53, 3731–3734 CrossRef CAS PubMed.
- H. Jeon, S. Yoon, M. M. Ahsan, S. Sung, G.-H. Kim, U. Sundaramoorthy, S.-K. Rhee and H. Yun, Catalysts, 2017, 7, 251 CrossRef.
- G. A. Aleku, J. Mangas-Sanchez, J. Citoler, S. P. France, S. L. Montgomery, R. S. Heath, M. P. Thompson and N. J. Turner, ChemCatChem, 2018, 10, 515–519 CrossRef CAS.
- P. F. Mugford, U. G. Wagner, Y. Jiang, K. Faber and R. J. Kazlauskas, Angew. Chem., Int. Ed., 2008, 47, 8782–8793 CrossRef CAS PubMed.
- C. V. Voss, C. C. Gruber, K. Faber, T. Knaus, P. Macheroux and W. Kroutil, J. Am. Chem. Soc., 2008, 130, 13969–13972 CrossRef CAS PubMed.
- C. E. Paul, I. Lavandera, V. Gotor-Fernández, W. Kroutil and V. Gotor, ChemCatChem, 2013, 5, 3875–3881 CrossRef CAS.
- Y.-P. Xue, Y.-G. Zheng, Y.-Q. Zhang, J.-L. Sun, Z.-Q. Liu and Y.-C. Shen, Chem. Commun., 2013, 49, 10706–10708 RSC.
- C. V. Voss, C. C. Gruber and W. Kroutil, Angew. Chem., Int. Ed., 2008, 47, 741–745 CrossRef CAS PubMed.
- B. Li, Y. Nie, X. Q. Mu and Y. Xu, J. Mol. Catal. B: Enzym., 2016, 129, 21–28 CrossRef CAS.
- M. C. Fragnelli, P. Hoyos, D. Romano, R. Gandolfi, A. R. Alcantara and F. Molinari, Tetrahedron, 2012, 68, 523–528 CrossRef CAS.
- S. M. Amrutkar, L. Banoth and U. C. Banerjee, Tetrahedron Lett., 2013, 54, 3274–3277 CrossRef CAS.
- S. Venkataraman and A. Chadha, J. Ind. Microbiol. Biotechnol., 2015, 42, 173–180 CrossRef CAS PubMed.
- T. Sivakumari and A. Chadha, RSC Adv., 2015, 5, 91594–91600 RSC.
- T. Saravanan, R. Selvakumar, M. Doble and A. Chadha, Tetrahedron: Asymmetry, 2012, 23, 1360–1368 CrossRef CAS.
- W. Adam, M. Lazarus, C. R. Saha-Möller and P. Schreier, Tetrahedron: Asymmetry, 1998, 9, 351–355 CrossRef CAS.
- Y.-P. Xue, H. Zeng, X.-L. Jin, Z.-Q. Liu and Y.-G. Zheng, Microb. Cell Fact., 2016, 15, 162 CrossRef PubMed.
- D. Mendez-Sanchez, J. Mangas-Sanchez, I. Lavandera, V. Gotor and V. Gotor-Fernandez, ChemCatChem, 2015, 7, 4016–4020 CrossRef CAS.
- A. Diaz-Rodriguez, N. Rios-Lombardia, J. H. Sattler, I. Lavandera, V. Gotor-Fernandez, W. Kroutil and V. Gotor, Catal. Sci. Technol., 2015, 5, 1443–1446 RSC.
- K. Kedziora, A. Diaz-Rodriguez, I. Lavandera, V. Gotor-Fernandez and V. Gotor, Green Chem., 2014, 16, 2448–2453 RSC.
- E. Liardo, N. Ríos-Lombardía, F. Morís, J. González-Sabín and F. Rebolledo, Eur. J. Org. Chem., 2018, 2018, 3031–3035 CrossRef CAS.
- I. Karume, M. Takahashi, S. M. Hamdan and M. M. Musa, ChemCatChem, 2016, 8, 1459–1463 CrossRef CAS.
- M. M. Musa, I. Karume, M. Takahashi, S. M. Hamdan and N. Ullah, ChemistrySelect, 2018, 3, 10205–10208 CrossRef CAS.
- D. Koszelewski, D. Pressnitz, D. Clay and W. Kroutil, Org. Lett., 2009, 11, 4810–4812 CrossRef CAS PubMed.
- D. Koszelewski, N. Muller, J. H. Schrittwieser, K. Faber and W. Kroutil, J. Mol. Catal. B: Enzym., 2010, 63, 39–44 CrossRef CAS.
- G. Shin, S. Mathew, M. Shon, B. G. Kim and H. Yun, Chem. Commun., 2013, 49, 8629–8631 RSC.
- S.-W. Han, Y. Jang and J.-S. Shin, ACS Catal., 2019, 9, 6945–6954 CrossRef CAS.
- E. O'Reilly, C. Iglesias and N. J. Turner, ChemCatChem, 2014, 6, 992–995 CrossRef.
- Y.-M. Seo, S. Mathew, H.-S. Bea, Y.-H. Khang, S.-H. Lee, B.-G. Kim and H. Yun, Org. Biomol. Chem., 2012, 10, 2482–2485 RSC.
- R. S. Heath, M. Pontini, S. Hussain and N. J. Turner, ChemCatChem, 2016, 8, 117–120 CrossRef CAS.
- V. Köhler, Y. M. Wilson, M. Durrenberger, D. Ghislieri, E. Churakova, T. Quinto, L. Knorr, D. Haussinger, F. Hollmann, N. J. Turner and T. R. Ward, Nat. Chem., 2013, 5, 93–99 CrossRef PubMed.
- F. Khorsand, C. D. Murphy, A. J. Whitehead and P. C. Engel, Green Chem., 2017, 19, 503–510 RSC.
- K. Tauber, M. Fuchs, J. H. Sattler, J. Pitzer, D. Pressnitz, D. Koszelewski, K. Faber, J. Pfeffer, T. Haas and W. Kroutil, Chem. – Eur. J., 2013, 19, 4030–4035 CrossRef CAS PubMed.
- J. H. Sattler, M. Fuchs, K. Tauber, F. G. Mutti, K. Faber, J. Pfeffer, T. Haas and W. Kroutil, Angew. Chem., Int. Ed., 2012, 51, 9156–9159 CrossRef CAS PubMed.
- C. M. Palacio, C. G. Crismaru, S. Bartsch, V. Navickas, K. Ditrich, M. Breuer, R. Abu, J. M. Woodley, K. Baldenius, B. Wu and D. B. Janssen, Biotechnol. Bioeng., 2016, 113, 1853–1861 CrossRef CAS PubMed.
- A. Lerchner, S. Achatz, C. Rausch, T. Haas and A. Skerra, ChemCatChem, 2013, 5, 3374–3383 CrossRef CAS.
- M. Pickl, M. Fuchs, S. M. Glueck and K. Faber, ChemCatChem, 2015, 7, 3121–3124 CrossRef CAS PubMed.
- M. Fuchs, K. Tauber, J. Sattler, H. Lechner, J. Pfeffer, W. Kroutil and K. Faber, RSC Adv., 2012, 2, 6262–6265 RSC.
- L. Martinez-Montero, V. Gotor, V. Gotor-Fernandez and I. Lavandera, Green Chem., 2017, 19, 474–480 RSC.
- F. G. Mutti, T. Knaus, N. S. Scrutton, M. Breuer and N. J. Turner, Science, 2015, 349, 1525–1529 CrossRef CAS PubMed.
- F. F. Chen, Y. Y. Liu, G. W. Zheng and J. H. Xu, ChemCatChem, 2015, 7, 3838–3841 CrossRef CAS.
- W. Böhmer, T. Knaus and F. G. Mutti, ChemCatChem, 2018, 10, 731–735 CrossRef PubMed.
- S. L. Montgomery, J. Mangas-Sanchez, M. P. Thompson, G. A. Aleku, B. Dominguez and N. J. Turner, Angew. Chem., Int. Ed., 2017, 56, 10491–10494 CrossRef CAS PubMed.
- M. P. Thompson and N. J. Turner, ChemCatChem, 2017, 9, 3833–3836 CrossRef CAS.
- V. Resch, W. M. F. Fabian and W. Kroutil, Adv. Synth. Catal., 2010, 352, 993–997 CrossRef CAS.
- T. Knaus, L. Cariati, M. F. Masman and F. G. Mutti, Org. Biomol. Chem., 2017, 15, 8313–8325 RSC.
- M. L. Contente and F. Paradisi, Nat. Catal., 2018, 1, 452–459 CrossRef CAS.
- M. Schober and K. Faber, Trends Biotechnol., 2013, 31, 468–478 CrossRef CAS PubMed.
- K. Matsumoto, K. Usuda, H. Okabe, M. Hashimoto and Y. Shimada, Tetrahedron: Asymmetry, 2013, 24, 108–115 CrossRef CAS.
- Y. Shimada, K. Usuda, H. Okabe, T. Suzuki and K. Matsumoto, Tetrahedron: Asymmetry, 2009, 20, 2802–2808 CrossRef CAS.
- W. Szymanski, A. Westerbeek, D. B. Janssen and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 10712–10715 CrossRef CAS PubMed.
- M. Schober, M. Toesch, T. Knaus, G. A. Strohmeier, B. van Loo, M. Fuchs, F. Hollfelder, P. Macheroux and K. Faber, Angew. Chem., Int. Ed., 2013, 52, 3277–3279 CrossRef CAS PubMed.
- M. Schober, P. Gadler, T. Knaus, H. Kayer, R. Birner-Grunberger, C. Gully, P. Macheroux, U. Wagner and K. Fabert, Org. Lett., 2011, 13, 4296–4299 CrossRef CAS PubMed.
- Y. Simeo and K. Faber, Tetrahedron: Asymmetry, 2006, 17, 402–409 CrossRef CAS.
- M. Fuchs, M. Schober, J. Pfeffer, W. Kroutil, R. Birner-Gruenberger and K. Faber, Adv. Synth. Catal., 2011, 353, 2354–2358 CrossRef CAS.
- S. Pedragosa Moreau, A. Archelas and R. Furstoss, Tetrahedron, 1996, 52, 4593–4606 CrossRef CAS.
- S. Hwang, C. Y. Choi and E. Y. Lee, Biotechnol. Lett., 2008, 30, 1219–1225 CrossRef CAS PubMed.
- S. Hwang, C. Y. Choi and E. Y. Lee, Biotechnol. Bioprocess Eng., 2008, 13, 453–457 CrossRef CAS.
- M. I. Monterde, M. Lombard, A. Archelas, A. Cronin, M. Arand and R. Furstoss, Tetrahedron: Asymmetry, 2004, 15, 2801–2805 CrossRef CAS.
- A. Steinreiber, S. F. Mayer, R. Saf and K. Faber, Tetrahedron: Asymmetry, 2001, 12, 1519–1528 CrossRef CAS.
- S. J. Lin, G. P. Horsman, Y. H. Chen, W. L. Li and B. Shen, J. Am. Chem. Soc., 2009, 131, 16410–16417 CrossRef CAS PubMed.
- Q.-Q. Zhu, W.-H. He, X.-D. Kong, L.-Q. Fan, J. Zhao, S.-X. Li and J.-H. Xu, Appl. Microbiol. Biotechnol., 2014, 98, 207–218 CrossRef CAS PubMed.
- Y.-W. Wu, X.-D. Kong, Q.-Q. Zhu, L.-Q. Fan and J.-H. Xu, Catal. Commun., 2015, 58, 16–20 CrossRef CAS.
- F.-L. Li, X.-D. Kong, Q. Chen, Y.-C. Zheng, Q. Xu, F.-F. Chen, L.-Q. Fan, G.-Q. Lin, J. Zhou, H.-L. Yu and J.-H. Xu, ACS Catal., 2018, 8, 8314–8317 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |