Catalytic activation of ethylene C–H bonds on uniform d8 Ir(i) and Ni(ii) cations in zeolites: toward molecular level understanding of ethylene polymerization on heterogeneous catalysts†
Abstract
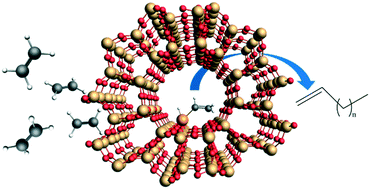
The homolytic activation of the strong C–H bonds in ethylene is demonstrated, for the first time, on d8 Ir(I) and Ni(II) single atoms in the cationic positions of zeolites H-FAU and H-BEA under ambient conditions. The oxidative addition of C2H4 to the metal center occurs with the formation of a d6 metal vinyl hydride, explaining the initiation of the olefin-polymerization cycle on d8 M(I/II) sites in the absence of pre-existing M–H bonds. Under mild reaction conditions (80–220 °C, 1 bar), the catalytic dimerization to butenes and dehydrogenative coupling of ethylene to butadiene occurs over these catalysts. 1-Butene is not converted to butadiene under the reaction conditions applied. Post-reaction characterization of the two materials reveals that the active metal cations remain site-isolated whereas deactivation occurs due to the formation of carbonaceous deposits on the zeolites. Our findings have significant implications for the molecular level understanding of ethylene conversion and the development of new ways to functionalize C–H bonds under mild conditions.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
