Reduction of carbon dioxide with mesoporous SnO2 nanoparticles as active photocatalysts under visible light in water†
Abstract
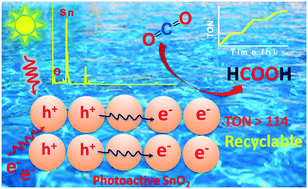
We demonstrated the photocatalytic reduction of CO2 to HCOOH using mesoporous SnO2 nanoparticles as active photocatalysts in water, which acted as a sacrificial electron source as well as a solvent under atmospheric pressure. Greater than 114 TON was achieved by using only 10 mg of the catalyst. The reaction did not occur in the absence of light (white LED) and could be readily controlled by light intensity alternation. We also demonstrated that a TON of 25 could be obtained with the use of sunlight using the catalytic cycle. These results open the door to a new class of protocols for the photocatalytic reduction of CO2 using SnO2 nanoparticles under visible light irradiation in green reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...