Open Access Article
Open Access ArticleNovozym 435: the “perfect” lipase immobilized biocatalyst?
Claudia
Ortiz
a,
María Luján
Ferreira
b,
Oveimar
Barbosa
c,
José C. S.
dos Santos
 d,
Rafael C.
Rodrigues
e,
Ángel
Berenguer-Murcia
d,
Rafael C.
Rodrigues
e,
Ángel
Berenguer-Murcia
 f,
Laura E.
Briand
f,
Laura E.
Briand
 *g and
Roberto
Fernandez-Lafuente
*g and
Roberto
Fernandez-Lafuente
 *h
*h
aEscuela de Microbiología, Universidad Industrial de Santander, Bucaramanga, Colombia
bPlanta Piloto de Ingeniería Química – PLAPIQUI, CONICET, Universidad Nacional del Sur, Camino La Carrindanga km 7, 8000 Bahía Blanca, Argentina
cDepartamento de Química, Facultad de Ciencias, Universidad del Tolima, Ibagué, 730006299, Colombia
dInstituto de Engenharias e Desenvolvimento Sustentável, Universidade da Integração Internacional da Lusofonia Afro-Brasileira, CEP 62790-970, Redenção, CE, Brazil
eBiotechnology, Bioprocess, and Biocatalysis Group, Food Science and Technology Institute, Federal University of Rio Grande do Sul, Av. Bento Gonçalves 9500, P. O. Box 15090, Porto Alegre, RS ZC 91501-970, Brazil
fInstituto Universitario de Materiales, Departamento de Química Inorgánica, Universidad de Alicante, Ap. 99-03080, Alicante, Spain
gCentro de Investigación y Desarrollo en Ciencias Aplicadas-Dr. Jorge J. Ronco, Universidad Nacional de La Plata, CONICET, CCT La Plata. Calle 47N 257, B1900AJK La Plata, Buenos Aires, Argentina. E-mail: briand@quimica.unlp.edu.ar
hDepartment of Biocatalysis, ICP-CSIC, Campus UAM-CSIC, Cantoblanco, Madrid, ZC 28049, Spain. E-mail: rfl@icp.csic.es
First published on 12th April 2019
Abstract
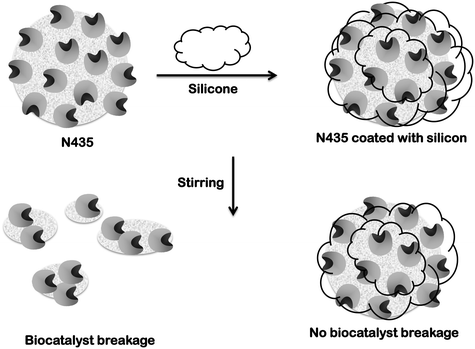
Novozym 435 (N435) is a commercially available immobilized lipase produced by Novozymes. It is based on immobilization via interfacial activation of lipase B from Candida antarctica on a resin, Lewatit VP OC 1600. This resin is a macroporous support formed by poly(methyl methacrylate) crosslinked with divinylbenzene. N435 is perhaps the most widely used commercial biocatalyst in both academy and industry. Here, we review some of the success stories of N435 (in chemistry, energy and lipid manipulation), but we focus on some of the problems that the use of this biocatalyst may generate. Some of these problems are just based on the mechanism of immobilization (interfacial activation) that may facilitate enzyme desorption under certain conditions. Other problems are specific to the support: mechanical fragility, moderate hydrophilicity that permits the accumulation of hydrophilic compounds (e.g., water or glycerin) and the most critical one, support dissolution in some organic media. Finally, some solutions (N435 coating with silicone, enzyme physical or chemical crosslinking, and use of alternative supports) are proposed. However, the N435 history, even with these problems, may continue in the coming future due to its very good properties if some simpler alternative biocatalysts are not developed.
1. Introduction
1.1. Lipases in biocatalysis
Chemistry is being continuously (and vigorously) pushed to become more environmentally friendly and compatible, therefore green chemistry is nowadays the final goal in most chemical industry developments.1–4 This is coupled with an ever-increasing demand for products with growing complexity, in many instances with multiple functions and chirality in many of them. In this environment, the use of enzymes as industrial catalysts is rising.5–7 Enzymes have many properties that make them very interesting: they are the most efficient catalysts in nature, performing their function under very mild conditions (at low pressure and temperature) in aqueous media. Moreover, they are very selective and specific, saving many protection/deprotection steps.8–13 However, enzymes are catalysts with a biological origin, and they have evolved under natural selection to be able to respond under stress conditions. Thus, enzymes are inhibited by diverse components, their stability is moderate even under physiological conditions and their excellent properties are only exhibited in physiological reactions and substrates.14 Besides, they are usually water soluble. These properties, although physiologically necessary, are a problem if they are going to be used as industrial biocatalysts, where they are expected to perform their function under standardized conditions. In this respect, maximal stability and activity will always be desired, and the substrates, as well as reaction conditions, may be quite far from the physiological ones.As a result, enzymes normally need to be improved in many instances before their industrial implementation. Thanks to the development of many different scientific areas, there are several ways of improving these enzyme limitations. Microbiological (metagenomics)15–20 and genetic (site-directed mutagenesis,21–23 directed evolution,24–28etc.) tools may provide high enzyme production with improved properties compared to the native enzyme. Immobilization was first a requirement to solve the issue of enzyme solubility, but it has recently become a powerful tool to improve many other enzyme properties like stability, activity, selectivity, specificity, purity, inhibition, or resistance to chemicals.29–39 As such, enzyme immobilization has evolved from a requirement to use these expensive catalysts to a tool to greatly enhance enzyme features.
Lipases are among the most widely used enzymes in biocatalysis.40,41 The biological function of lipases is the hydrolysis of triglycerides to produce free fatty acids and glycerol.42 The heterogeneity of the natural substrate43–48 has converted lipases in enzymes with a very broad specificity, accepting substrates very different from glycerides (even amides). Thus, lipases are used in vitro to catalyze reactions different from those of the natural hydrolase function,49–54 such as esterification,55–61 acidolysis,62–67 interesterificaton,68–71 transesterification,72–76 aminolysis,77–81 perhydrolysis,82,83etc., together with a collection of the so-called promiscuous reactions.84–92
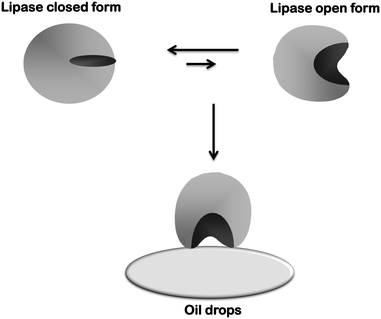
Lipases are usually quite stable, and so they have been used in diverse media, like aqueous media, organic solvents,93–95 ionic liquids,96–99 supercritical fluids,100–102 and deep eutectic solvents.103–106 This way, lipases have a huge range of possibilities in industrial biocatalysis. Besides, they have a peculiar mechanism of action called “interfacial activation”, which will be explained below.
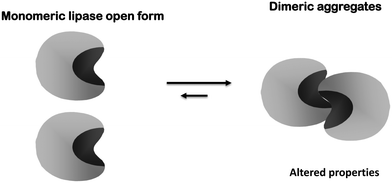
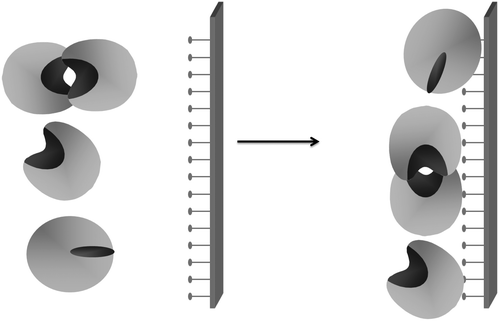
This catalytic mechanism may be a problem for lipase handling.116 Lipases are adsorbed on any hydrophobic surface. As an example, lipases tend to form dimeric aggregates by interaction between two open forms of lipases (giving altered properties)117–119 (Fig. 2) or may interact with hydrophobic proteins on the crude extract (also altering enzyme properties).120 This may also be a problem when immobilizing a lipase on a solid support, as the lipase molecules will be isolated from the external medium and external drops of the hydrophobic substrate can hardly interact with the enzyme, making their interfacial activation impossible.
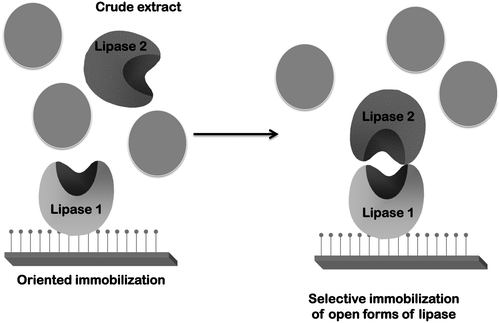
However, once this phenomenon is known, it can be utilized for some purposes. For example, properly oriented immobilized lipases have been used as a chromatography matrix to purify other lipases via interaction between the open forms of two lipase molecules120,121 (Fig. 3).
Moreover, as described later, this allowed development of one of the most utilized protocols for lipase immobilization: the immobilization of lipases on hydrophobic supports via interfacial activation.122
1.2. Lipase B from Candida antarctica
CALB has a molecular weight of 33 kDa, with a pI of 6.0. CALB is an α/β protein with many features similar to those of other lipases. The structure of CALB has been fully resolved and shows that the enzyme has a Ser-His-Asp catalytic triad in its active site, with a very small lid that is unable to fully seclude the active center.123,124 Even with that small lid, CALB retains its capacity to be adsorbed on hydrophobic surfaces, that is, it remains an interfacial enzyme. For this reason it is considered a true lipase, although the closed form is not really closed as in other lipases.125,126 This may make its handling simpler than using other lipases, as it does not have the strong tendency of other lipases to form dimeric aggregates.This enzyme is among the most stable commercialized lipases127,128 and has been used in a wide range of reactions; it is in fact very likely the most widely used lipase.129–131 This lipase has been utilized in almost all areas of lipase utilization, from triglyceride modification to biodiesel production, from resolution of racemic mixtures to regioselective reactions, production and degradation of polymers, promiscuous reactions, etc.132–152
This good stability has made CALB one of the most intensively researched enzymes in ionic liquids.153–155 CALB properties have been improved via genetic tools.156–159 Some papers are based on the comparison of commercial CALB and some other recombinant CALB expressed in different hosts (mainly Pichia pastoris) presenting in some cases very different properties.160–166
1.3. Immobilization of lipases
As previously stated, immobilization of enzymes is a requirement for most industrial enzyme uses, and the same thing occurs when using lipases. As discussed above, immobilization may be a tool to improve enzyme features; the objective should be to have a reusable, active and, if possible, improved biocatalyst.29–39 This way, the costs associated with immobilization may be fully compensated. Here, we will not review the immobilization; there are some excellent review papers that may be used for that purpose.29–39,167–172 However, in the case of lipases some points must be carefully considered.The first point is that an enzyme immobilized on porous supports will not be exposed to external interfaces. This is positive because enzyme inactivation is not possible by interaction with these interfaces,33 but if the enzyme is inside a porous support, interfacial activation is not possible, (except when using nearly anhydrous media that can penetrate the support porous system).
Second, as previously explained, lipases tend to form bimolecular aggregates involving the open forms of two lipase molecules.117–119,121,173–175 If immobilization is performed under conditions where this is favored, the effect may be quite negative because these dimers will be immobilized together with the monomeric enzyme molecules (Fig. 4). These dimeric lipase forms generally presented altered properties and lower activity than the monomeric enzyme.117,173 Moreover, the percentage of dimers and monomers will depend on the exact immobilization conditions, making it difficult to reproduce the results when using different enzyme batches. Additionally, if only one of the enzyme molecules forming the dimer is attached to the support, some enzyme leakage may be produced contaminating the final product (Fig. 5). The use of detergents during immobilization may solve this problem: it will break the dimers and allow the immobilization of monomeric enzymes.117–119,121,173–175 (Fig. 6) Moreover, if immobilization is via an intense enough enzyme–support interaction, the presence of detergent during immobilization can also improve enzyme activity by maintaining the open form of the lipase when the detergent is eliminated176–183 (Fig. 6).
 | ||
| Fig. 4 Problems generated in lipase immobilization by the tendency of lipases to form enzyme dimeric aggregates. | ||
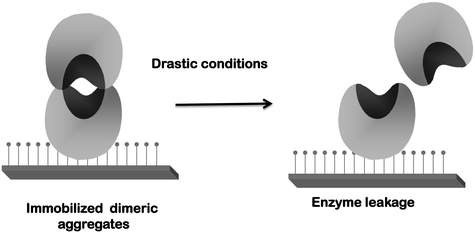 | ||
| Fig. 5 Risk of enzyme desorption if dimeric aggregates are immobilized via one enzyme molecule only. | ||
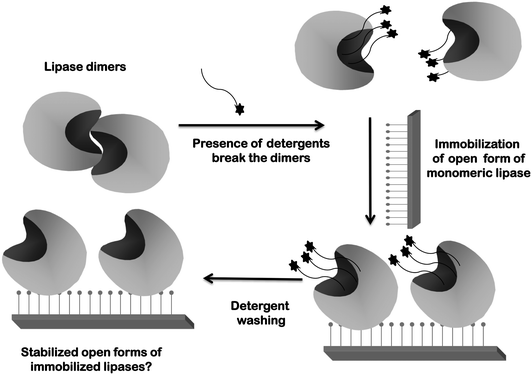 | ||
| Fig. 6 Immobilization of lipases in the presence of detergents to break the dimers ensures immobilization of monomeric forms. | ||
On the other hand, the active center of lipases is very flexible, and it has been shown in many instances that lipase properties may be strongly modulated via immobilization.184–204 However this also has a counterpart: if the enzyme already has the desired specificity of selectivity, keeping the properties of the lipase after immobilization may be quite difficult.32 Thus, lipase immobilization must consider some points that are not required for other enzymes.
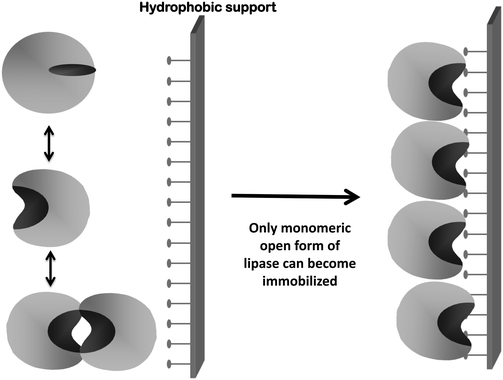 | ||
| Fig. 7 Immobilization of lipases on hydrophobic supports at low ionic strength via interfacial activation: immobilization of monomeric and open forms of lipases. | ||
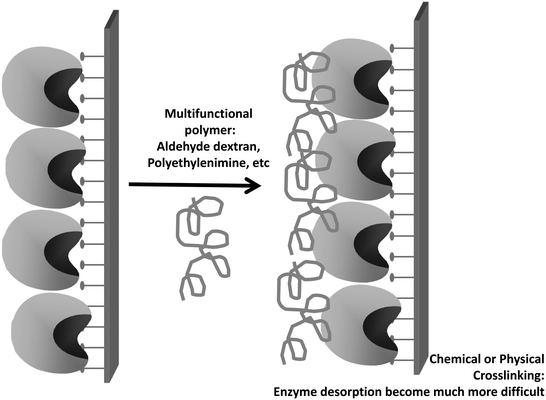
Thus, lipases immobilized by interfacial adsorption may almost fully retain their activity even under very high ionic strength, while a conventional immobilized enzyme will have the active center closed under these conditions.122 However, the method also has some problems. Immobilization is reversible and based on hydrophobic interactions, which means that lipase molecules may be released to the medium at high temperatures, in the presence of co-solvents or detergents.210 Special care must be taken with some substrates or products with detergent properties,211 for example triglyceride hydrolysis will release free fatty acids and di- or mono-glycerides, which have recognized detergent properties and can favor enzyme release. It has been reported that even diacylglycerols from short chain carboxylic acids such as diacetin or dibutyrin may be enough to favor enzyme release from the support.212,213 This problem may be reduced if some intermolecular crosslinking (covalent or physical) is performed,214–218 or using heterofunctional supports (supports having the acyl moiety and some reactive groups able to give rise to an additional covalent or ionic immobilization).210,219–227 Later on, this will be discussed in detail in this review.
Thus, the immobilization of lipases via interfacial activation may be the most popular immobilization strategy, using silica (and other inorganic materials) nanoparticles or natural hydrophilic supports coated with long acyl groups, hydrophobic polymeric supports coated (or uncoated) with hydrophobic groups, etc.191,228–247
This is the most used lipase preparation in the literature. A search in Scopus on 27th November 2018 revealed 1500 papers related to this commercial immobilized enzyme. This review will present some examples of successful use of this biocatalyst (focused from 2016 onwards), very likely one of the most commercially available stable and active preparations that have permitted many studies to be performed. Later, a deep discussion on the problems in the use of this biocatalyst will be performed; some will be related to the immobilization mechanism of the enzyme and shared with any other lipase immobilized just by the lipase interfacial activation mechanism. Other problems will be related to the support. Finally, some alternatives to solve the problems will be presented.
2. Lewatit VP OC 1600
The N435 support, Lewatit VP OC 1600, is a macroporous matrix with a spherical bead morphology. This organic carrier is made of macroporous poly(methyl methacrylate) crosslinked with divinylbenzene, and it is currently marketed by Lanxess (Germany) as Lewatit VP OC 1600.249,253The chemistry of the synthesis of the Lewatit support has not been described in detail. However, it is presumed that the polymethacrylate divinylbenzene copolymer matrix is produced through condensation polymerization reactions or by addition between methacrylic esters and divinylbenzene.254 Lewatit VP OC 1600 has an average particle size, surface area, and pore diameter of 315–1000 μm, 130 m2 g−1, and 150 Å, respectively (product information Lewatit VP OC 1600, Lanxess, edition: 2011-10-13). These characteristics have allowed its application to enzyme immobilization, especially for immobilizing lipases due to its relative hydrophobicity. This support has not been exclusively used by Novozymes to produce N435.
For example, CALB has been immobilized on this support in some laboratories. Thus, the enzyme was immobilized on different hydrophobic supports, including Lewatit VP OC 1600. The home-made preparation obtained using this support did not differ in its properties from N435, suggesting that industrial immobilization did not modify the enzyme.252 However, both preparations differ in the enzyme catalytic properties from CALB immobilized on other hydrophobic supports (giving inverse enantiospecificity in the hydrolysis of rac-2-O-butyryl-2-phenylacetic acid and different selectivity in the hydrolysis of 3-phenylglutaric acid dimethyl diester).252
Considering the success of N435, it could be expected that many other lipases have been immobilized on the same support. However, only some examples may be found.
For instance, a lipase from R. arrhizus was immobilized on Lewatit VP OC 1600, Duolite A568, Amberlite X. A.D 761 and O-pentynyl dextran and used to prepare geranyl octanoate in organic solvent. The results showed that O-pentynyl dextran was clearly superior compared to adsorbents like Lewatit VP OC 1600, for which only a loading of 1.1% protein was obtained, additionally it was 3 times less active in the esterification of geraniol and octanoic acid.255
In another instance, phospholipase A1 was immobilized on Lewatit VP OC 1600, showing a good immobilization efficiency (79% global immobilization yield) and a very high specific activity (6.7 × 10−3 μmol per g protein per min) in the modification of phosphatidylcholine with n-3 polyunsaturated fatty acids.256 These results surpassed those obtained using other hydrophobic supports such as silica coated with octyl groups, Accurel MP 1000 or Celite 545, among others.256
Rhizopus oryzae and Carica papaya lipases were also immobilized on Lewatit, and used in biodiesel production by transesterification of Jatropha oil with methanol. The results obtained showed that the highest immobilization yield was achieved with the lipase from Carica papaya (98%), while for lipase from Rhizopus oryzae the immobilization yield was only 77.2%.257 However, the percentage of methyl ester production was 65%, w/w when using the lipase from Rhizopus oryzae while for Carica papaya lipase it was 51.7%.257
In a last example, the lipase from Penicillium sp. (CBMAI 1583) was immobilized on a battery of hydrophobic supports including agarose-butyl, agarose-phenyl and agarose-octyl, acrylic Toyopearl, octadecyl Sepabeads and Lewatit VP OC 1600. The biocatalysts were used in the hydrolysis of fish oil to get omega-3 fatty acids and ethanolysis to produce the respective ethyl esters. Immobilization yields were very high in all cases (over 75%) and the expressed activity ranged from 54.2% to 144.9% compared to the free enzyme. However, the least stable preparation at drastic pH values was that prepared using Lewatit.258
As such, the great success of Lewatit VP OC 1600 with CALB cannot be extrapolated to other lipases. It has been clearly showed that the selection of the “optimal” support may depend on the enzyme, the specific reaction (e.g., to determine the activity, selectivity or specificity) and the experimental conditions (e.g., to determine the enzyme stability, activity, selectivity or specificity).185,191,234,252,259
3. Novozym 435: a history of success
3.1. Use in chemistry and fine chemistry
Many simple esters have been produced via esterification catalyzed by N435 using different media. As an advantage of enzyme specificity, the specific acylation of primary alcohols in the presence of secondary alcohols and phenols was demonstrated using ethyl acetate as a medium and acylating agent and N435 as a catalyst.261 For example, butyl butyrate was produced by esterification in a solvent-less medium.262 Optimization via the response surface methodology gave a yield of butyl butyrate near 100%. In a very interesting paper, N435 was used to esterify 1-butanol and butyric acid using diesel as the reaction medium, with a 90% yield.263 The diesel thus modified has some improved properties. Another paper shows that butyric acid and ethanol were esterified in n-hexane using N435.59 In another paper, the esterification of L-ascorbic acid and n-octanoic acid or n-caprylic acid catalyzed by N435 permitted yields above 85% to be reached.264 N435 was used to modify Konjac glucomannan via esterification with oleic acid in isooctane.265 Butyl caprylate was produced from caprylic acid and butanol in a solvent-free system employing a stirred batch reactor catalyzed by N435 (yield was 92%).266 N435 was used to study the thermodynamics in the esterification of succinic acid with ethanol.267 More than 95% citronellyl palmitate ester from palmitic acid was produced by esterification catalyzed by N435 using hexane as a solvent.268 Azelaic acid was diesterified with lauric alcohol using N435 and used as a bacteria growth inhibitor.269 Another paper shows that methyl caffeate production via esterification catalyzed by N435 was improved via a microfluidic strategy in a continuous-flow reactor (yields surpassed 98% in 2.5 h).270 N435 was also used in the esterification of oleic acid and kojic acid in a stirred tank reactor with a yield near 43%.271 Using a fluidized tank reactor the yield decreased, but the operational stability of the biocatalyst was enhanced. Esters of some free fatty acids and picolinol were produced using N435 in toluene as the biocatalyst for the determination of free fatty acids, but they could not be applied for some epoxy free fatty acids, fatty wax, or parinaric acid.272 The esterification of furfuryl alcohol and castor oil fatty acid catalyzed by N435 in a solvent-free system gave a yield of 88.64% (% w/w) at 5 h.273
Ionic liquids, deep eutectic solvents and supercritical fluids have been used in some instances as reaction media in N435 esterification reactions. Several alkyl dihydrocaffeates were synthesized in ionic liquids by the esterification of dihydrocaffeic acid with methanol, hexanol octanol and dodecanol catalyzed by N435.274 Lauryl ferulate was produced via esterification of ferulic acid and lauryl alcohol catalyzed by N435 in ionic liquids (yields were higher than 90%).275 This biocatalyst was used in the esterification of n-butanol and D,L-lactic acid in supercritical trifluoromethane/ionic liquid and supercritical carbon dioxide/ionic liquid medium.276 Butyl stearate and ethyl stearate were produced via esterification catalyzed by N435 (92% yield).277 Enzyme reuses were not satisfactory (activity decreased after 3 or 5 reuses). The esterification of dihydrocaffeic acid with hexanol in ionic liquids was statistically optimized using N435 as a catalyst (yield of 84.4%).278 Oleic acid was esterified with different alcohols in supercritical carbon dioxide using N435.279
Ultrasound has been used to intensify some of these esterification reactions. The solvent-free ultrasound-assisted synthesis of citronellol laurate via esterification catalyzed by N435 gave more than 95% conversion and allowed the enzyme to be reused for 5 cycles.280 N435 was used under ultrasonic irradiation to produce L-ascorbyl fatty acid esters as antioxidant materials.281 Ascorbyl linoleate was obtained by the esterification reaction between linoleic acid and ascorbic acid catalyzed by N435, using an ultrasound bath.282 The yields reached 90% using tert-butanol as an organic solvent.
Microwave irradiation has been used in many examples as a heating strategy. n-Butyl propionate was synthesized by esterification of propionic acid with n-butanol under microwave irradiation by N435, Lipozyme TL-IM and Lipozyme RM-IM.283 N435 was the most active biocatalyst, reaching 92% conversion. In another paper, esterification of valeric acid and ethanol in solvent-free medium was intensified by microwave irradiation of N435, Lipozyme TL-IM and Lipozyme RM-IM.284 N435 gave almost 70% conversion and could be reused. The solvent-free microwave-assisted production of isoamyl acetate via esterification (acetic acid) or transesterification (acetic anhydride and ethyl acetate) catalyzed by N435 and Lipozyme RM-IM has been studied.285 N435 was found to be the optimal catalyst, using acetic anhydride; however it did not exhibit a good operational stability.
N435 has been compared with other lipases in some instances. For example, benzyl propionate was synthesized by lipase catalyzed esterification, comparing N435, Lipozyme TL-IM, Lipozyme RM-IM and a home-made biocatalyst from CALB.286 Among them, N435 exhibited the best performance in solvent-free medium, with a yield of more than 40%. Esterification of geraniol with some acids was performed using different catalysts, with N435 being the most active one.144 Geraniol and butanoic acid were esterified by N435 in a solvent-free system with a yield of over 95%, higher than the yield observed using a home-made biocatalyst.287 N435 was also the best enzyme preparation among the assayed ones in the synthesis of several geraniol esters via an esterification process in a continuous-flow packed-bed reactor (e.g., 87% of geranyl propionate).288 Ethyl lactate was produced via esterification catalyzed by N435, with a yield near 90%.289 N435 presented better results than some homemade biocatalysts or Lipozyme RM-IM. 5-Hydroxymethylfurfural and levulinic acid were used to produce 5-hydroxymethylfurfuryl levulinate by esterification catalyzed by N435 (the best enzyme among several assayed).290 A yield of 85% was achieved using 2-methyltetrahydrofuran as a reaction solvent. Levulinic acid was esterified with 1-butanol, ethanol, and methanol catalyzed by N435.291 In another paper, n-butyl levulinate was synthesized by esterification using Lipozyme RM-IM, Lipozyme TL-IM, and N435 in both a stirred tank batch reactor and a continuous flow packed bed tubular microreactor.292 N435 was the best catalyst (yields of 85%). Solvent-free production of cetyl laurate, myristate, palmitate and stearate has been also reported to be catalyzed by N435 (conversion was higher than 98.5%).293 The same groups reported that a cetyl ester mixture was obtained via esterification of myristic acid, palmitic acid or stearic acid (95%) and cetyl alcohol.294 The reaction was catalyzed by different enzyme preparations, with N435 being the reference, and not overtaken by any of the new biocatalysts (with a product cost of 56.5 € per kg versus 58 € per kg for the best new catalyst).287
In other cases, a transesterification reaction has been used to get the target product. For example, eugenyl acetate has been synthesized in supercritical carbon dioxide by transesterification of eugenol and acetic anhydride catalyzed by N435.295 More than 55% of ferulyl oleins were produced using N435 in toluene with ethyl ferulate and triolein as substrates.296 Methyl gallate was produced using N435 as a catalyst and propyl gallate and methanol as substrates, in a deep eutectic solvent.297 N435 was found to be the most efficient among the analyzed enzymes in the production via transesterification of the sorbitol ester of norbixin (50% total reaction yield).298 N435 was used to catalyze the synthesis of L-ascorbyl phenolates via a transesterification reaction using the corresponding vinyl phenolates.299 The transesterification of butyl acetate with hexanol was studied with N435 to analyze the causes of catalyst activity loss in enzymatic catalyzed reactive distillation.300 The transesterification of epoxidized soybean oil catalyzed by N435 permitted the production of epoxidized soybean oil methyl esters with a 95.7% yield.301 The biocatalyst was reutilized in 10 cycles maintaining the activity. Vinyl acetate and 2-phenethyl alcohol have been used to produce 2-phenylethyl acetate in hexane employing N435 as a catalyst.302 Aromatic aldehyde oximes were acetylated by reaction with vinyl and isopropenyl acetates catalyzed by N435 (the best one among the assayed ones) to produce aromatic aldehyde oxime esters, with the conversion being almost quantitative.303 N435 was used in the production of wax esters using microbial oils via transesterification with behenyl or cetyl esters, with conversion yields up to 87.3% and 69.1%, respectively.304
Methacrylated trimethylolpropane cyclic carbonates were produced by two-step transesterifications catalyzed by N435 followed by thermal cyclization.305 Six-membered cyclic carbonates with methoxycarbonyloxy and hydroxyl functionalities were obtained via transesterification of trimethylolpropane or dimethyl carbonate in a solvent-free medium flow reaction using N435, followed by thermal cyclization (yields over 80%).306 Octyl ethanoate has been produced via ultrasound-assisted transesterification catalyzed by N435 and using vinyl acetate as an activated acyl donor in a solvent-free medium (yield over 97%).307
Other papers compared both esterification and transesterification routes. The synthesis of phenethyl acetate was studied using a free acid and different activated acyl donors, utilizing N435 as a catalyst.308 The authors obtained yields of 99.12% and 98.44% employing acetic anhydride and vinyl acetate as the activated acyl donors, respectively (results were only slightly lower after 20 cycles). L-Ascorbyl flurbiprofenate was produced by both esterification and transesterification catalyzed by N435.309 Using flurbiprofen as an acyl donor, 61.0% of L-ascorbic acid was converted, while only 46.4% was obtained by employing a flurbiprofen methyl ester (very likely due to the competition of methanol with L-ascorbic acid).
In some cases, two lipases have been combined to reach the desired result. An isosorbide diester was synthesized using a mixture of N435 and Ylip2 (77.4% of the diester).310 In another case, N435 is coupled to other kinds of enzymes. For example, chiral diols and siloxane were coupled (66% yield) using dioxygenase and N435 (Lipozyme RM-IM and Lipozyme TL-IM were not active in this reaction).311
Ethyl 2-((4R,6S)-2,2-dimethyl-6-((E)-styryl)-1,3-dioxan-4-yl)acetate and the hydrolyzed (4S,6R)-acid have been produced by enantiospecific hydrolysis of racemic syn-ethyl (E)-2-(2,2-dimethyl-6-styryl-1,3-dioxan-4-yl)acetate catalyzed by Novozym-435.312 Enantiomerically pure β-halohydrin (1S)-2-chloro-1-(2,4-dichlorophenyl)-1-ethanol was produced via kinetic resolution of the corresponding racemic acetate catalyzed by Lipozyme TL-IM or N435.313 N435 was more efficient in producing (S)-β-halohydrin (ee of 99%). The kinetic resolution of flurbiprofen (R,S)-[2-(3-fluro-4-phenyl)phenyl] propionic acid using N435 as a catalyst and microwave irradiation has been reported.314 The reaction permitted the conversion of the R-enantiomer into an ester with high enantioselectivity (eeP was 98.9%).
Benzoxazole derivatives were synthesized using chiral alcohols and esters which were previously resolved by N435 catalyzed transesterification or hydrolysis.315 N435 was used in the resolution of trans-2-phenylcyclopropyl azolides via hydrolysis or alcoholysis in methyl, giving trans-2-phenylcyclopropyl 1,2,4-azolide (trans-2-PCPT) of high optical purity.316
N435 sequential acetylation/hydrolysis has permitted the production of (S)-C5- lipidic dialkynylcarbinols in 97% ee and (R)-C5- lipidic dialkynylcarbinols in 99% ee from racemic mixtures.317
2-Phenylpropionic acid was esterified by N435 in bio-based solvents (e.g., p-cymene) in a continuous flow reactor, enabling its kinetic resolution.318 Racemic octahydroindolizine (indolizidine) was resolved using N435 to produce (7R, 8aS)-octahydro-5,5-dimethylindolizin-7-amine and (7S, 8aS)-octahydro-5,5-dimethylindolizin-7-ol 9, amine.319 The resolution of (R,S)-1-(4-chlorophenyl)ethylamine was achieved employing N435.320 The target unreacted product (S)-1-(4-chlorophenyl)ethylamine was obtained with an ee of 99% after a conversion of 52%. N435 was used as an example of the use of CO2-expanded bio-based liquids.321 The model reaction was the resolution of rac-1-adamantylethanol via esterification, which failed using standard solvents but gave very good results using CO2-expanded methyltetrahydrofuran (enantiospecificity was 200).
Transesterification is perhaps the most popular strategy. N435 was the optimal catalyst in the resolution of (±)-1-methyl-3-phenylpropylamine (almost absolute specificity) using methyl benzoate as an activated acyl donor.322 N435 was used in the enantioselective resolution of (R,S)-α-methyl-4-pyridinemethanol via transesterification.323 The kinetic resolution of racemic-2-pentanol using vinyl butyrate as a co-substrate was investigated, comparing several enzymes.324 N435 gave 50% conversion and 99% enantiomeric excess of (S)-2-pentanol after only 30 min. The kinetic resolution of (R,S)-α-tetralol via transesterification with vinyl acetate catalyzed by N435 was carried out in a packed-bed and a stirred-tank batch bioreactor.325 While the continuous-flow packed-bed reactor needed a residence time of only 3 minutes to reach a 50% conversion for (R)-α-tetralol, after 8 h the conversion obtained using the stirred-tank batch reactor was 43.6%, although in both cases eep ≥ 99.99% was achieved. Enantioresolution of 1-phenylethanol in reaction with corn germ oil (E > 1000) has been performed using N435 in supercritical carbon dioxide with a conversion near 90%.326 3-(RS)-Hydroxy-2-methylenebutanenitrile was resolved using several lipases via transesterification, and N435 offered the best results.327
In some instances, two lipases allowed access to both enantiomers. The opposite enantioselectivities of N435 lipase and lipase AK in the acetylation reaction of (2,6,6-trimethyltetrahydro-2H-pyran-2-yl)methanol have permitted the production of the two enantiomeric forms of the alcohol.328 In other cases, a metal-enzymatic combocatalysis was proposed. Pd/C and N435 were used in the dynamic kinetic resolution of 1,1,1-trifluoroisopropylamine, optimized via the response surface methodology to give a conversion higher than 95% under optimum conditions.329
In other instances, the enantioselectivity of the enzyme was exploited using prochiral substrates. For example, cryptocaryalactones were chemically produced after Novozym-435 enantioselective hydrolysis of the prochiral anti-ethyl-(E)-2-(2,2-dimethyl-6-styryl-1,3-dioxan-4-yl)acetate.330
N435 was used in the regioselective hydrolysis 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl hydroxybenzoate or 2,3,4-tri-O-acetyl-α-D-xylopyranosyl hydroxybenzoate.331 Using β-D-glucopyranosyl hydroxybenzoates, the hydrolysis involves C-4 and C-6 positions; if β-D-xylopyranosyl hydroxybenzoate is the substrate, the deacetylation takes place at the C-4 position. In another paper, 92% yield of isosorbide-2-acetate was obtained via N435 catalyzed hydrolysis of isosorbide-2,5-diacetate, thanks to the high regioselectivity of the biocatalyst.332 N435 was the best among the studied lipases for the hydrolysis of the C-6′ acetoxy group of macrolactonic sophorolipid.333 This product was later acetylated.
Glucose was modified with palmitic acid via esterification catalyzed by N435 in different ionic liquids with a 77% yield in the best case.334 Xylose caproate has been produced by esterification catalyzed by N435 (yield 64%).335 Direct esterification of methyl glucoside with fatty acids has been studied using N435 with ionic liquids or deep eutectic solvents as reaction media.336 1,6-Di-O-octanomannitol was produced with a purity of 90% using N435 as a catalyst of the esterification reaction between mannitol and octanoic acid in a reaction medium composed of acetone and n-hexane.337 N435 catalyzed the acylation of flavonoid glycosides from bamboo leaves with oleic acid, giving isoorientin-6′′-oleate and isovitexin-6′′-oleate.338 N435 catalyzed the selective production of 5-O-acetyl-4-C-hydroxymethyl-1,2-O-isopropylidene-α-D-ribo- and xylofuranose which can be utilized for the convergent synthesis of two different types of bicyclic nucleosides.339 Moreover, N435 has been utilized to produce glucosyl monoester surfactants using N-fatty acyl amino acid and D-glucose.340 N435 has been used to produce 2′,3′,5′-tri-O-acetyl-4′-C-p-toluenesulfonyloxymethyl-β-D-xylofuranosylthymine and 2′,3′,5′-tri-O-acetyl-4′-C-p-toluenesulfonyloxymethyl-β-D-xylofuranosyluracil was used to produce C-4′-spiro-oxetano-α-L-ribonucleosides.341 Three citrus fruit-derived flavonoids (grapefruit extract, naringin, and neohesperidin dihydrochalcone) have been esterified with different fatty acids (e.g., omega-3 polyunsaturated fatty acids obtained from fish oil) in a reaction catalyzed by N435.342 The conversions were over 85%, and the modification was in the primary alcohol of the glucose moiety of the flavonoids. Different chain length saturated fatty acids were used to acylate cyanidin-3-O-galactoside using N435, with the best results obtained using lauric acid.343 The product was identified as cyanidin-3-O-(6′′-dodecanoyl)galactoside. Quercetin-3-O-β-D-glucopyranoside was acylated using phenyl propanoate, phenyl acetate, benzoate and cinnamate vinyl esters with N435, which failed when using hydroxyaromatic acids, but with good results in the other cases.344,345 In another research effort, quercetin-3-O-glucoside and phloretin-2′-glucoside were regioselectively esterified with several fatty acids under sonication employing N435.346 12-Vinyl dodecanedioate-23-O-silybin was regioselectively produced using N435.347 6-O-Acylglucose esters have been produced by N435 catalyzed esterifications between D-glucose and seven different fatty acid vinyl esters which were used as emulsifiers.348 N435 was one of the few biocatalysts with activity in the production of (3R,6R)-6-acetoxy-7-hydroxylinalool or (3R,6S)-6-acetoxy-7-hydroxylinalool via esterification of 6,7-dihydroxy-linalool stereoisomers.349
Transesterification has also been used for this purpose. D-Xylose and L-arabinose lauryl mono- and diesters have been produced using N435 and the transesterification reaction was performed in an organic medium. The reaction used vinyl laurate and L-arabinose or D-xylose.350 Using L-arabinose, a 57% overall yield of one monoester and one diester was achieved. Using D-xylose, a 74.9% global yield of modified products was achieved, but the reaction regioselectivity was lower and two monoesters and two diesters were synthesized.350 Modification of lactulose with vinyl laurate was studied with 10 lipases.351 N435 modified mainly the 1-O-position, while Lipozyme TL-IM and Lipozyme RM-IM mainly modified the 6-O-position. The regioselective synthesis of 3-O-acyl monoester lutein using N435 and vinyl propionate or vinyl stearate as activated acyl donors has been also successfully reported.352 N435 was found to be the most efficient lipase to catalyze the production of Agave fructans mono- and diacylated with lauric acid via a transesterification reaction.353 1,3-Di-O-benzyl-myo-inositol was selectively acetylated using vinyl acetate.354 When Lipozyme RM-IM and Lipozyme TL-IM were utilized, l-(+)-6-O-acetyl-1,3-di-O-benzyl-myo-inositol was obtained. However, N435 produced the non-chiral 5-O-acetylated product.354 Fluorescent glycolipids were produced using vinyl esters and functionalized sugars employing N435 as a catalyst.355
Modification of glycerol is also performed, usually via glycerolysis of esters of the target acid. Glycerolysis of phenolic acid ethyl esters under ultrasound irradiation and in a solvent-free system was used to get monoglyceryl phenolic acids using N435 (yields over 97%).356 Glyceryl monocaffeate has been produced by glycerolysis of ethyl caffeate catalyzed by N435, and after optimization, the yields were over 95%.357
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.358 This group later studied the use of supercritical fluids in this reaction, with lower yields and molecular size (around 60 wt% and 33
000.358 This group later studied the use of supercritical fluids in this reaction, with lower yields and molecular size (around 60 wt% and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).359 Another paper shows the polymerization of ε-caprolactone using lipase N435 as a catalyst.360 The successful enzymatic copolymerizations via ring opening of ε-thiocaprolactone and ε-caprolactone catalyzed by N435 have been reported.361 The polyester poly(ω-pentadecalactone) was synthesized using N435 and a bifunctional initiator/chain transfer agent, with ω-pentadecalactone as the substrate.362 Polycaprolactone was produced via enzymatic ring-opening polymerization. Irgacure-2959 was utilized as the nucleophilic initiator and N435 as the catalyst.363 N435 exhibited better activity than NS 88011 (a commercial product also from CALB) in the production of aliphatic polyesters using globalide and ω-pentadecalactone as substrates, although the length of the polymer was smaller.364 Poly(ε-caprolactone) has been synthesized via ring-opening polymerization of ε-caprolactone catalyzed by N435 in ionic liquids.365
000 g mol−1).359 Another paper shows the polymerization of ε-caprolactone using lipase N435 as a catalyst.360 The successful enzymatic copolymerizations via ring opening of ε-thiocaprolactone and ε-caprolactone catalyzed by N435 have been reported.361 The polyester poly(ω-pentadecalactone) was synthesized using N435 and a bifunctional initiator/chain transfer agent, with ω-pentadecalactone as the substrate.362 Polycaprolactone was produced via enzymatic ring-opening polymerization. Irgacure-2959 was utilized as the nucleophilic initiator and N435 as the catalyst.363 N435 exhibited better activity than NS 88011 (a commercial product also from CALB) in the production of aliphatic polyesters using globalide and ω-pentadecalactone as substrates, although the length of the polymer was smaller.364 Poly(ε-caprolactone) has been synthesized via ring-opening polymerization of ε-caprolactone catalyzed by N435 in ionic liquids.365
Other polymers are based on 2,5-furandicarboxylic acid. For example, 2,5-furandicarboxylic acid-based semi-aromatic polyamides have been produced by polycondensation of dimethyl 2,5-furandicarboxylate and aliphatic diamines of diverse lengths, catalyzed by N435.366 The best results were obtained using 1,8-octanodiamine. 2,5-Furandicarboxylic acid-based semi-aromatic polyamides have been produced via polymerization of dimethyl 2,5-furandicarboxylate catalyzed by N435.367
In other instances, glycerol based polymers are produced. For example, N435 was used to produce polyglycerol fatty acid esters of different-chain-length fatty acids in a solvent-free system.368 In another instance, poly-(glycerol adipate) was produced from divinyl adipate and glycerol by N435 catalyzed condensation, controlling the branching of the polymer with the temperature.369
Sugar-based polymers may also be found. Sugar-poly-ethylene glycol amphiphilic copolymers were synthesized using N435 as a catalyst for the transesterification, introducing decanoic and myristic acids.370D-Fructose (99%) and D-glucose (34%) were modified with 2,2,2-trifluoroethyl methacrylate in tert-butanol using N435, and methacryloyl-D-fructose was polymerized using as the crosslinker ethylene glycol dimethacrylate.371
Glycerolysis of certain oils are also important to get monomers useful in polymer production. For instance, andiroba oil was subject to glycerolysis catalyzed by N435 to produce a polyol in a tubular fixed bed reactor using t-butanol as the reaction medium (monoacylglycerol yield was over 65%).372 This was used later for polyurethane foam production. In another study, enzymatic glycerolysis of castor oil catalyzed by N435 in a solvent-free system gave a mixture of mono and diglycerides that were used in the stabilization and synthesis of poly(urea-urethane) nanoparticles via miniemulsion polymerization.373 Later, a polyurethane foam was produced by this research group using mono- and diacylglycerols obtained by the glycerolysis of castor oil (64% yield).374 A polymer was prepared from glycerol and oleic di-acid using N435 or classical thermo-chemical methods.375 The enzyme polymerized product was found to be more biodegradable than the chemical one.
However, the range of materials used to produce polymers using N435 is very wide. Trimethylolpropane, 1,8-octanediol, and adipic acid were first pre-polymerized via the automatic catalytic effect of the reactants themselves to obtain an appropriate reaction substrate mixture.376 Dimer acid cyclocarbonate was produced by the N435 catalyzed esterification of glycerol carbonate and dimer acid from Sapium sebiferum oil.377 This compound could be used in the synthesis of bio-based non-isocyanate polyurethane ions. Chiral poly(ester amide) polymers were produced using N435, and the polymer successively presented hydroxyhexanoic and aspartate acids.378 The reactions started using L or DN-(6-hydroxyhexanoyl) aspartate diesters, and methyl or benzyl ester groups at the α or β-carbonyl positions of the aspartic acid.
N435 has been used also to modify some polymers, mainly starch. For example, N435 was used to modify starch with myristic acid to alter its physicochemical properties.379 In another paper from the same group, N435 was utilized in the production of myristic acid starch ester in a solvent-free system.380 The modified starch exhibited good hydrophobicity and emulsion stability, and the gel strength was reduced. In another paper, lauric acid was esterified with starch using ionic liquids as reaction media and N435 as a catalyst, in order to improve the starch hydrophobicity.381 Octenyl succinic anhydride starch production in ionic liquids using N435 has been also reported.382
Novozym 4435 does not always afford the best results. For example, CALB immobilized on polypropylene beads was compared to N435 in the polymerization of dimethyl adipate and 1,4-butanediol (BDO) using an optimized preparation.383 In this case, higher molecular weight polyesters (4 kDa versus 3.1 kDa) were obtained using the home-made catalyst.
In other cases, N435 was combined with a chemo-catalyst. For example, this biocatalyst, the 1,5,7-triazabicyclo [4.4.0] dec-5-ene biocatalyst and an organocatalyst were employed in the polymerizations of ε-caprolactone, δ-valerolactone, L-lactide and trimethylene carbonate.384 N435 did not work using L-lactide while the organocatalyst had low activity using caprolactone. The enzyme and organocatalyst were combined in an assembled tandem microreactor system, producing different well-defined triblock copolymers.384
Finally, N435 can be used to degrade some polymers. As an example, N435 was used in the successfully degradation of poly (butylene succinate-co-diethylene glycol succinate) and poly (butylene succinate-co-butylene diglycolic acid).385
Novozym has been shown in the last few years to exhibit some of these promiscuous activities. For example, the Morita–Baylis–Hillman reaction between 2, 4-dinitrobenzaldehyde and cyclohexenone was catalyzed by N435 with isonicotinamide as a necessary co-catalyst and β-cyclodextrin as an additive to improve the enzyme activity with a yield of 43.4% in 2 days.392 In another example, the amidation of anilines with 1,3-diketones via C–C bond cleavage has been reported using also N435 as a catalyst.393 The yields ranged from 64.3% to 96.2%, retaining more than 80% of the initial yield after seven reuses.
Lipase-mediated Dakin reactions have been reported. A broad variety of hydroxylated benzaldehydes were oxidized with high yields (from 90% to 97%) using N435, which could be reutilized in 10 cycles while maintaining its activity intact.394
Epoxidation of unsaturated oils is one of the most popular promiscuous reactions catalyzed by lipases249,395 and this reaction has been also studied using N435. In this reaction, the lipase is responsible for the perhydrolysis of the oil using as the nucleophile hydrogen peroxide, forming a peracid that is actually responsible for the epoxidation reaction. For example, a research study shows how different lipases were assayed in the chemoenzymatic epoxidation of Karanja oil and N435 was found to be the most efficient one (epoxide conversion of 80%,) but hydrogen peroxide compromises the biocatalyst reuse due to enzyme inactivation.396 In a similar way, monoepoxidated linoleic acid was produced by employing N435 with a reaction yield of 82.14%.397 N435 epoxidation of acid sunflower oil was improved by introducing butyric acid as an active oxygen carrier (reaching an oxirane conversion of 96.4 ± 3.0%).398,399 Finally, ultrasonic irradiation was used for enhancing N435 activity in the epoxidation of soybean oil.400 A relative percentage conversion to oxirane oxygen of 91.22% was achieved within 5 h. The lipase was reused six times to produce epoxidized soybean oil. The functionalization of lignin from Organosolv and Kraft pulping processes to obtain oxirane rings was analyzed using N435 as a catalyst for the peroxidation of caprylic acid to peroxycaprylic acid (90% yield).138 This peracid reacted with the unsaturated C–C bonds to form the oxirane ring, with a yield of 55% after optimization.
3.2. Food technology: glyceride modifications and production
Thus, the hydrolysis of waste cooking oil under solvent-free conditions was performed using N435 under ultrasound irradiation to produce free fatty acids.401 After 2 h, a yield of 75.19% was obtained. Novozym-435 and Lipozyme TL-IM lipases were used to hydrolyze anhydrous milk fat and anhydrous buffalo milk fat and to enhance the flavor of milk, with N435 giving the higher production of butanoic and hexanoic acids.402
In another paper, virgin coconut oil (very rich in lauric acid and myristic acid) was subjected to glycerolysis catalyzed by N435 to produce mono- and diacylglycerols (MAGs and DAGs) to reinforce its antibacterial functionality.403 Another example is the hydrolysis of triacylglycerols from anhydrous milk catalyzed by Novozym-435 to decrease the percentage of two short or medium chain fatty acids while the percentage of triglycerides with at least two long-chain fatty acids (CN 44–54) enhanced the melting and crystallization profiles of the product.404 In another instance, hydrolysis of oils by using some organic co-solvents produced a monophasic system that reached 88% using N435, while using Lipozyme TL-IM or Lipozyme RM-IM the reaction reached only 66%.405
N435 and Thermomyces lanuginosus lipase were compared in the hydrolysis of anhydrous milk cow fat or anhydrous buffalo milk fat using ultrasonic microwave-assisted extraction to eliminate short chain fatty acids.406 N435 produced a significant decrease of triglycerides with short-chain fatty acids, altering the melting point of the products.
N435 was also used in the glycerolysis of ratfish liver oil to produce bioactive lipid carriers with potential self-emulsifying properties.408 The same group reported later that the N435 catalyzed glycerolysis of ratfish liver oil allowed the process to be studied in a pilot plant, showing a catalyst half-life of 145 h (enzyme activity cannot be fully restored by hexane washings), and that glycerolysis of triacylglycerol was 1.5 times faster than that of diacylglycerol.409 N435 was used for the glycerolysis of n-3 polyunsaturated fatty acid-rich ethyl oil as the first step in a new two-step process designed for the production of pure triacylglycerols enriched in n-3 polyunsaturated fatty acids.410 Diacylglycerol-enriched soybean oil was produced via glycerolysis of soybean oil catalyzed by N435 in a solvent-free system using a modified bubble column reactor.411 Almost 50% diacylglycerol content was obtained and the enzyme can be reused in 10 cycles. Sardine oil was subjected to glycerolysis to produce mono and diglycerides rich in unsaturated fatty acids using supercritical CO2 and organic solvents.412 47.6% of monolaurin was produced by glycerolysis of methyl laurate, with N435 being more efficient for this reaction than the lipase from Aspergillus oryzae.413
Polyunsaturated fatty acids in the form of 2-monoacylglycerols were prepared by ethanolysis catalyzed by N435 (yield of 27% of 2-monoacylglycerols).414 Low temperature crystallization allowed 90% of 2-monoacylglycerol to be obtained, while molecular distillation gave a polyunsaturated fatty acid concentration of 72% while decreasing the content of 2-monoacylglycerols to 69.81%.
Soybean oil and ethanol were used in a reaction catalyzed by N435 to produce 2-monoacylglycerols and then, after esterification again catalyzed by N435 (yield around 65%) with acetic acid, a low energy lipid was produced having 55% of the energy of the initial oil.415 The molecular distillation gave 94.3% purity of the desired low energy oil. The synthesis of 2-docosahexaenoylglycerol was performed via ethanolysis of algal oil using several lipases, with N435 being the most efficient biocatalyst and giving 27–31% of monoglycerides. In this case the catalyst could be reused for 7 cycles without any significant inactivation.416
Monoacylglycerols rich in ω-3 polyunsaturated fatty acids were obtained by glycerolysis of sardine oil catalyzed by N435 (67% MAGs) and further purified via short path distillation.417 In another example, the performances of N435, Lipozyme TL-IM, and Lipozyme RM-IM in producing 2-monoacylglycerols rich in ω-3 polyunsaturated fatty acids (PUFAs) via ethanolysis of supercritical carbon dioxide extracted Pacific oyster oil were compared.418 N435 gave a yield of 43.03%, very similar to that obtained using Lipozyme TL-IM (45.95%).
In another case, short- and medium-chain 1,3-diacylglycerols were synthesized as products with very low-calorie features via transesterification reactions between short- and medium-chain fatty acid ethyl esters and glycerol.419 Different enzymes were assayed and N435 did not need the previous adsorption of glycerol on silica gel to form acylglycerols and gave a yield just behind that of Lipozyme RM-IM (52 versus 60.7%). Using N435, the reaction rate can be increased by adding 1% (w/w) of lecithin.
In some cases, N435 is combined with other lipases to achieve the objective. Ethanolysis of low-grade fish oil was subsequently performed using Novozyme NS 81006 and N435, to flexibly produce fatty acid ethyl esters or concentrated polyunsaturated fatty acids.420 First, most of the fatty acid glycerides were transformed into ethyl esters (yield of 70–80%), while less than 20% of docosahexaenoic fatty acids were modified using Novozyme NS 81006. Using molecular distillation to eliminate the esters, the amount of glycerides containing polyunsaturated fatty acids increased from ∼18% of crude fish oil to 34%. A second ethanolysis step catalyzed by N435 converted these glycerides to ethyl esters with 80–100% yield.420
In other reactions, N435 was not the best catalyst. For example, N435, Lipozyme TL-IM, Lipozyme RM-IM, and Lipase DF were evaluated in the synthesis of 2-monoacylglycerol enriched in omega-3 polyunsaturated fatty acids in supercritical carbon dioxide using salmon frame bone via ethanolysis.421 In this instance, Lipozyme TL-IM showed the highest activity. Also, in the glycerolysis of lard, Lipozyme RM-IM worked better than N435.422
Eicosapentaenoic and docosahexaenoic acid enriched fish oil triacylglycerols were prepared by a two-step process.423 Using AY “Amano” 400SD, fish oil was partially hydrolyzed, increasing the content of the target acids in the acylglycerols from 19.30% and 13.09 wt% to 25.95 wt% and 22.06 wt%, respectively. Subsequently, N435 was used in a transesterification reaction of the product with a stock enriched in eicosapentaenoic and docosahexaenoic ethyl esters. The final products prepared presented more than 95% of triacylglycerols, with high content of eicosapentaenoic and docosahexaenoic acids (28.20% and 25.61%, respectively).423
Caprylic acid and glycerol were esterified via ultrasound-assisted intensification, comparing Lipozyme RM-IM and N435; both enzymes showed their (yields just under 95%) applicability and were reused for 10 cycles.424 Caprylic acid/polyunsaturated fatty acids/caprylic acid structured lipids were synthesized via esterification of omega-3 concentrate fatty acids with dicaprylic glycerol catalyzed by N435.425 N435 was used to improve the camellia seed oil quality by esterification of the free fatty acids of the oil with epicatechin (the main products were epicatechin oleate and epicatechin palmitate).426
In another paper, medium chain fatty acids from different sources and glycerol were used to produce structured mono- and diacylglycerols using N435, Lipozyme RM-IM and Lipozyme TL-IM as catalysts.427 N435 gave the highest incorporation of free fatty acids into glycerol (90% conversion of medium chain fatty acids into glycerol was obtained in 30 min). Diacylglycerols were rapidly synthesized in a solvent-free system via esterification of glycerol with a palm oil deodorizer distillate and 40 wt% oleic acid using N435 in a bubble column reactor.428 The content of diacylglycerols was near 60%, while around 25% of monoglycerols and less than 3% of free fatty acids were found in the products.
N435 has been described as the most efficient catalyst in the esterification of glycerophosphorylcholine and conjugated linoleic acid.429 However, an immobilized mutant lipase (MAS1-H108A) improved the results from 70 mol% to 89.10 mol%. In another paper, the esterification of glycerol and caprylic acid in supercritical carbon dioxide catalyzed by N435 and Lipozyme RM-IM has been studied.430 N435 exhibited better performance, with a conversion of free fatty acids to tricaprylin of 97.3% in 6 h reaction time at 50 °C; 15 reuses had a small effect on the enzyme activity.
In some cases, several lipases are sequentially used to get the desired product. For example, a stearidonic acid rich triacylglycerol was produced via hydrolysis, followed by a two-step lipase-catalyzed esterification under vacuum, each catalyzed by a lipase.431 A stearidonic acid rich stock was obtained by the hydrolysis of echium oil by using Candida rugosa lipase. For the esterification, N435 was used to esterify the stearidonic acid rich stock with glycerin and later, Lipozyme TL-IM continued this esterification. This gave an 86.4% yield. In another example, triglycerides enriched in n-3 polyunsaturated fatty acids were prepared using a multi-step process. N435 was used to esterify n-3 polyunsaturated fatty acids and glycerol and these partial glycerides were subjected to hydrolysis using an immobilized lipase from Malassezia globosa.432
Linoleic, conjugated linoleic and pinolenic acids were esterified, using N435 as a catalyst, with a solvent-free system to prepare triacylglycerols with anti-obesity effects. A triglyceride content of 98.9% was obtained.433 N435 showed pronounced selectivity to pinolenic acid > conjugated linoleic acid > linoleic acid.
In some cases, other components of the oils are used in the esterification to get the target products. For example, esterification of tyrosol and hydroxytyrosol extracted from olive mill wastewater with various fatty acids (caprate, laurate, and palmitate) catalyzed by N435 was successfully performed and shown to be efficient to avoid lipid oxidation.434
For example, different oil blends have been interesterified. Thus, different lipases (Lipozyme RM-IM and N435) were used in the interesterification of mixtures of lard and rapeseed oil containing 35 and 25% of lard.436 The reaction was performed faster and at higher temperature using N435 but the Sn-2 and Sn-1,3 distributions of the product were nearly random when N435 was used, while Lipozyme RM-IM did not modify the Sn-2 position. In another research study, the increase in omega-3 content at the Sn-2 position of high oleic sunflower and sardine oil via enzymatic interesterification was studied using Lipozyme TL-IM and N435, with Lipozyme TL-IM being more adequate.437 N435 stood out from other catalysts (solid acid, sodium hydroxide and methoxide) in the production of low trans margarine fat analogs by interesterification of soybean oil and fully hydrogenated palm oil.438 Medium-chain triacylglycerol rich structured lipids were synthesized by lipase-catalyzed interesterification of ARASCO with medium-chain triacylglycerols, comparing four commercial immobilized lipases, with N435 being the most efficient one.439 Glycolipids were produced using α-chloralose and various vinyl esters as substrates and N435 as a catalyst.440
In other cases, oils and simple free fatty acid esters have been used. For instance, medium and long chain triacylglycerols were synthesized in a solvent-free system by interesterification of soybean oil with medium chain esters using N435 as a catalyst.441 In another case, menhaden oil and ethyl caprate were used to produce structured lipids using two different lipases as catalysts. Results were better than those using a free acid.442 N435 (almost 31%) gave better results than Lipozyme RM-IM (almost 20%). Feruloylated shea butter and feruloylated coconut oil were produced by interesterification of vegetable oil/fat with ethyl ferulate employing N435 as a catalyst in a packed-bed bioreactor.443
As in all other reactions, N435 did not always present the best properties. A blend of palm stearin and vegetable oil was interesterified to enhance the plastic range, comparing N435 and Lipozyme TL-IM.444 In this case, the Lipozyme TL-IM product favored more the formation of β′ crystals.
In some cases, combi-lipases have been used (see the biodiesel section for the concept of combi-lipases). High Sn-2 docosahexaenoic and arachidonic acid oils were produced independently via enzymatic interesterification of Sn-2 docosahexaenoic and arachidonic acid oil rich single cell oils using a mixture of immobilized lipases, Lipozyme TL-IM and N435.445
Thus, moringa oil (formed mainly long chains) was subjected to acidolysis with different medium chain fatty acids in supercritical CO2, comparing the performance of N435 and Lipozyme RM-IM.446 N435 gave the highest yield (63.2%). The biocatalyst could be reused for 15 cycles.
Acidolysis of camellia oil by lauric acid revealed that N435 was nearly non-selective due to its susceptibility to solvent systems (the enzyme was more selective in hydrolysis in aqueous medium).447
Human milk fat substitutes with four types of n-3 fatty acids for infant formula were produced via acidolysis of Nannochloropsis oculata rich oil by free fatty acids from Isochrysis galbana in a solvent-free SYSTEM using N435, TL-IM and RM-IM as biocatalysts.448 A product containing a total of n-3 PUFAs of 13.92–17.12 wt% in the Sn-2 position under optimal conditions could be obtained using N435 and Lipozyme TL-IM.
In another research effort, castor oil was reacted with caffeic acid to produce castor oil-based caffeoyl structured lipids with a conversion and yield of monoglycerides bearing caffeic acid of near 95%.449
Menhaden oil and capric acid were used to produce structured lipids using two different lipases as catalysts.442 N435 gave a yield of 28.63 mol%, while Lipozyme RM-IM gave a yield of only 9.81 mol% of incorporation of capric acid.
In another case, oleic acid and corn oil were reacted in a bubble column reactor system using N435 as a catalyst, to produce highly unsaturated glycerides; the final product contained 46.67 wt% of monoglycerides and 35.56 wt% of diglycerides.450 This treatment decreased the oil crystallization rate significantly.
The synthesis of conjugated linoleic acid partial glycerides was performed using different lipase commercial preparations. N435 offered much better results than Lipozyme RM-IM or Lipozyme TL-IM.451 Results could be improved by using other immobilization techniques, and the lipase from R. miehei immobilized on another hydrophobic support became more active and selective than N435.
Citronellic acid was used in an acidolysis process of egg-yolk phosphatidylcholine using five commercially available immobilized lipases as biocatalysts.67 The best results were achieved using N435, with 33% yield of a phospholipid fraction enriched with citronellic acid in the Sn-1 position (39% incorporation in this fraction).
As in other cases, N435 is not always the recommended catalyst. For example, Lipozyme TL-IM, Lipozyme RM-IM and N435 were compared in the synthesis of caprylic or capric acids/long chain fatty acids/caprylic or capric acid triglycerides via batch acidolysis in solvent-free medium.452 As an oil substrate, grapeseed oil (rich in linoleic acid) was utilized. The best results were obtained using capric acid and Lipozyme RM-IM.
3.3. Energy: biodiesel production
Similar to all lipases, N435 has been intensively used in biodiesel production.12,72–74,145–147 Here, we have collected some of the most representative examples since 2016.For example, biodiesel production from Ceiba pentandra oil using N435 was optimized to reach a 78.0% yield via stepwise addition of 9-fold methanol excess, although a decrease to less than 70% was observed after only 3 cycles.453 In another research study, ethanolysis of triglycerides in a solvent-free reaction medium catalyzed by N435 as a biocatalyst was studied, trying to understand the acyl migration that results in 100% yield.454 The paper shows that long-chain fatty acids with unsaturation have limitations in their access to the active site of the lipase. Triolein ethanolysis was performed in a fixed-bed reactor operated in circulating batch mode using N435.455 Triolein was also methanolyzed by N435 using dimethyl ether (DME) as the reaction medium in a batch reactor and a continuous pipe reactor.456 In another example, oil obtained from the seeds of Manilkara zapota (L.) was trans-esterified with methanol, comparing different lipases as biocatalysts, with the best results achieved using N435.457 Novozym-435 gave 96% biodiesel yield after 12 h, but the activity decreased progressively (after 6 cycles, the yield was only 72%). However, the activity can be recovered by incubating it in soybean oil, 2-butanol or tert-butanol. In another research effort, N435 was also employed to analyze the advantages of a micro packed-bed reactor based on a two-parallel-plate configuration to produce biodiesel.458
In another investigation, Eruca sativa oil was used as a substrate in a comparison between N435 and Aspergillus niger lipase, obtaining a much higher yield using N435 (98.3% versus 56.4%).459 N435 was also used to produce biodiesel from degummed crude palm oil and optimized by the response surface methodology.460 Ethanolysis of babassu oil catalyzed by N435 gave yields over 98% in a fluidized bed reactor.461 N435 gave promising values of 85 and 76% of biodiesel from waste cooking oil and M. circinelloides oil, respectively.462 Fish oil was transformed into biodiesel and enriched in polyunsaturated fatty acids by methanolysis catalyzed by NS 81006 followed by hydrolysis by N435.463
Different lipases were compared in biodiesel production using sunflower oil and methanol.464 Although N435, Lipozyme TL-IM and Lipozyme 62350 showed similar reaction rates, N435 was more stable and gave the highest yields. Biodiesel from crude Citrullus colocynthis oil and methanol was produced in tert-butanol with a yield of 97.8% using N435.465
No standard oils have been used for biodiesel production using N435. For example, black soldier fly larvae fat and methanol were used to produce biodiesel, comparing different lipases, and N435 showed the highest activity.466 Using the response surface methodology, the process was optimized, giving a biodiesel yield of over 96%. N435 could be reused 20 times, decreasing the biodiesel yield to 92.5%. Using this oil, methyl acetate was proposed to obtain biodiesel via interesterification catalyzed by N435 with a yield of almost 97%.467 This avoided methanol enzyme inactivation and the biocatalyst could be used 20 cycles while maintaining the activity. In order to avoid methanol lipase inactivation, in another study N435 was utilized as a successful model to produce biodiesel from soybean oil by drip-feeding of methanol, obtaining a yield as high as 98.75%.468 Spent coffee oil was also used to produce biodiesel, comparing many different biodiesels, with Novozym-435 offering better results (around 96%).469
In another case, microalgal oil extracted from Nannochloropsis gaditana was used, comparing N435 and Rhizopus oryzae in tert-butanol medium.470 The highest reaction rate was obtained using the least polar lipid content. The same group using the same algae and N435 showed direct transesterification (in the presence of the biomass) in tert-butanol.471 A biodiesel yield of more than 99% was achieved and the product was recovered by hexane extraction. However, the enzyme activity rapidly decreased. Another microalgae oil, extracted from Aurantiochytrium sp., KRS101, was also used in biodiesel production catalyzed by N435, taking advantage of the high concentration of free fatty acids to produce a biodiesel free of glycerol with 89.5% conversion under optimal conditions.472 Glycerol was undetectable in the biodiesel.
In a very interesting work, waste cooking oil and dimethyl carbonate (DMC) were used as reactants and N435 as a catalyst to simultaneously produce biodiesel and glycerol carbonate as a very interesting by-product.473,474 The enzyme maintained 88% of its activity after six reaction cycles.
Not only has a suitable biodiesel been produced using N435, but also some biodiesel additives. For example, an n-butyl oleate ester using N435 in a stirred basket reactor was produced with a yield of 98%, as a biodiesel additive.475
As N435 is regarded as a highly effective biocatalyst in biodiesel production, N435 is used to show the advantages of new preparations. Sometimes the new preparations seem to offer better possibilities. For example, a polyacrylonitrile (PAN) hollow membrane was activated with nitrile-click chemistry and treated with sodium alginate and CaCl2. The immobilized enzyme was 2.5 fold more active than N435.476
Ionic liquids have been also used to produce biodiesel using N435.480 The paper established some rules on the effect of ionic liquids on enzyme activity. In another case, N435 was used with zwitter-type ionic liquids as a cocatalyst to improve the reaction rate using sunflower oil, obtaining a 64% biodiesel yield.481 Using the same system with a slurry of whole-cell Chlorella zofingiensis in water as a substrate, the biodiesel yield reached up to 16%. The causes of this decrease are discussed in the paper.
In another example, but where the enzymes are not mixed, a very acidic and heterogeneous (having monoglycerides, diglycerides and triglycerides) oil from rice bran oil soapstock in a solvent-free system has been transformed into biodiesel, showing that the successive use of N435, Lipozyme TL-IM or Lipozyme RM-IM and Lipozyme TL-IM resulted in similar or higher levels of yield of the individual lipases (around 92%).488
4. Problems of N435
Thus far, N435 has a strong history of success in diverse applications, and after many years it has remained among the most used biocatalysts at least by academic researchers.132–152 However, this seemingly “golden” biocatalyst has some serious problems that in many instances are ignored.First, there is a general problem that is common to all biocatalysts prepared using interfacial activation of lipases versus supports bearing a hydrophobic surface: the enzyme may be released from the support at high temperature, in the presence of organic co-solvents, detergents, etc.210 And in many instances, the substrates/products of the lipases are detergent-like molecules (e.g., fatty acids, partial glycerides, and phospholipids)211 (Fig. 8).
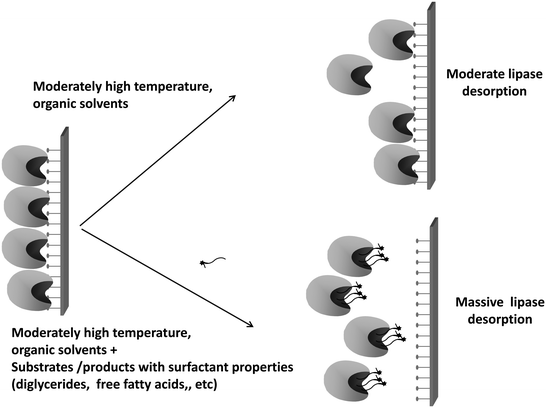 | ||
| Fig. 8 Lipase release from the support is favored in the presence of detergent-like substrates or products. | ||
The other problems are a direct consequence of some negative specific features of the utilized support, the Lewatit support. And some of them are so serious that there should be thoughtful consideration before selecting N435 as an industrial catalyst.
The first problem is that even though the surface of Lewatit is hydrophobic enough to permit the immobilization of CALB via interfacial activation, it is relatively hydrophilic. As such, it can retain hydrophilic by-products (e.g., water in esterifications and glycerin in biodiesel production), producing an apparent enzyme inactivation due to the formation of glycerin or water layers inside the biocatalyst.236
The second problem is related to the fragility of the particles of the support under stirring. This may produce problems with the filters of the system.
However, the most important problem is something reported many years ago but did not have the relevance that this issue deserves: the support may be dissolved in some media (that is, the crosslinking may not be efficient enough).489,490 This implies that not only may the enzyme be released from the support, but also the polymeric components of the support could be incorporated to the reaction media, contaminating the product.
Now, we will go in more detail into each of these problems, suggesting some solutions for each of them.
4.1. Enzyme leaching
Since 2002, there have been some papers alerting us to the risks of enzyme “leaching”.131,249,491,492 This obviously affects the studies on the operational stability and reuses of N435, as well as the implementation of N435 on a large scale. The reasons for this enzyme leakage are general to all lipases immobilized using interfacial activation versus hydrophobic surfaces, not specific to N435 (Fig. 8). Although the enzyme–support interaction is very strong122 due to the very large size of the hydrophobic pocket of the lipase, a high temperature may weaken this interaction and result in the release of the enzyme to the medium.210 Usually, the enzyme immobilized by this strategy is much more stable than the free enzyme and even multipoint covalently immobilized lipases,193,493 and it is not clear if the released enzyme molecules are previously inactivated or become inactivated after the enzyme release, but the fact is that it is possible to observe the decrease of the enzyme on the support and the appearance of the enzyme in the supernatant (Fig. 8).210In a similar way, organic co-solvents may weaken the enzyme–support hydrophobic interactions and facilitate enzyme release. As such, while in thermal inactivation the lipases immobilized via interfacial activation tend to be more stable than the covalently immobilized enzymes, in the presence of organic co-solvents the situation is reversed and the covalently immobilized enzyme becomes more stable.193,493 The problem is accentuated if the reaction involves detergent-like compounds.211 These compounds need not necessarily be as long as free fatty acids or di- or monoglycerides. It has been shown that even dibutyrin or diacetin may greatly facilitate the lipase release from hydrophobic supports.212,213
N435 has all these problems. However, the U. K. Food Standards Agency did not find any detectable contamination from N435 on simulated food (containing isooctane and 95% (v/v) ethanol) and diverse acrylic materials.494
However, this enzyme release under certain conditions can be considered a proved fact. The incubation of N435 in dimethyl sulfoxide resulted in the quantitative release of the enzyme.495 Using N435, Chen et al.249 have reported that enzyme leaching from N435 can become a serious problem for the application of this biocatalyst in the production of pharmaceutical compounds, and that enzyme leaching becomes a serious problem for enzyme reuse.253 Using N435 in the production of polymers, the enzyme leakage was again reported to be a real problem.496 Enzyme release from N435 was also reported in the solvent-free esterification of polyglycerol-3 and related compounds, due to their surfactant features.491 Enzyme release even in its first use has been reported in oil chemistry497 and polymer production.383
Enzyme release from N435 in organic solvents and ionic liquids has been also reported to be a real problem, as “active” traces of the active material were released from the catalyst and hindered the control of the reaction (because some reactions continued after eliminating N435)498,499 Thus, this enzyme release has been in fact considered as one of the main problems for the industrial implementation of N435.500
4.2. Support solubility in organic media
The solubility of a support in any reaction media may become a serious problem. The pioneering investigations regarding the possibility of solubility of Lewatit from N435 in some media started from some of the coauthors of this review, who observed that the operational stability of the biocatalyst in the enantiospecific esterification of ibuprofen in ethanol was much lower than expected.501 The research pointed that the support became solubilized in the presence of some solvents such as ethanol.489,490 They detected that the mass of the polymer in the biocatalyst decreased and that polymethacrylate and divinylbenzene, components of Lewatit, could be found dissolved in the medium. The problems persisted even in aqueous/ethanol mixtures. Later, this effect was also found using other alcohols.502,503Table 1 shows the main conclusions of these studies. In fact, it is not possible to fully discard that part of the protein decrease may be due to the dissolution of the support in the reaction medium.
| Alcohol | Global mass loss (%) | Protein loss (%) | % alcohol adsorbed |
|---|---|---|---|
| Methanol | 11.6 | 1.93 | 2.12 |
| Ethanol | 16.6 | 1.27 | 3.81 |
| 1-propanol | 5.9 | 0.57 | 4.04 |
| 2-propanol | 1.2 | 0.79 | 3.68 |
This is a very serious problem, as the polymer can go to the medium, be incorporated to the product, and produce serious problems in all the reactor operation and the recovery of the products. However, this serious problem has scarce impact in the literature.
The adsorption of alcohols on Novozym-435 was analyzed in some detail by programed thermic desorption.503 The study revealed a very strong physical adsorption, but the existence of dimethyl ether and propylene suggested the possibility of chemisorption, which can produce methoxy and propoxy species that may be dehydrated on acid active sites, inactivating the enzyme.
The modification of the support was also studied using environmental scanning electron microscopy; the exposure of N435 to alcohols decreased the fractal value and increased the minimal cell size, showing the internal modification of the biocatalyst porous structure.503,504 Later, the same methodologies were used to explain the operational stability of N435 in biodiesel production, showing that at 65 °C some polymers from the support could be found in the product (mainly if water was continuously eliminated from the medium). In fact, the size of the particles of the biocatalyst increased when incubated in biodiesel for 2 minutes (from an average diameter of 539 μm to 626 μm).
4.3. Support mechanical fragility
Another problem of Lewatit is its mechanical fragility under stirring.33,505 This fragility does not seem to be very relevant at the laboratory scale, where the immobilized enzyme may be recovered in operational stability studies using centrifugation and the reaction may be performed in a beaker, but becomes a great problem at the industrial level.At the academic level, this fragility may have confusing results, for example if the reaction has reduced activity due to a very high activity that results in diffusion limitations of the substrate. When the catalyst starts to break down, the size of the particles will decrease and the diffusion limitation problems will decrease. As such, apparent “hyperactivation” or a higher stability of the biocatalyst may be found by this artifact.32
At the industrial level, this may be a critical point, as the production of fine powders can block the filters of the reactor and result in the necessity to discard the biocatalyst even if it is fully active. Fig. 9 shows the fine powder production under stirring using N435.
 | ||
| Fig. 9 Images of N435 (a) before and (b) after 6 h under magnetic stirring at 30 °C and 700 rpm in an esterification reaction in hexane. | ||
Therefore, this may be a very important negative feature when considering the use of N435 in mechanically stirred batch reactors, although it may be solved using other stirred reactor configurations which are less aggressive with the mechanical structure of the beads (e.g., vortex reactors).506–508
4.4. Retention of hydrophilic compounds
Many uses of lipases are in anhydrous media (e.g., hydrophobic solvents) and during reaction some hydrophilic compounds may be released. This can lead to the accumulation of these compounds in the biocatalyst,509 which will be more hydrophilic than the reaction medium in many instances, mainly after being coated with the very hydrophilic molecules of lipase.510For example, in esterification, water is a side-product of the reaction.511 In many instances, water is adsorbed on molecular sieves to shift the equilibrium to the direction of the synthesis (this is a thermodynamically controlled synthesis).512,513
However, if the water formation is more rapid than the diffusion, and the support pores or matrix is more hydrophilic than the reaction medium (imagine that the medium is octane), water can be accumulated inside the biocatalyst forming an aqueous phase.152 This way, acids can also be accumulated in this environment, exposing the enzyme to very low pH values and promoting enzyme inactivation by these drastic conditions.151 Moreover, water accumulation may make the thermodynamics of the process unfavorable in the enzyme proximity, reducing the enzyme activity.
In a transesterification reaction to produce biodiesel, glycerin may be the problematic side-product that can form a glycerin phase.514,515 If this occurs, this will hinder the access of the hydrophobic substrate to the enzyme, and it can also capture water as it is a hygroscopic material. Moreover, glycerin, as a likely nucleophile in the deacylation of the acyl enzyme, may compete with methanol, or ethanol.
As such, Lewatit, even having a moderate hydrophilicity, causes some problems on the use of N435 in esterification/biodiesel production, studied in detail only in some specific cases.236 Some solutions to this problem will be presented later.
In other cases, a perhydrolysis is desired,83,516 and hydrogen peroxide is able to impair the enzyme activity by diverse reasons.517–519 Although N435 is very stable even in 1 M hydrogen peroxide,520 the use of a more hydrophobic support was found to be favorable to force a partition of this deleterious compound.521
5. Some solutions to N435 problems
5.1. Enzyme leakage prevention
The leakage of enzymes adsorbed by interfacial activation is a general problem of immobilized lipases (Fig. 9).211–213 The most obvious solution is to cross-link the enzymes to prevent enzyme desorption. This has been tried in a covalent way using glutaraldehyde495,522 or aldehyde dextran (Fig. 10).218,523 A simpler solution is by physically cross-linking the proteins using ionic polymers like polyethylenimine or dextran sulfate (Fig. 10).214,215,524,525 A combination of polyethylenimine coating and glutaraldehyde cross-linking has also proved to be efficient.216 Now, to release the enzyme from the support, the cross-linked proteins must be simultaneously released from the support, which is much more difficult.These strategies have been also utilized in the case of N435. In fact, the treatment of the biocatalyst with polyethylenimine or glutaraldehyde has an unexpected effect: in biodiesel production using N435 with camelina oil, a deposit can be found in the N435 preparation which cannot be found after modification.526–528 Intermolecular glutaraldehyde cross-linking is not efficient enough to fully prevent enzyme release from the support.491,529
The use of heterofunctional supports, adding some ions or chemically reactive groups, is another strategy that proved to be efficient with some hydrophobic supports.210,219–222,225–227,241,530 However, this has not been tried using N435, as the enzyme is already in the support and modifying the support may be problematic.
5.2. Biocatalyst mechanical fragility
The biocatalyst fragility (Fig. 9) is mainly relevant on the industrial scale. To avoid the support breakage, together with using stirring methods which are less aggressive towards the support physical integrity, one of the strategies is the coating of the biocatalyst with some rigid cover that may avoid the Lewatit breakage. For example, N435 was covered with silicone and this allowed support destruction to be almost fully avoided under vigorous stirring (Fig. 11).531 Furthermore, this treatment also reduces enzyme leaching from the support, if the biocatalyst is fully closed in a silicone matrix.531 The biocatalyst maintained more than 90% of its activity after silicone coating.532 In another instance, the silicone-coated biocatalyst was used in a complex chemo-enzymatic epoxidation reaction in a three-phase system.533 The biocatalyst kept 50% of the activity after 5 days in 5 mM hydrogen peroxide. In another research study, the syntheses of poly(ethylene glycol) 400-coconut fatty acid monoester, myristyl myristate and propylene oxide copolymer-oleic acid, and ethylene oxide diester were studied with N435 and N435 treated with silicone.534 The turnover numbers increased by a factor of up to 50.534 The treatment did not alter the specificity of the enzyme; this way the enantiospecific acylation of racemic 1-phenylethanol with vinyl butyrate retains the excellent resolution.534 Later the coating was performed in a fluidized reactor and the Pd catalyst necessary for silicone polymerization was removed.535It should be expected that similar protection effects may be found using other materials used to trap some weak biocatalysts, like the crosslinked enzyme aggregates trapped in silicates,536–538 in sol–gel539 or in LentiKats (polymers of polyvinyl alcohol).540
5.3. Hydrophilic compound adsorption and support dissolution
These points can have a more complex solution. Water or glycerin accumulation on Lewatit may be partially reduced using ultrasound and molecular sieves.44,152,541–550 However, the moderate hydrophilicity of the support may complicate the understanding of the results and reuses of the biocatalyst.The dissolution in organic solvents should be related to an inadequate crosslinking in the polymerization step. This could be (at least partially) solved after some modification of the biocatalyst which can prevent the release of polymer fragments, perhaps in a similar way to the prevention of the enzyme release. However, there are no studies in this regard.
Thus, the solution to these problems may be quite complex when keeping Lewatit as a support. The alternative is to change the immobilization matrix, using some hydrophobic ones that can be more resistant to solvent dissolution and that may be more hydrophobic to prevent water accumulation.150,236,551,552
However, N435 has some special features,252 perhaps due to the existence of some acid groups in the matrix that make this a peculiar biocatalyst. It must also be considered that even if the immobilization follows an interfacial adsorption mechanism, CALB properties may be altered by changing the support hydrophobicity, internal morphology, etc.185,191,259,553 We can assume the idea that a hydrophobic support may be convenient for CALB immobilization, purification and stabilization209 A hydrophobic support that is intended to be an alternative for Lewatit should show some critical features. First, the support must not be dissolved in any media (that is, a suitable crosslinking must be performed) and it must be hydrophobic enough to reduce the adsorption of hydrophilic compounds (and perhaps allow a stronger enzyme adsorption), without affecting CALB stability. Exhibiting a high mechanical resistance will also be a desirable feature. Finally, it should keep or improve the high enzyme loading, stability and versatility that N435 has shown even with these significant problems. Moreover, it should have an adequate particle size and particle size distribution, a competitive price and also, offer some solutions towards disposal (even if immobilization is reversible, support breakage may be promoted that the reuse may no longer be convenient, mainly if the enzyme is very stable and can be used for months). If the support is compatible with some heterofunctionality that permits reduction in enzyme leaching, this will be an advantage to be considered.
Although there are many hydrophobic supports in the market,191,234,247 a systematic study of all these properties has not been performed (perhaps because academically it is not very interesting). Until that moment, the successful history of N435 will very likely continue.
6. Conclusions
This review shows that N435, despite being a very successful biocatalyst with applications in many different areas, has some very relevant problems that in some cases may be avoided using relatively simple techniques (e.g., enzyme leaching and mechanical fragility may be solved by encapsulation of the biocatalyst in silicone), while others may be much more intricate to work around, like the dissolution of the support in certain media. As N435 is mainly used in chemistry, and in these cases the final product is usually fully purified and/or crystalized, perhaps this is not a key problem at the industrial level, except that it may interfere in product purification. However this may be a critical problem in food modification, where the contaminant will be incorporated to the food. Whatever the reason, even with this problem, which was already described many years ago, N435 remains as one of the most used biocatalysts. The best solution may be to change the current support to another one with fewer problems, or to improve the crosslinking step of Lewatit (this may both reduce support dissolution and increase its mechanical resistance). However, it seems that for some reason, Lewatit VP OC 1600 and CALB are a dynamic duo that may be difficult to alter, even when nowadays there are many new alternative hydrophobic supports that have been reported to improve CALB loading, activity and stability. Price and simplicity may be among the points that make replacing Lewatit VP OC 1600 by some more suitable supports difficult. Whatever the case, it seems evident that the success of N435 will carry on at least into the near future.Conflicts of interest
There are not conflicts to declare.Acknowledgements
We gratefully recognize the financial support from MINECO from the Spanish Government (project number CTQ2017-86170-R, Colciencias, Ministerio de Educación Nacional, Ministerio de Industria, Comercio y Turismo e ICETEX, Convocatoria Ecosistema Científico – Colombia Científica. Fondo Francisco José de Caldas, Contrato RC-FP44842-212-2018 and Colciencias (Colombia) (project number FP44842-076-2016), Generalitat Valenciana (PROMETEO/2018/076), FAPERGS (project number 17/2551-0000939-8), CONICET (R. Argentina), FUNCAP (project number BP3-0139-00005.01.00/18) and ANPCyT (PICT 2015-0932 and PICT CABBIO 4687). We acknowledge Dr Daniel Sánchez (PLAPIQUI, R. Argentina) for the photographs before and after stirring of Novozym435.References
- R. A. Sheldon, Metrics of Green Chemistry and Sustainability: Past, Present, and Future, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- J. Li, J. Albrecht, A. Borovika and M. D. Eastgate, Evolving Green Chemistry Metrics into Predictive Tools for Decision Making and Benchmarking Analytics, ACS Sustainable Chem. Eng., 2018, 6, 1121–1132 CrossRef CAS.
- P. Anastas and N. Eghbali, Green Chemistry: Principles and Practice, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr. and R. J. Linderman, et al., Key green chemistry research areas - A perspective from pharmaceutical manufacturers, Green Chem., 2007, 9, 411–420 RSC.
- N. Ran, L. Zhao, Z. Chen and J. Tao, Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale, Green Chem., 2008, 10, 361–372 RSC.
- S. Sen and J. E. Puskas, Green polymer chemistry: Enzyme catalysis for polymer functionalization, Molecules, 2015, 20, 9358–9379 CrossRef CAS PubMed.
- V. S. Ferreira-Leitão, M. C. Cammarota, E. C. G. Aguieiras, L. R. V. de Sá, R. Fernandez-Lafuente and D. M. G. Freire, The protagonism of biocatalysis in green chemistry and its environmental benefits, Catalysts, 2017, 7, 9 CrossRef.
- A. Schmid, J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts and B. Witholt, Industrial biocatalysis today and tomorrow, Nature, 2001, 409, 258–268 CrossRef CAS PubMed.
- D. J. Pollard and J. M. Woodley, Biocatalysis for pharmaceutical intermediates: the future is now, Trends Biotechnol., 2007, 25, 66–73 CrossRef CAS PubMed.
- M. T. Reetz, Biocatalysis in organic chemistry and biotechnology: Past, present, and future, J. Am. Chem. Soc., 2013, 135, 12480–12496 CrossRef CAS PubMed.
- J. M. Woodley, New opportunities for biocatalysis: making pharmaceutical processes greener, Trends Biotechnol., 2008, 26, 321–327 CrossRef CAS PubMed.
- A. Robles-Medina, P. A. González-Moreno, L. Esteban-Cerdán and E. Molina-Grima, Biocatalysis: Towards ever greener biodiesel production, Biotechnol. Adv., 2009, 27, 398–408 CrossRef CAS PubMed.
- R. N. Patel, Synthesis of chiral pharmaceutical intermediates by biocatalysis, Coord. Chem. Rev., 2008, 252, 659–701 CrossRef CAS.
- H. E. Schoemaker, D. Mink and M. G. WubboLts, Dispelling the Myths--Biocatalysis in Industrial Synthesis, Science, 2003, 299, 1694–1697 CrossRef CAS PubMed.
- I. Castilla, D. Woods, F. J. Reen and F. O'Gara, Harnessing Marine Biocatalytic Reservoirs for Green Chemistry Applications through Metagenomic Technologies, Mar. Drugs, 2018, 16, 227 CrossRef PubMed.
- C. Peña-García, M. Martínez-Martínez, D. Reyes-Duarte and M. Ferrer, High throughput screening of esterases, lipases and phospholipases in mutant and metagenomic libraries: A review, Comb. Chem. High Throughput Screening, 2016, 19, 605–615 CrossRef.
- D. A. Cowan, J.-B. Ramond, T. P. Makhalanyane and P. De Maayer, Metagenomics of extreme environments, Curr. Opin. Microbiol., 2015, 25, 97–102 CrossRef CAS PubMed.
- L. Fernández-Arrojo, M.-E. E. Guazzaroni, N. López-Cortés, A. Beloqui and M. Ferrer, Metagenomic era for biocatalyst identification, Curr. Opin. Biotechnol., 2010, 21, 725–733 CrossRef PubMed.
- M. Tuffin, D. Anderson, C. Heath and D. A. Cowan, Metagenomic gene discovery: How far have we moved into novel sequence space?, Biotechnol. J., 2009, 4, 1671–1683 CrossRef CAS PubMed.
- M. Ferrer, A. Beloqui, K. N. Timmis and P. N. Golyshin, Metagenomics for mining new genetic resources of microbial communities, J. Mol. Microbiol. Biotechnol., 2008, 16, 109–123 CrossRef PubMed.
- C. Vieille and G. J. Zeikus, Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability, Microbiol. Mol. Biol. Rev., 2001, 65, 1–43 CrossRef CAS PubMed.
- U. T. Bornscheuer and M. Pohl, Improved biocatalysts by directed evolution and rational protein design, Curr. Opin. Chem. Biol., 2001, 5, 137–143 CrossRef CAS PubMed.
- K. L. Morley and R. J. Kazlauskas, Improving enzyme properties: When are closer mutations better?, Trends Biotechnol., 2005, 23, 231–237 CrossRef CAS PubMed.
- N. J. Turner, Directed evolution drives the next generation of biocatalysts, Nat. Chem. Biol., 2009, 5, 567–573 CrossRef CAS PubMed.
- J. R. Cherry and A. L. Fidantsef, Directed evolution of industrial enzymes: An update, Curr. Opin. Biotechnol., 2003, 14, 438–443 CrossRef CAS PubMed.
- P. A. Romero and F. H. Arnold, Exploring protein fitness landscapes by directed evolution, Nat. Rev. Mol. Cell Biol., 2009, 10, 866–876 CrossRef CAS PubMed.
- V. G. H. Eijsink, S. Gåseidnes, T. V. Borchert and B. van den Burg, Directed evolution of enzyme stability, Biomol. Eng., 2005, 22, 21–30 CrossRef CAS PubMed.
- F. H. Arnold and A. A. Volkov, Directed evolution of biocatalysts, Curr. Opin. Chem. Biol., 1999, 3, 54–59 CrossRef CAS PubMed.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Improvement of enzyme activity, stability and selectivity via immobilization techniques, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- R. A. Sheldon, Enzyme immobilization: The quest for optimum performance, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- O. Barbosa, C. Ortiz, Á. Berenguer-Murcia, R. Torres, R. C. Rodrigues and R. Fernandez-Lafuente, Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts, Biotechnol. Adv., 2015, 33, 435–456 CrossRef CAS PubMed.
- R. C. Rodrigues, C. Ortiz, Á. Berenguer-Murcia, R. Torres and R. Fernández-Lafuente, Modifying enzyme activity and selectivity by immobilization, Chem. Soc. Rev., 2013, 42, 6290–6307 RSC.
- C. Garcia-Galan, Á. Berenguer-Murcia, R. Fernandez-Lafuente and R. C. Rodrigues, Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance, Adv. Synth. Catal., 2011, 353, 2885–2904 CrossRef CAS.
- D. Brady and J. Jordaan, Advances in enzyme immobilisation, Biotechnol. Lett., 2009, 31, 1639–1650 CrossRef CAS PubMed.
- D. N. Tran and K. J. Balkus, Perspective of recent progress in immobilization of enzymes, ACS Catal., 2011, 1, 956–968 CrossRef CAS.
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop and R. A. Wahab, An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes, Biotechnol. Biotechnol. Equip., 2015, 29, 205–220 CrossRef CAS PubMed.
- U. Guzik, K. Hupert-Kocurek and D. Wojcieszynska, Immobilization as a strategy for improving enzyme properties- Application to oxidoreductases, Molecules, 2014, 19, 8995–9018 CrossRef PubMed.
- A. A. Homaei, R. Sariri, F. Vianello and R. Stevanato, Enzyme immobilization: An update, J. Chem. Biol., 2013, 6, 185–205 CrossRef PubMed.
- R. K. Singh, M. K. Tiwari, R. Singh and J.-K. Lee, From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes, Int. J. Mol. Sci., 2013, 14, 1232–1277 CrossRef PubMed.
- M. T. Reetz, Lipases as practical biocatalysts, Curr. Opin. Chem. Biol., 2002, 6, 145–150 CrossRef CAS PubMed.
- P. Villeneuve, J. M. Muderhwa, J. Graille and M. J. Haas, Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches, J. Mol. Catal. B: Enzym., 2000, 9, 113–148 CrossRef CAS.
- R. Zechner, R. Zimmermann, T. O. Eichmann, S. D. Kohlwein, G. Haemmerle and A. Lass, et al., FATSIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling, Cell Metab., 2012, 15, 279–291 CrossRef CAS PubMed.
- R. C. Rodrigues and M. A. Z. Ayub, Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil, Process Biochem., 2011, 46, 682–688 CrossRef CAS.
- J. K. Poppe, C. R. Matte, R. Fernandez-Lafuente, R. C. Rodrigues and M. A. Z. Ayub, Transesterification of Waste Frying Oil and Soybean Oil by Combi-lipases Under Ultrasound-Assisted Reactions, Appl. Biochem. Biotechnol., 2018, 186, 576–589 CrossRef CAS PubMed.
- J. K. Poppe, C. R. Matte, V. O. de Freitas, R. Fernandez-Lafuente, R. C. Rodrigues and M. A. Z. Ayub, Enzymatic synthesis of ethyl esters from waste oil using mixtures of lipases in a plug-flow packed-bed continuous reactor, Biotechnol. Prog., 2018, 34, 952–959 CrossRef PubMed.
- H. Qiao, F. Zhang, W. Guan, J. Zuo and D. Feng, Optimisation of combi-lipases from Aspergillus niger for the synergistic and efficient hydrolysis of soybean oil, Anim. Sci. J., 2017, 88, 772–780 CrossRef CAS.
- J. K. Poppe, C. R. Matte, M. D. C. R. Peralba, R. Fernandez-Lafuente, R. C. Rodrigues and M. A. Z. Ayub, Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases, Appl. Catal., A, 2015, 490, 50–56 CrossRef CAS.
- J. S. Alves, N. S. Vieira, A. S. Cunha, A. M. Silva, M. A. Z. Ayub and R. Fernandez-Lafuente, et al., Combi-lipase for heterogeneous substrates: a new approach for hydrolysis of soybean oil using mixtures of biocatalysts, RSC Adv., 2014, 4, 6863–6868 RSC.
- F. Tavares, J. Petry, P. R. Sackser, C. E. Borba and E. A. Silva, Use of castor bean seeds as lipase source for hydrolysis of crambe oil, Ind. Crops Prod., 2018, 124, 254–264 CrossRef CAS.
- N. Matuoog, K. Li and Y. Yan, Thermomyces lanuginosus lipase immobilized on magnetic nanoparticles and its application in the hydrolysis of fish oil, J. Food Biochem., 2018, 42, e12549 CrossRef.
- S. Sun, J. Liu and X. Li, A novel and rapid method for fatty acid preparation by the lipase-catalyzed hydrolysis of Phoenix tree seeds, 3 Biotech., 2018, 8, 403 CrossRef PubMed.
- C.-H. Su, H. C. Nguyen, M. L. Nguyen, P. T. Tran, F.-M. Wang and Y.-L. Guan, Liquid lipase-catalyzed hydrolysis of gac oil for fatty acid production: Optimization using response surface methodology, Biotechnol. Prog., 2018, 34, 1129–1136 CrossRef CAS PubMed.
- D. Goswami, J. K. Basu and S. De, Lipase applications in oil hydrolysis with a case study on castor oil: A review, Crit. Rev. Biotechnol., 2013, 33, 81–96 CrossRef CAS PubMed.
- K. Ramani, L. John Kennedy, M. Ramakrishnan and G. Sekaran, Purification, characterization and application of acidic lipase from Pseudomonas gessardii using beef tallow as a substrate for fats and oil hydrolysis, Process Biochem., 2010, 45, 1683–1691 CrossRef CAS.
- E. C. G. Aguieiras, D. S. N. de Barros, R. Fernandez-Lafuente and D. M. G. Freire, Production of lipases in cottonseed meal and application of the fermented solid as biocatalyst in esterification and transesterification reactions, Renewable Energy, 2019, 130, 574–581 CrossRef.
- H. Kim, N. Choi, Y. Kim, H.-R. Kim, J. Lee and I.-H. Kim, Immobilized lipase-catalyzed esterification for synthesis of trimethylolpropane triester as a biolubricant, Renewable Energy, 2019, 130, 489–494 CrossRef CAS.
- M. Huemmer, S. Kara, A. Liese, I. Huth, J. Schrader and D. Holtmann, et al., Synthesis of (−)-menthol fatty acid esters in and from (−)-menthol and fatty acids - novel concept for lipase catalyzed esterification based on eutectic solvents, Mol. Catal., 2018, 458, 67–72 CrossRef CAS.
- S. Cebrián-García, A. M. Balu, A. García and R. Luque, Sol-gel immobilisation of lipases: Towards active and stable biocatalysts for the esterification of valeric acid, Molecules, 2018, 23, 2283 CrossRef PubMed.
- A. Foukis, O. A. Gkini, P.-Y. Stergiou and E. M. Papamichael, New insights and tools for the elucidation of lipase catalyzed esterification reaction mechanism in n-hexane: The synthesis of ethyl butyrate, Mol. Catal., 2018, 455, 159–163 CrossRef CAS.
- P. Y. Stergiou, A. Foukis, M. Filippou, M. Koukouritaki, M. Parapouli and L. G. Theodorou, et al., Advances in lipase-catalyzed esterification reactions, Biotechnol. Adv., 2013, 31, 1846–1859 CrossRef CAS PubMed.
- P. Pires-Cabral, M. M. R. da Fonseca and S. Ferreira-Dias, Esterification activity and operational stability of Candida rugosa lipase immobilized in polyurethane foams in the production of ethyl butyrate, Biochem. Eng. J., 2010, 48, 246–252 CrossRef.
- C. A. Palla and M. E. Carrín, Kinetics modeling of the acidolysis with immobilized Rhizomucor miehei lipases for production of structured lipids from sunflower oil, Biochem. Eng. J., 2014, 90, 184–194 CrossRef CAS.
- S. M. Abed, X. Zou, A. H. Ali, Q. Jin and X. Wang, Synthesis of 1,3-dioleoyl-2-arachidonoylglycerol-rich structured lipids by lipase-catalyzed acidolysis of microbial oil from Mortierella alpina, Bioresour. Technol., 2017, 243, 448–456 CrossRef CAS PubMed.
- S. M. Abed, W. Wei, A. H. Ali, S. A. Korma, A. H. Mousa and H. M. Hassan, et al., Synthesis of structured lipids enriched with medium-chain fatty acids via solvent-free acidolysis of microbial oil catalyzed by Rhizomucor miehei lipase, LWT--Food Sci. Technol., 2018, 93, 306–315 CrossRef.
- A. Chojnacka and W. Gładkowski, Production of Structured Phosphatidylcholine with High Content of Myristic Acid by Lipase-Catalyzed Acidolysis and Interesterification, Catalysts, 2018, 8, 281 CrossRef.
- R. Sneha and T. Jeyarani, Lipase-catalysed acidolysis of mango kernel fat with capric acid to obtain medium- and long-chain triacylglycerols, Int. J. Food Sci. Technol., 2018, 53, 1527–1534 CrossRef.
- M. Rychlicka, N. Niezgoda and A. Gliszczyńska, Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Citronellic Acid. New Insight into Synthesis of Isoprenoid-Phospholipids, Molecules, 2018, 23, 314 CrossRef PubMed.
- W. Xie and X. Zang, Immobilized lipase on core–shell structured Fe3O4–MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard, Food Chem., 2016, 194, 1283–1292 CrossRef CAS.
- M. M. Soumanou, M. Pérignon and P. Villeneuve, Lipase-catalyzed interesterification reactions for human milk fat substitutes production: A review, Eur. J. Lipid Sci. Technol., 2013, 115, 270–285 CrossRef.
- J. H. Lee, J. M. Son, C. C. Akoh, M. R. Kim and K.-T. Lee, Optimized synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich triacylglycerol via interesterification catalyzed by a lipase from Thermomyces lanuginosus, New Biotechnol., 2010, 27, 38–45 CrossRef PubMed.
- W. Xie and X. Zang, Covalent immobilization of lipase onto aminopropyl-functionalized hydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles: A magnetic biocatalyst for interesterification of soybean oil, Food Chem., 2017, 227, 397–403 CrossRef CAS PubMed.
- A. Bajaj, P. Lohan, P. N. Jha and R. Mehrotra, Biodiesel production through lipase catalyzed transesterification: An overview, J. Mol. Catal. B: Enzym., 2010, 62, 9–14 CrossRef.
- L. Li, W. Du, D. Liu, L. Wang and Z. Li, Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium, J. Mol. Catal. B: Enzym., 2006, 43, 58–62 CrossRef CAS.
- S. Shah, S. Sharma and M. N. Gupta, Biodiesel preparation by lipase-catalyzed transesterification of Jatropha oil, Energy Fuels, 2004, 18, 154–159 CrossRef CAS.
- N. Choi, D. S. No, H. Kim, B. H. Kim, J. Kwak and J.-S. Lee, et al., In situ lipase-catalyzed transesterification in rice bran for synthesis of fatty acid methyl ester, Ind. Crops Prod., 2018, 120, 140–146 CrossRef CAS.
- I. A. Modenez, D. Sastre, F. C. Moraes and C. M. Netto, Influence of Glutaraldehyde Cross-Linking Modes on the Recyclability of Immobilized Lipase B from Candida antarctica for Transesterification of Soy Bean Oil, Molecules, 2018, 23, 2230 CrossRef.
- S. Zeng, J. Liu, S. Anankanbil, M. Chen, Z. Guo and J. P. Adams, et al., Amide Synthesis via Aminolysis of Ester or Acid with an Intracellular Lipase, ACS Catal., 2018, 8, 8856–8865 CrossRef CAS.
- C.-H. Kuo, J.-A. Lin, C.-M. Chien, C.-H. Tsai, Y.-C. Liu and C.-J. Shieh, Formation of amide bond catalyzed by lipase in aqueous phase for peptide synthesis, J. Mol. Catal. B: Enzym., 2016, 129, 15–20 CrossRef CAS.
- F. Van Rantwijk, M. A. P. J. Hacking and R. A. Sheldon, Lipase-catalyzed synthesis of carboxylic amides: Nitrogen nucleophiles as acyl acceptor, Monatsh. Chem., 2000, 131, 549–569 CrossRef CAS.
- M. C. De Zoete, A. C. Kock-Van Dalen, F. Van Rantwijk and R. A. Sheldon, Lipase-catalysed ammoniolysis of lipids. A facile synthesis of fatty acid amides, J. Mol. Catal. B: Enzym., 1996, 1, 109–113 CrossRef CAS.
- M. Quirós, V. M. Sánchez, R. Brieva, F. Rebolledo and V. Gotor, Lipase-catalyzed synthesis of optically active amides in organic media, Tetrahedron: Asymmetry, 1993, 4, 1105–1112 CrossRef.
- X.-P. Wang, P.-F. Zhou, Z.-G. Li, B. Yang, F. Hollmann and Y.-H. Wang, Engineering a lipase B from Candida antactica with efficient perhydrolysis performance by eliminating its hydrolase activity, Sci. Rep., 2017, 7, 44599 CrossRef CAS.
- D. Méndez-Sánchez, N. Ríos-Lombardía, V. Gotor and V. Gotor-Fernández, Chemoenzymatic epoxidation of alkenes based on peracid formation by a Rhizomucor miehei lipase-catalyzed perhydrolysis reaction, Tetrahedron, 2014, 70, 1144–1148 CrossRef.
- B. P. Dwivedee, S. Soni, M. Sharma, J. Bhaumik, J. K. Laha and U. C. Banerjee, Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update, ChemistrySelect, 2018, 3, 2441–2466 CrossRef CAS.
- S. Guezane-Lakoud, M. Toffano and L. Aribi-Zouioueche, Promiscuous lipase catalyzed a new P-C bond formation: Green and efficient protocol for one-pot synthesis of α-aminophosphonates, Heteroat. Chem., 2017, 28, e21408 CrossRef.
- X. Hua, Y. Xing and X. Zhang, Enhanced Promiscuity of Lipase-Inorganic Nanocrystal Composites in the Epoxidation of Fatty Acids in Organic Media, ACS Appl. Mater. Interfaces, 2016, 8, 16257–16261 CrossRef CAS PubMed.
- D. González-Martínez, V. Gotor and V. Gotor-Fernández, Application of Deep Eutectic Solvents in Promiscuous Lipase-Catalysed Aldol Reactions, Eur. J. Org. Chem., 2016, 2016, 1513–1519 CrossRef.
- W. Li, R. Li, X. Yu, X. Xu, Z. Guo and T. Tan, et al., Lipase-catalyzed Knoevenagel condensation in water-ethanol solvent system. Does the enzyme possess the substrate promiscuity?, Biochem. Eng. J., 2015, 101, 99–107 CrossRef CAS.
- M. Kapoor, A. B. Majumder and M. N. Gupta, Promiscuous Lipase-Catalyzed C-C Bond Formation Reactions between 4 Nitrobenzaldehyde and 2-Cyclohexen-1-one in Biphasic Medium: Aldol and Morita-Baylis-Hillman Adduct Formations, Catal. Lett., 2015, 145, 527–532 CrossRef CAS.
- D. F. Izquierdo, O. Barbosa, M. I. Burguete, P. Lozano, S. V. Luis and R. Fernandez-Lafuente, et al., Tuning lipase B from Candida antarctica C-C bond promiscuous activity by immobilization on poly-styrene-divinylbenzene beads, RSC Adv., 2014, 4, 6219–6225 RSC.
- F. Yang, Z. Wang, H. Wang, H. Zhang, H. Yue and L. Wang, Enzyme catalytic promiscuity: Lipase catalyzed synthesis of substituted 2H-chromenes by a three-component reaction, RSC Adv., 2014, 4, 25633–25636 RSC.
- M. Kapoor and M. N. Gupta, Lipase promiscuity and its biochemical applications, Process Biochem., 2012, 47, 555–569 CrossRef CAS.
- A. Kumar, K. Dhar, S. S. Kanwar and P. K. Arora, Lipase catalysis in organic solvents: Advantages and applications, Biol. Proced. Online, 2016, 18, 1–11 CrossRef PubMed.
- T. Kobayashi and S. Adachi, Reaction equilibrium for lipase-catalyzed condensation in organic solvent systems, Biotechnol. Lett., 2004, 26, 1461–1468 CrossRef CAS PubMed.
- A. Ghanem and H. Y. Aboul-Enein, Lipase-mediated chiral resolution of racemates in organic solvents, Tetrahedron: Asymmetry, 2004, 15, 3331–3351 CrossRef CAS.
- L.-E. Meyer, J. von Langermann and U. Kragl, Recent developments in biocatalysis in multiphasic ionic liquid reaction systems, Biophys. Rev., 2018, 10, 901–910 CrossRef CAS PubMed.
- A. A. Elgharbawy, F. A. Riyadi, M. Z. Alam and M. Moniruzzaman, Ionic liquids as a potential solvent for lipase-catalysed reactions: A review, J. Mol. Liq., 2018, 251, 150–166 CrossRef CAS.
- T. Itoh, Ionic liquids as tool to improve enzymatic organic synthesis, Chem. Rev., 2017, 117, 10567–10607 CrossRef CAS PubMed.
- R. A. Sheldon, Biocatalysis and Biomass Conversion in Alternative Reaction Media, Chem. – Eur. J., 2016, 22, 12984–12999 CrossRef CAS PubMed.
- A. L. B. Dias, P. dos Santos and J. Martínez, Supercritical CO2 technology applied to the production of flavor ester compounds through lipase-catalyzed reaction: A review, J. CO2 Util., 2018, 23, 159–178 CrossRef CAS.
- R. E. Gumba, S. Saallah, M. Misson, C. M. Ongkudon and A. Anton, Green biodiesel production: A review on feedstock, catalyst, monolithic reactor, and supercritical fluid technology, Biofuel Res. J., 2016, 3, 431–447 CrossRef CAS.
- T. Matsuda, Recent progress in biocatalysis using supercritical carbon dioxide, J. Biosci. Bioeng., 2013, 115, 233–241 CrossRef CAS PubMed.
- I. Juneidi, M. Hayyan and M. A. Hashim, Intensification of biotransformations using deep eutectic solvents: Overview and outlook, Process Biochem., 2018, 66, 33–60 CrossRef CAS.
- P. Xu, G.-W. Zheng, M.-H. Zong, N. Li and W.-Y. Lou, Recent progress on deep eutectic solvents in biocatalysis, Bioresour. Bioprocess., 2017, 4, 34 CrossRef PubMed.
- H. Taher and S. Al-Zuhair, The use of alternative solvents in enzymatic biodiesel production: a review, Biofuels, Bioprod. Biorefin., 2017, 11, 168–194 CrossRef CAS.
- E. Durand, J. Lecomte and P. Villeneuve, Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions, Eur. J. Lipid Sci. Technol., 2013, 115, 379–385 CrossRef.
- R. D. Schmid and R. Verger, Lipases: Interfacial enzymes with attractive applications, Angew. Chem., Int. Ed., 1998, 37, 1609–1633 CrossRef CAS.
- A. M. Brzozowski, U. Derewenda, Z. S. Derewenda, G. G. Dodson, D. M. Lawson and J. P. Turkenburg, et al., A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex, Nature, 1991, 351, 491–494 CrossRef CAS PubMed.
- H. Van Tilbeurgh, M. P. Egloff, C. Martinez, N. Rugani, R. Verger and C. Cambillau, Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography, Nature, 1993, 362, 814–820 CrossRef CAS PubMed.
- P. Grochulski, Y. Li, J. D. Schrag, F. Bouthillier, P. Smith and D. Harrison, et al., Insights into interfacial activation from an open structure of Candida rugosa lipase, J. Biol. Chem., 1993, 268, 12843–12847 CAS.
- M. Martinelle, M. Holmquist and K. Hult, On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase, Biochim. Biophys. Acta, Lipids Lipid Metab., 1995, 1258, 272–276 CrossRef.
- C. Chapus, M. Semeriva, C. Bovier-Lapierre and P. Desnuelle, Mechanism of pancreatic lipase action. 1. Interfacial activation of pancreatic lipase, Biochemistry, 1976, 15, 4980–4987 CrossRef CAS.
- M. L. Jennens and M. E. Lowe, A surface loop covering the active site of human pancreatic lipase influences interfacial activation and lipid binding, J. Biol. Chem., 1994, 269, 25470–25474 CAS.
- N. Yaacob, N. Ahmad Kamarudin, A. Leow, A. Salleh, R. Raja Abd Rahman and M. Mohamad Ali, The Role of Solvent-Accessible Leu-208 of Cold-Active Pseudomonas fluorescens Strain A. M.S8 Lipase in Interfacial Activation, Substrate Accessibility and Low-Molecular Weight Esterification in the Presence of Toluene, Molecules, 2017, 22, 1312 CrossRef PubMed.
- C. Cheng, T. Jiang, Y. Wu, L. Cui, S. Qin and B. He, Elucidation of lid open and orientation of lipase activated in interfacial activation by amphiphilic environment, Int. J. Biol. Macromol., 2018, 119, 1211–1217 CrossRef CAS PubMed.
- R. Verger, Interfacial activation of lipases: Facts and artifacts, Trends Biotechnol., 1997, 15, 32–38 CrossRef CAS.
- J. M. Palomo, M. Fuentes, G. Fernández-Lorente, C. Mateo, J. M. Guisan and R. Fernández-Lafuente, General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality, Biomacromolecules, 2003, 4, 1–6 CrossRef CAS PubMed.
- G. Fernandez-Lorente, J. M. Palomo, M. Fuentes, C. Mateo, J. M. Guisan and R. Fernandez-Lafuente, Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties, Biotechnol. Bioeng., 2003, 82, 232–237 CrossRef CAS PubMed.
- L. Wilson, J. M. Palomo, G. Fernández-Lorente, A. Illanes, J. M. Guisán and R. Fernández-Lafuente, Effect of lipase-lipase interactions in the activity, stability and specificity of a lipase from Alcaligenes sp, Enzyme Microb. Technol., 2006, 39, 259–264 CrossRef.
- J. M. Palomo, M. M. Peñas, G. Fernández-Lorente, C. Mateo, A. G. Pisabarro and R. Fernández-Lafuente, et al., Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases, Biomacromolecules, 2003, 4, 204–210 CrossRef PubMed.
- G. Volpato, M. Filice, M. A. Z. Ayub, J. M. Guisan and J. M. Palomo, Single-step purification of different lipases from Staphylococcus warneri, J. Chromatogr. A, 2010, 1217, 473–478 CrossRef CAS PubMed.
- E. A. Manoel, J. C. S. dos Santos, D. M. G. Freire, N. Rueda and R. Fernandez-Lafuente, Immobilization of lipases on hydrophobic supports involves the open form of the enzyme, Enzyme Microb. Technol., 2015, 71, 53–57 CrossRef PubMed.
- J. Uppenberg, N. Oehrner, M. Norin, K. Hult, G. J. Kleywegt and S. Patkar, et al., Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols, Biochemistry, 1995, 34, 16838–16851 CrossRef.
- J. Uppenberg, M. T. Hansen, S. Patkar and T. A. Jones, The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica, Structure, 1994, 2, 293–308 CrossRef CAS PubMed.
- T. Zisis, P. L. Freddolino, P. Turunen, M. C. F. van Teeseling, A. E. Rowan and K. G. Blank, Interfacial Activation of Candida antarctica Lipase B: Combined Evidence from Experiment and Simulation, Biochemistry, 2015, 54, 5969–5979 CrossRef CAS.
- B. Stauch, S. J. Fisher and M. Cianci, Open and closed states of Candida antarctica lipase B: protonation and the mechanism of interfacial activation, J. Lipid Res., 2015, 56, 2348–2358 CrossRef CAS PubMed.
- S. Arana-Peña, Y. Lokha and R. Fernández-Lafuente, Immobilization of Eversa Lipase on Octyl Agarose Beads and Preliminary Characterization of Stability and Activity Features, Catalysts, 2018, 8, 511 CrossRef.
- S. Arana-Peña, Y. Lokha and R. Fernández-Lafuente, Immobilization on octyl-agarose beads and some catalytic features of commercial preparations of lipase a from Candida antarctica (Novocor ADL): Comparison with immobilized lipase B from Candida antarctica, Biotechnol. Prog., 2019, 35, e2735 CrossRef PubMed.
- E. M. Anderson, K. M. Larsson and O. Kirk, One biocatalyst - many applications: The use of Candida antarctica B-lipase in organic synthesis, Biocatal. Biotransform., 1998, 16, 181–204 CrossRef.
- V. Gotor-Fernández, E. Busto and V. Gotor, Candida antarctica lipase B: An ideal biocatalyst for the preparation of nitrogenated organic compounds, Adv. Synth. Catal., 2006, 348, 797–812 CrossRef.
- O. Kirk and M. W. Christensen, Lipases from Candida antarctica : Unique Biocatalysts from a Unique Origin, Org. Process Res. Dev., 2002, 6, 446–451 CrossRef.
- B. Kovács, E. Forró and F. Fülöp, Candida antarctica lipase B catalysed kinetic resolution of 1,2,3,4-tetrahydro-β-carbolines: Substrate specificity, Tetrahedron, 2018, 74, 6873–6877 CrossRef.
- P. Ghahremanifard, N. Rezaeinezhad, G. Rigi, F. Ramezani and G. Ahmadian, Designing a novel signal sequence for efficient secretion of Candida antarctica lipase B in E. coli: The molecular dynamic simulation, codon optimization and statistical analysis approach, Int. J. Biol. Macromol., 2018, 119, 291–305 CrossRef CAS PubMed.
- N. Nyari, A. Paulazzi, R. Zamadei, C. Steffens, G. L. Zabot and M. V. Tres, et al., Synthesis of isoamyl acetate by ultrasonic system using Candida antarctica lipase B immobilized in polyurethane, J. Food Process Eng., 2018, 41, e12812 CrossRef.
- A. Pellis, J. W. Comerford, A. J. Maneffa, M. H. Sipponen, J. H. Clark and T. J. Farmer, Elucidating enzymatic polymerisations: Chain-length selectivity of Candida antarctica lipase B towards various aliphatic diols and dicarboxylic acid diesters, Eur. Polym. J., 2018, 106, 79–84 CrossRef CAS.
- M. P. Pinheiro, N. S. Rios, S. Fonseca T de, A. Bezerra F de, E. Rodríguez-Castellón and R. Fernandez-Lafuente, et al., Kinetic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350, Biotechnol. Prog., 2018, 34, 878–889 CrossRef CAS PubMed.
- A. Wcisłek, A. Sonseca Olalla, A. McClain, A. Piegat, P. Sobolewski and J. Puskas, et al., Enzymatic Degradation of Poly(butylene succinate) Copolyesters Synthesized with the Use of Candida antarctica Lipase B, Polymer, 2018, 10, 688 Search PubMed.
- F. Vásquez-Garay, R. Teixeira Mendonça and S. W. Peretti, Chemoenzymatic lignin valorization: Production of epoxidized pre-polymers using Candida antarctica lipase B, Enzyme Microb. Technol., 2018, 112, 6–13 CrossRef PubMed.
- B. Wang, C. Zhang, Q. He, H. Qin, G. Liang and W. Liu, Efficient resolution of (R,S)-1-(1-naphthyl)ethylamine by Candida antarctica lipase B in ionic liquids, Mol. Catal., 2018, 448, 116–121 CrossRef CAS.
- M. G. Yadav, M. R. Kavadia, R. N. Vadgama, A. A. Odaneth and A. M. Lali, Production of 6-O-l-Ascorbyl Palmitate by Immobilized Candida antarctica Lipase B, Appl. Biochem. Biotechnol., 2018, 184, 1168–1186 CrossRef CAS PubMed.
- K. Zhang, Z. Pan, Z. Diao, S. Liang, S. Han and S. Zheng, et al., Kinetic resolution of sec-alcohols catalysed by Candida antarctica lipase B displaying Pichia pastoris whole-cell biocatalyst, Enzyme Microb. Technol., 2018, 110, 8–13 CrossRef CAS PubMed.
- A. Kundys, E. Białecka-Florjańczyk, A. Fabiszewska and J. Małajowicz, Candida antarctica Lipase B as Catalyst for Cyclic Esters Synthesis, Their Polymerization and Degradation of Aliphatic Polyesters, J. Polym. Environ., 2018, 26, 396–407 CrossRef CAS.
- J. Vázquez-Martínez, E. Nieto-Álvarez, E. Ramírez-Chávez and J. Molina-Torres, Enzymatic Method for N-Acyl Homoserine Lactones Synthesis Using Immobilized Candida antarctica Lipase, Catal. Lett., 2018, 148, 62–67 CrossRef.
- G. D. Yadav and M. P. Kamble, A Green Process for Synthesis of Geraniol Esters by Immobilized Lipase from Candida antarctica B Fraction in Non-Aqueous Reaction Media: Optimization and Kinetic Modeling, Int. J. Chem. React. Eng., 2018, 16, 2017017 Search PubMed.
- Ö. Köse, M. Tüter and H. A. Aksoy, Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium, Bioresour. Technol., 2002, 83, 125–129 CrossRef.
- Y. Watanabe, Y. Shimada, A. Sugihara, H. Noda, H. Fukuda and Y. Tominaga, Continuous production of biodiesel fuel from vegetable oil using immobilized Candida antarctica lipase, J. Am. Oil Chem. Soc., 2000, 77, 355–360 CrossRef.
- Y. Shimada, Y. Watanabe, T. Samukawa, A. Sugihara, H. Noda and H. Fukuda, et al., Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase, J. Am. Oil Chem. Soc., 1999, 76, 789–793 CrossRef CAS.
- C. Wang, N. Wang, X. Liu, P. Wan, X. He and Y. Shang, Expanding Application of Immobilized Candida antarctica Lipase B: A Green Enzyme Catalyst for Knoevenagel Condensation Reaction, Fibers Polym., 2018, 19, 1611–1617 CrossRef CAS.
- M. Woudenberg-Van Oosterom, F. Van Rantwijk and R. A. Sheldon, Regioselective acylation of disaccharides in tert-butyl alcohol catalyzed by Candida antarctica lipase, Biotechnol. Bioeng., 1996, 49, 328–333 CrossRef CAS PubMed.
- N. G. Graebin, A. B. Martins, A. S. G. Lorenzoni, C. Garcia-Galan, R. Fernandez-Lafuente and M. A. Z. Ayub, et al., Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis, Biotechnol. Prog., 2012, 28, 406–412 CrossRef CAS PubMed.
- A. B. Martins, N. G. Graebin, A. S. G. Lorenzoni, R. Fernandez-Lafuente, M. A. Z. Ayub and R. C. Rodrigues, Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology, Process Biochem., 2011, 46, 2311–2316 CrossRef.
- A. B. Martins, M. F. Schein, J. L. R. Friedrich, R. Fernandez-Lafuente, M. A. Z. Ayub and R. C. Rodrigues, Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability, Ultrason. Sonochem., 2013, 20, 1155–1160 CrossRef CAS PubMed.
- F. van Rantwijk, F. Secundo and R. A. Sheldon, Structure and activity of Candida antarctica lipase B in ionic liquids, Green Chem., 2006, 8, 282–286 RSC.
- R. Madeira Lau, M. J. Sorgedrager, G. Carrea, F. van Rantwijk, F. Secundo and R. A. Sheldon, Dissolution of Candida antarctica lipase B in ionic liquids: effects on structure and activity, Green Chem., 2004, 6, 483–487 RSC.
- P. Lozano, T. De Diego, D. Carrié, M. Vaultier and J. L. Iborra, Over-stabilization of Candida antarctica lipase B by ionic liquids in ester synthesis, Biotechnol. Lett., 2001, 23, 1529–1533 CrossRef CAS.
- N. Zhang, W.-C. Suen, W. Windsor, L. Xiao, V. Madison and A. Zaks, Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution, Protein Eng., 2003, 16, 599–605 CrossRef CAS.
- K. Zorn, I. Oroz-Guinea, H. Brundiek, M. Dörr and U. T. Bornscheuer, Alteration of Chain Length Selectivity of Candida antarctica Lipase A by Semi-Rational Design for the Enrichment of Erucic and Gondoic Fatty Acids, Adv. Synth. Catal., 2018, 360, 4115–4131 CrossRef CAS PubMed.
- H. Höck, S. Engel, S. Weingarten, H. Keul, U. Schwaneberg and M. Möller, et al., Comparison of Candida antarctica Lipase B Variants for Conversion of ε-Caprolactone in Aqueous Medium - Part 2, Polymer, 2018, 10, 524 Search PubMed.
- J.-W. Shen, J.-M. Qi, X.-J. Zhang, Z.-Q. Liu and Y.-G. Zheng, Significantly increased catalytic activity of Candida antarctica lipase B for the resolution of cis-(±)-dimethyl 1-acetylpiperidine-2,3-dicarboxylate, Catal. Sci. Technol., 2018, 8, 4718–4725 RSC.
- J. C. Rotticci-Mulder, M. Gustavsson, M. Holmquist, K. Hult and M. Martinelle, Expression in Pichia pastoris of Candida antarctica Lipase B and Lipase B Fused to a Cellulose-Binding Domain, Protein Expression Purif., 2001, 21, 386–392 CrossRef CAS PubMed.
- M. W. Larsen, U. T. Bornscheuer and K. Hult, Expression of Candida antarctica lipase B in Pichia pastoris and various Escherichia coli systems, Protein Expression Purif., 2008, 62, 90–97 CrossRef CAS PubMed.
- J.-K. Yang, L.-Y. Liu, J.-H. Dai and Q. Li, de novo Design and Synthesis of Candida antarctica Lipase B Gene and α-Factor Leads to High-Level Expression in Pichia pastoris, PLoS One, 2013, 8, e53939 CrossRef CAS PubMed.
- E. P. Cipolatti, M. C. C. Pinto, M. Robert J de, T. P. da Silva, C. Beralto T da and J. G. F. Santos, et al., Pilot-scale development of core-shell polymer supports for the immobilization of recombinant lipase B from Candida antarctica and their application in the production of ethyl esters from residual fatty acids, J. Appl. Polym. Sci., 2018, 135, 46727 CrossRef.
- J. M. Robert, F. S. Lattari, A. C. Machado, A. M. de Castro, R. V. Almeida and F. A. G. Torres, et al., Production of recombinant lipase B from Candida antarctica in Pichia pastoris under control of the promoter PGK using crude glycerol from biodiesel production as carbon source, Biochem. Eng. J., 2017, 118, 123–131 CrossRef.
- E. A. Manoel, J. M. Robert, M. C. C. Pinto, A. C. O. Machado, M. D. Besteti and M. A. Z. Coelho, et al., Evaluation of the performance of differently immobilized recombinant lipase B from Candida antarctica preparations for the synthesis of pharmacological derivatives in organic media, RSC Adv., 2016, 6, 4043–4052 RSC.
- M. V. H. Moura, G. P. da Silva, A. C. de O. Machado, F. A. G. Torres, D. M. G. Freire and R. V. Almeida, Displaying Lipase B from Candida antarctica in Pichia pastoris Using the Yeast Surface Display Approach: Prospection of a New Anchor and Characterization of the Whole Cell Biocatalyst. Silman I, editor, PLoS One, 2015, 10, e0141454 CrossRef PubMed.
- C. Bernal, K. Rodríguez and R. Martínez, Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts, Biotechnol. Adv., 2018, 36, 1470–1480 CrossRef CAS PubMed.
- J. D. Cui and S. R. Jia, Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges, Crit. Rev. Biotechnol., 2015, 35, 15–28 CrossRef CAS PubMed.
- S. Velasco-Lozano and F. López-Gallego, Wiring step-wise reactions with immobilized multi-enzyme systems, Biocatal. Biotransform., 2018, 36, 184–194 CrossRef CAS.
- J. Zdarta, A. Meyer, T. Jesionowski and M. Pinelo, A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility, Catalysts, 2018, 8, 92 CrossRef.
- M. Bilal, T. Rasheed, Y. Zhao, H. M. N. Iqbal and J. Cui, Smart chemistry and its application in peroxidase immobilization using different support materials, Int. J. Biol. Macromol., 2018, 119, 278–290 CrossRef PubMed.
- C. Silva, M. Martins, S. Jing, J. Fu and A. Cavaco-Paulo, Practical insights on enzyme stabilization, Crit. Rev. Biotechnol., 2018, 38, 335–350 CrossRef CAS PubMed.
- J. M. Palomo, C. Ortiz, M. Fuentes, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Use of immobilized lipases for lipase purification via specific lipase-lipase interactions, J. Chromatogr. A, 2004, 1038, 267–273 CrossRef PubMed.
- J. M. Palomo, C. Ortiz, G. Fernández-Lorente, M. Fuentes, J. M. Guisán and R. Fernández-Lafuente, Lipase-lipase interactions as a new tool to immobilize and modulate the lipase properties, Enzyme Microb. Technol., 2005, 36, 447–454 CrossRef.
- L. N. de Lima, C. C. Aragon, C. Mateo, J. M. Palomo, R. L. C. Giordano and P. W. Tardioli, et al., Immobilization and stabilization of a bimolecular aggregate of the lipase from Pseudomonas fluorescens by multipoint covalent attachment, Process Biochem., 2013, 48, 118–123 CrossRef.
- M. G. Sánchez-Otero, G. Valerio-Alfaro, H. S. García-Galindo and R. M. Oliart-Ros, Immobilization in the presence of Triton X-100: modifications in activity and thermostability of Geobacillus thermoleovorans CCR11 lipase, J. Ind. Microbiol. Biotechnol., 2008, 35, 1687–1693 CrossRef PubMed.
- M. Filice, M. Marciello, L. Betancor, A. V. Carrascosa, J. M. Guisan and G. Fernandez-Lorente, Hydrolysis of fish oil by hyperactivated Rhizomucor miehei lipase immobilized by multipoint anion exchange, Biotechnol. Prog., 2011, 27, 961–968 CrossRef CAS PubMed.
- D. Weiser, P. L. Sóti, G. Bánóczi, V. Bódai, B. Kiss and Á. Gellért, et al., Bioimprinted lipases in PVA nanofibers as efficient immobilized biocatalysts, Tetrahedron, 2016, 72, 7335–7342 CrossRef CAS.
- G. Fernandez-Lorente, J. M. Palomo, C. Mateo, R. Munilla, C. Ortiz and Z. Cabrera, et al., Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance, Biomacromolecules, 2006, 7, 2610–2615 CrossRef PubMed.
- I. Mingarro, C. Abad and L. Braco, Interfacial activation-based molecular bioimprinting of lipolytic enzymes, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 3308–3312 CrossRef CAS.
- H. Gonzalez-Navarro and L. Braco, Lipase-enhanced activity in flavour ester reactions by trapping enzyme conformers in the presence of interfaces, Biotechnol. Bioeng., 1998, 59, 122–127 CrossRef PubMed.
- P. López-Serrano, L. Cao, F. Van Rantwijk and R. A. Sheldon, Cross-linked enzyme aggregates with enhanced activity: Application to lipases, Biotechnol. Lett., 2002, 24, 1379–1383 CrossRef.
- J. C. S. dos Santos, C. Garcia-Galan, R. C. Rodrigues, H. B. de Sant'Ana, L. R. B. Gonçalves and R. Fernandez-Lafuente, Stabilizing hyperactivated lecitase structures through physical treatment with ionic polymers, Process Biochem., 2014, 49, 1511–1515 CrossRef.
- J. M. Palomo, G. Fernandez-Lorente, C. Mateo, C. Ortiz, R. Fernandez-Lafuente and J. M. Guisan, Modulation of the enantioselectivity of lipases via controlled immobilization and medium engineering: hydrolytic resolution of mandelic acid esters, Enzyme Microb. Technol., 2002, 31, 775–783 CrossRef CAS.
- G. Fernandez-Lorente, Z. Cabrera, C. Godoy, R. Fernandez-Lafuente, J. M. Palomo and J. M. Guisan, Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties, Process Biochem., 2008, 43, 1061–1067 CrossRef CAS.
- J. M. Palomo, G. Fernández-Lorente, C. Mateo, M. Fuentes, R. Fernández-Lafuente and J. M. Guisan, Modulation of the enantioselectivity of Candida antarctica B lipase via conformational engineering. Kinetic resolution of (±)-α-hydroxy-phenylacetic acid derivatives, Tetrahedron: Asymmetry, 2002, 13, 1337–1345 CrossRef CAS.
- G. Fernandez-Lorente, J. M. Palomo, Z. Cabrera, J. M. Guisán and R. Fernández-Lafuente, Specificity enhancement towards hydrophobic substrates by immobilization of lipases by interfacial activation on hydrophobic supports, Enzyme Microb. Technol., 2007, 41, 565–569 CrossRef CAS.
- J. M. Palomo, G. Muñoz, G. Fernández-Lorente, C. Mateo, M. Fuentes and J. M. Guisan, et al., Modulation of Mucor miehei lipase properties via directed immobilization on different hetero-functional epoxy resins: Hydrolytic resolution of (R,S)-2-butyroyl-2-phenylacetic acid, J. Mol. Catal. B: Enzym., 2003, 21, 201–210 CrossRef CAS.
- J. M. Palomo, R. L. Segura, C. Mateo, M. Terreni, J. M. Guisan and R. Fernández-Lafuente, Synthesis of enantiomerically pure glycidol via a fully enantioselective lipase-catalyzed resolution, Tetrahedron: Asymmetry, 2005, 16, 869–874 CrossRef CAS.
- W. G. de Morais Júnior, A. Moura Maia, P. Alves Martins, G. Fernández-Lorente, J. M. Guisán and B. C. Pessela, Influence of different immobilization techniques to improve the enantioselectivity of lipase from Geotrichum candidum applied on the resolution of mandelic acid, Mol. Catal., 2018, 458, 89–96 CrossRef.
- V. G. Tacias-Pascacio, S. Peirce, B. Torrestiana-Sanchez, M. Yates, A. Rosales-Quintero and J. J. Virgen-Ortíz, et al., Evaluation of different commercial hydrophobic supports for the immobilization of lipases: tuning their stability, activity and specificity, RSC Adv., 2016, 6, 100281–100294 RSC.
- J. C. S. dos Santos, N. Rueda, R. Torres, O. Barbosa, L. R. B. Gonçalves and R. Fernandez-Lafuente, Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties, Process Biochem., 2015, 50, 918–927 CrossRef CAS.
- J. C. S. dos Santos, N. Rueda, A. Sanchez, R. Villalonga, L. R. B. Gonçalves and R. Fernandez-Lafuente, Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization, RSC Adv., 2015, 5, 35801–35810 RSC.
- V. R. Moure, C. Fabrício, G. Frensch, F. A. Marques, D. A. Mitchell and N. Krieger, Enhancing the enantioselectivity of the lipase from Burkholderia cepacia LTEB11 towards the resolution of secondary allylic alcohols, Biocatal. Agric. Biotechnol., 2014, 3, 146–153 CrossRef.
- O. Barbosa, R. Torres, C. Ortiz and R. Fernandez-Lafuente, Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica, Process Biochem., 2012, 47, 1220–1227 CrossRef CAS.
- M. Naya and M. Imai, Regulation of the hydrolysis reactivity of immobilized Candida rugosa lipase with the aid of a hydrophobic porous carrier, Asia-Pac. J. Chem. Eng., 2012, 7, S157–S165 CrossRef CAS.
- C. Du, B. Zhao, C. Li, P. Wang, Z. Wang and J. Tang, et al., Improvement of the enantioselectivity and activity of lipase from Pseudomonas sp. via adsorption on a hydrophobic support: kinetic resolution of 2-octanol, Biocatal. Biotransform., 2009, 27, 340–347 CrossRef CAS.
- G. Yang, J. Wu, G. Xu and L. Yang, Enhancement of the activity and enantioselectivity of lipase in organic systems by immobilization onto low-cost support, J. Mol. Catal. B: Enzym., 2009, 57, 96–103 CrossRef CAS.
- P.-Y. Wang, S.-W. Tsai and T.-L. Chen, Improvement of enantioselectivity and stability of Klebsiella oxytoca hydrolase immobilized on Eupergit C 250L, J. Chem. Technol. Biotechnol., 2008, 83, 1518–1525 CrossRef CAS.
- S. Takaç and M. Bakkal, Impressive effect of immobilization conditions on the catalytic activity and enantioselectivity of Candida rugosa lipase toward S-Naproxen production, Process Biochem., 2007, 42, 1021–1027 CrossRef.
- D. Koszelewski, A. Redzej and R. Ostaszewski, The study on efficient hydrolases immobilization for the kinetic resolution of the α-acetoxyamides, J. Mol. Catal. B: Enzym., 2007, 47, 51–57 CrossRef CAS.
- A. Chaubey, R. Parshad, S. Koul, S. C. Taneja and G. N. Qazi, Enantioselectivity modulation through immobilization of Arthrobacter sp. lipase: Kinetic resolution of fluoxetine intermediate, J. Mol. Catal. B: Enzym., 2006, 42, 39–44 CrossRef CAS.
- S. Sabbani, E. Hedenström and O. Nordin, The enantioselectivity of Candida rugosa lipase is influenced by the particle size of the immobilising support material Accurel, J. Mol. Catal. B: Enzym., 2006, 42, 1–9 CrossRef CAS.
- H. Yu, J. Wu and B. C. Chi, Enhanced activity and enantioselectivity of Candida rugosa lipase immobilized on macroporous adsorptive resins for ibuprofen resolution, Biotechnol. Lett., 2004, 26, 629–633 CrossRef CAS PubMed.
- K. K. Kim, H. K. Song, D. H. Shin, K. Y. Hwang and S. W. Suh, The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor, Structure, 1997, 5, 173–185 CrossRef CAS PubMed.
- K.-E. Jaeger, S. Ransac, H. B. Koch, F. Ferrato and B. W. Dijkstra, Topological characterization and modeling of the 3D structure of lipase from Pseudomonas aeruginosa, FEBS Lett., 1993, 332, 143–149 CrossRef CAS PubMed.
- M. Cygler and J. D. Schrag, Structure and conformational flexibility of Candida rugosa lipase, Biochim. Biophys. Acta, 1999, 1441, 205–214 CrossRef CAS.
- A. Bastida, P. Sabuquillo, P. Armisen, R. Fernández-Lafuente, J. Huguet and J. M. Guisán, A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports, Biotechnol. Bioeng., 1998, 58, 486–493 CrossRef CAS PubMed.
- R. Fernandez-Lafuente, P. Armisén, P. Sabuquillo, G. Fernández-Lorente and J. M. Guisán, Immobilization of lipases by selective adsorption on hydrophobic supports, Chem. Phys. Lipids, 1998, 93, 185–197 CrossRef CAS PubMed.
- N. Rueda, J. C. S. dos Santos, R. Torres, C. Ortiz, O. Barbosa and R. Fernandez-Lafuente, Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads, RSC Adv., 2015, 5, 11212–11222 RSC.
- J. J. Virgen-Ortíz, V. G. Tacias-Pascacio, D. B. Hirata, B. Torrestiana-Sanchez, A. Rosales-Quintero and R. Fernandez-Lafuente, Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports, Enzyme Microb. Technol., 2017, 96, 30–35 CrossRef PubMed.
- D. B. Hirata, T. L. Albuquerque, N. Rueda, J. J. Virgen-Ortíz, V. G. Tacias-Pascacio and R. Fernandez-Lafuente, Evaluation of different immobilized lipases in transesterification reactions using tributyrin: Advantages of the heterofunctional octyl agarose beads, J. Mol. Catal. B: Enzym., 2016, 133, 117–123 CrossRef CAS.
- D. B. Hirata, T. L. Albuquerque, N. Rueda, J. M. Sánchez-Montero, E. Garcia-Verdugo and R. Porcar, et al., Advantages of Heterofunctional Octyl Supports: Production of 1,2-Dibutyrin by Specific and Selective Hydrolysis of Tributyrin Catalyzed by Immobilized Lipases, ChemistrySelect, 2016, 1, 3259–3270 CrossRef CAS.
- L. Fernandez-Lopez, S. G. Pedrero, N. Lopez-Carrobles, J. J. Virgen-Ortíz, B. C. Gorines and C. Otero, et al., Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers: Avoiding enzyme release from the support, Process Biochem., 2017, 54, 81–88 CrossRef CAS.
- L. Fernandez-Lopez, J. J. Virgen-OrtÍz, S. G. Pedrero, N. Lopez-Carrobles, B. C. Gorines and C. Otero, et al., Optimization of the coating of octyl-CALB with ionic polymers to improve stability and decrease enzyme leakage, Biocatal. Biotransform., 2018, 36, 47–56 CrossRef CAS.
- H. Zaak, L. Fernandez-Lopez, C. Otero, M. Sassi and R. Fernandez-Lafuente, Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde, Enzyme Microb. Technol., 2017, 106, 67–74 CrossRef CAS PubMed.
- S. Peirce, V. Tacias-Pascacio, M. Russo, A. Marzocchella, J. Virgen-Ortíz and R. Fernandez-Lafuente, Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI), Molecules, 2016, 21, 751 CrossRef PubMed.
- G. Fernandez-Lorente, M. Filice, D. Lopez-Vela, C. Pizarro, L. Wilson and L. Betancor, et al., Cross-linking of lipases adsorbed on hydrophobic supports: Highly selective hydrolysis of fish oil catalyzed by RML, J. Am. Oil Chem. Soc., 2011, 88, 801–807 CrossRef CAS.
- C. Bernal, A. Illanes and L. Wilson, Heterofunctional hydrophilic-hydrophobic porous silica as support for multipoint covalent immobilization of lipases: Application to lactulose palmitate synthesis, Langmuir, 2014, 30, 3557–3566 CrossRef CAS PubMed.
- C. Bernal, A. Illanes and L. Wilson, Improvement of Efficiency in the Enzymatic Synthesis of Lactulose Palmitate, J. Agric. Food Chem., 2015, 63, 3716–3724 CrossRef CAS PubMed.
- N. Guajardo, C. Bernal, L. Wilson and Z. Cabrera, Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase, Catal. Today, 2015, 255, 21–26 CrossRef CAS.
- N. Guajardo, C. Bernal, L. Wilson and Z. Cabrera, Selectivity of R-α-monobenzoate glycerol synthesis catalyzed by Candida antarctica lipase B immobilized on heterofunctional supports, Process Biochem., 2015, 50, 1870–1877 CrossRef CAS.
- Z. Boros, D. Weiser, M. Márkus, E. Abaháziová, Á. Magyar and A. Tomin, et al., Hydrophobic adsorption and covalent immobilization of Candida antarctica lipase B on mixed-function-grafted silica gel supports for continuous-flow biotransformations, Process Biochem., 2013, 48, 1039–1047 CrossRef CAS.
- V. Vescovi, R. Giordano, A. Mendes and P. Tardioli, Immobilized Lipases on Functionalized Silica Particles as Potential Biocatalysts for the Synthesis of Fructose Oleate in an Organic Solvent/Water System, Molecules, 2017, 22, 212 CrossRef PubMed.
- A. Suescun, N. Rueda, J. C. S. dos Santos, J. J. Castillo, C. Ortiz and R. Torres, et al., Immobilization of lipases on glyoxyl-octyl supports: Improved stability and reactivation strategies, Process Biochem., 2015, 50, 1211–1217 CrossRef CAS.
- N. Rueda, J. C. S. dos Santos, M. D. Rodriguez, T. L. Albuquerque, O. Barbosa and R. Torres, et al., Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization, J. Mol. Catal. B: Enzym., 2016, 128, 10–18 CrossRef CAS.
- N. Rueda, T. Albuquerque, R. Bartolome-Cabrero, L. Fernandez-Lopez, R. Torres and C. Ortiz, et al., Reversible Immobilization of Lipases on Heterofunctional Octyl-Amino Agarose Beads Prevents Enzyme Desorption, Molecules, 2016, 21, 646 CrossRef PubMed.
- D.-G. Lee, K. M. Ponvel, M. Kim, S. Hwang, I.-S. Ahn and C.-H. Lee, Immobilization of lipase on hydrophobic nano-sized magnetite particles, J. Mol. Catal. B: Enzym., 2009, 57, 62–66 CrossRef CAS.
- Y. Li, F. Gao, W. Wei, J.-B. Qu, G.-H. Ma and W.-Q. Zhou, Pore size of macroporous polystyrene microspheres affects lipase immobilization, J. Mol. Catal. B: Enzym., 2010, 66, 182–189 CrossRef CAS.
- Y. Z. Chen, C. T. Yang, C. B. Ching and R. Xu, Immobilization of Lipases on Hydrophobilized Zirconia Nanoparticles: Highly Enantioselective and Reusable Biocatalysts, Langmuir, 2008, 24, 8877–8884 CrossRef CAS PubMed.
- A. A. Mendes, P. C. Oliveira, A. M. Vélez, R. C. Giordano, R. D. L. C. Giordano and H. F. de Castro, Evaluation of immobilized lipases on poly-hydroxybutyrate beads to catalyze biodiesel synthesis, Int. J. Biol. Macromol., 2012, 50, 503–511 CrossRef CAS PubMed.
- P. Adlercreutz, Immobilisation and application of lipases in organic media, Chem. Soc. Rev., 2013, 42, 6406–6436 RSC.
- B. Al-Duri and Y. P. Yong, Lipase immobilisation: an equilibrium study of lipases immobilised on hydrophobic and hydrophilic/hydrophobic supports, Biochem. Eng. J., 2000, 4, 207–215 CrossRef CAS.
- K. Hernandez, C. Garcia-Galan and R. Fernandez-Lafuente, Simple and efficient immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads, Enzyme Microb. Technol., 2011, 49, 72–78 CrossRef CAS PubMed.
- A. Galarneau, M. Mureseanu, S. Atger, G. Renard and F. Fajula, Immobilization of lipase on silicas. Relevance of textural and interfacial properties on activity and selectivity, New J. Chem., 2006, 30, 562–571 RSC.
- E. Séverac, O. Galy, F. Turon, C. A. Pantel, J. S. Condoret and P. Monsan, et al., Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact, Enzyme Microb. Technol., 2011, 48, 61–70 CrossRef PubMed.
- K. Kawakami, Y. Oda and R. Takahashi, Application of a Burkholderia cepacia lipase-immobilized silica monolith to batch and continuous biodiesel production with a stoichiometric mixture of methanol and crude Jatropha oil, Biotechnol. Biofuels, 2011, 4, 42 CrossRef CAS PubMed.
- M. P. Guauque Torres, M. L. Foresti and M. L. Ferreira, Cross-linked enzyme aggregates (CLEAs) of selected lipases: A procedure for the proper calculation of their recovered activity, AMB Express, 2013, 3, 1–11 CrossRef PubMed.
- M. L. Foresti, G. A. Alimenti and M. L. Ferreira, Interfacial activation and bioimprinting of Candida rugosa lipase immobilized on polypropylene: effect on the enzymatic activity in solvent-free ethyl oleate synthesis, Enzyme Microb. Technol., 2005, 36, 338–349 CrossRef CAS.
- M. H. Sörensen, J. B. S. Ng, L. Bergström and P. C. A. Alberius, Improved enzymatic activity of Thermomyces lanuginosus lipase immobilized in a hydrophobic particulate mesoporous carrier, J. Colloid Interface Sci., 2010, 343, 359–365 CrossRef PubMed.
- T. L. d. Albuquerque, N. Rueda, J. C. S. dos Santos, O. Barbosa, C. Ortiz and B. Binay, et al., Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment, Process Biochem., 2016, 51, 865–874 CrossRef.
- C. Garcia-Galan, O. Barbosa, K. Hernandez, J. C. S. dos Santos, R. C. Rodrigues and R. Fernandez-Lafuente, Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases, Molecules, 2014, 19, 7629–7645 CrossRef PubMed.
- E. A. Manoel, M. F. P. Ribeiro, J. C. S. dos Santos, M. A. Z. Coelho, A. B. C. Simas and R. Fernandez-Lafuente, et al., Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia : Application to the kinetic resolution of myo -inositol derivatives, Process Biochem., 2015, 50, 1557–1564 CrossRef CAS.
- Y. Z. Chen, C. B. Ching and R. Xu, Lipase immobilization on modified zirconia nanoparticles: Studies on the effects of modifiers, Process Biochem., 2009, 44, 1245–1251 CrossRef CAS.
- B. Chen, C. Yin, Y. Cheng, W. Li, Z. Cao and T. Tan, Using silk woven fabric as support for lipase immobilization: The effect of surface hydrophilicity/hydrophobicity on enzymatic activity and stability, Biomass Bioenergy, 2012, 39, 59–66 CrossRef CAS.
- R. Reshmi and S. Sugunan, Superior activities of lipase immobilized on pure and hydrophobic clay supports: Characterization and catalytic activity studies, J. Mol. Catal. B: Enzym., 2013, 97, 36–44 CrossRef CAS.
- R. C. Rodrigues, K. Hernandez, O. Barbosa, N. Rueda, C. Garcia-Galan and J. C. S. dos Santos, et al., Immobilization of proteins in poly-styrene-divinylbenzene matrices: Functional properties and applications, Curr. Org. Chem., 2015, 19, 1707–1718 CrossRef CAS.
- C. R. Johnson and S. J. Bis, Enzymatic asymmetrization of meso-2-cycloalken-1,4-diols and their diacetates in organic and aqueous media, Tetrahedron Lett., 1992, 33, 7287–7290 CrossRef CAS.
- B. Chen, J. Hu, E. M. Miller, W. Xie, M. Cai and R. A. Gross, Candida antarctica Lipase B Chemically Immobilized on Epoxy-Activated Micro- and Nanobeads: Catalysts for Polyester Synthesis, Biomacromolecules, 2008, 9, 463–471 CrossRef CAS.
- B. Chen, M. E. Miller and R. A. Gross, Effects of porous polystyrene resin parameters on Candida antarctica lipase B adsorption, distribution, and polyester synthesis activity, Langmuir, 2007, 23, 6467–6474 CrossRef CAS PubMed.
- Y. Mei, L. Miller, W. Gao and R. A. Gross, Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy, Biomacromolecules, 2003, 4, 70–74 CrossRef CAS PubMed.
- Z. Cabrera, G. Fernandez-Lorente, R. Fernandez-Lafuente, J. M. Palomo and J. M. Guisan, Novozym 435 displays very different selectivity compared to lipase from Candida antarctica B adsorbed on other hydrophobic supports, J. Mol. Catal. B: Enzym., 2009, 57, 171–176 CrossRef CAS.
- A. Tambe, R. Vyasarayani and A. Datla, Macroporous Poly(Vinyl Acetate-Co-Divinyl Benzene) Copolymer Beads as Adsorptive Support for the Direct Immobilization of Candida antarctica Lipase B, Enzyme Eng., 2015, 4, 1000130 Search PubMed.
- H. K. Albeladi, A. N. Al-Romaizan and M. A. Hussein, Role of Cross-Linking Process on the Performance of PMMA, Int. J. Biosens. Bioelectron., 2017, 3, 279–284 Search PubMed.
- M. N. Tahir, A. Adnan, E. Strömberg and P. Mischnick, Stability of lipase immobilized on O-pentynyl dextran, Bioprocess Biosyst. Eng., 2012, 35, 535–544 CrossRef CAS PubMed.
- T. Zhao, D. S. No, B. H. Kim, H. S. Garcia, Y. Kim and I. H. Kim, Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid, Food Chem., 2014, 157, 132–140 CrossRef CAS PubMed.
- J. Rodrigues, A. Canet, I. Rivera, N. M. Osório, G. Sandoval and F. Valero, et al., Biodiesel production from crude Jatropha oil catalyzed by non-commercial immobilized heterologous Rhizopus oryzae and Carica papaya lipases, Bioresour. Technol., 2016, 213, 88–95 CrossRef CAS PubMed.
- D. Turati, W. Morais Júnior, C. Terrasan, S. Moreno-Perez, B. Pessela and G. Fernandez-Lorente, et al., Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties, Molecules, 2017, 22, 339 CrossRef PubMed.
- V. G. Tacias-Pascacio, J. J. Virgen-Ortíz, M. Jiménez-Pérez, M. Yates, B. Torrestiana-Sanchez and A. Rosales-Quintero, et al., Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support, Fuel, 2017, 200, 1–10 CrossRef CAS.
- V. Kasche, U. Haufler and L. Riechmann, Equilibrium and kinetically controlled synthesis with enzymes: Semisynthesis of penicillins and peptides, Methods Enzymol., 1987, 136, 280–292 CAS.
- E. C. de Souza, M. Romero-Ortega and H. F. Olivo, Lipase-mediated selective acetylation of primary alcohols in ethyl acetate, Tetrahedron Lett., 2018, 59, 287–290 CrossRef CAS.
- I. N. A. Rahman, F. M. A. Manan, N. H. C. Marzuki, N. A. Mahat, N. Attan and A. S. A. Keyon, et al., A statistical approach for optimizing the high yield green production of the flavor ester butyl butyrate, J. Teknol., 2017, 79, 141–151 Search PubMed.
- M. Sjöblom, P. Risberg, A. Filippova, O. G. W. Öhrman, U. Rova and P. Christakopoulos, In Situ Biocatalytic Synthesis of Butyl Butyrate in Diesel and Engine Evaluations, ChemCatChem, 2017, 9, 4529–4537 CrossRef.
- S. Zhang, L. Cao, X. Wu, Y. Mo and L. Pan, Research on enzymatic synthesis and property of L-ascorbyl octanoate, Zhongguo Liangyou Xuebao, 2016, 31, 107–113 Search PubMed.
- L. XiaoYan, P. JiuXiang and Y. LinQiu, Lipase-catalyzed esterification of konjac glucomannan in isooctane, Environ. Prog. Sustainable Energy, 2016, 35, 1149–1155 CrossRef.
- M. T. Sose, S. R. Bansode and V. K. Rathod, Solvent free lipase catalyzed synthesis of butyl caprylate, J. Chem. Sci., 2017, 129, 1755–1760 CrossRef CAS.
- E. Altuntepe, T. Greinert, F. Hartmann, A. Reinhardt, G. Sadowski and C. Held, Thermodynamics of enzyme-catalyzed esterifications: I. Succinic acid esterification with ethanol, Appl. Microbiol. Biotechnol., 2017, 101, 5973–5984 CrossRef CAS PubMed.
- A. Rashid, N. A. Virk, A. R. S. Butt, A. Adnan, M. Pervaiz and M. A. Raza, et al., Enzymatic synthesis of citronellyl palmitate in organic media: Process optimization and kinetic evaluation, Asian J. Chem., 2016, 28, 298–300 CrossRef CAS.
- N. Khairudin, M. Basri, H. Fard Masoumi, S. Samson and S. Ashari, Enhancing the Bioconversion of Azelaic Acid to Its Derivatives by Response Surface Methodology, Molecules, 2018, 23, 397 CrossRef PubMed.
- S.-S. Wang, Z.-J. Li, S. Sheng, F.-A. Wu and J. Wang, Microfluidic biocatalysis enhances the esterification of caffeic acid and methanol under continuous-flow conditions, J. Chem. Technol. Biotechnol., 2016, 91, 555–562 CrossRef CAS.
- A. F. B. Lajis, M. Hamid, S. Ahmad and A. B. Ariff, Comparative study of stirred and fluidized tank reactor for hydroxyl-kojic acid derivatives synthesis and their biological activities, Turk Biyokim. Derg., 2018, 43, 205–219 CAS.
- C.-S. Kim, D.-H. Lim and Y. S. Keum, Lipase-Catalyzed Synthesis of Fatty Acid Pyridylcarbinol Ester for the Analysis of Seed Lipids, J. Am. Oil Chem. Soc., 2016, 93, 339–346 CrossRef CAS.
- S. Mukherjee and M. Ghosh, Studies on performance evaluation of a green plasticizer made by enzymatic esterification of furfuryl alcohol and castor oil fatty acid, Carbohydr. Polym., 2017, 157, 1076–1084 CrossRef CAS PubMed.
- S. Gholivand, O. Lasekan, C. P. Tan, F. Abas and L. S. Wei, Comparative study of the antioxidant activities of some lipase-catalyzed alkyl dihydrocaffeates synthesized in ionic liquid, Food Chem., 2017, 224, 365–371 CrossRef CAS PubMed.
- Y.-G. Shi, Y. Wu, X.-Y. Lu, Y.-P. Ren, Q. Wang and C.-M. Zhu, et al., Lipase-catalyzed esterification of ferulic acid with lauryl alcohol in ionic liquids and antibacterial properties in vitro against three food-related bacteria, Food Chem., 2017, 220, 249–256 CrossRef CAS PubMed.
- M. Primožič, S. Kavčič, Ž. Knez and M. Leitgeb, Enzyme-catalyzed esterification of d,l-lactic acid in different SCF/IL media, J. Supercrit. Fluids, 2016, 107, 414–421 CrossRef.
- G. N. Pereira, J. P. Holz, P. P. Giovannini, J. V. Oliveira, D. de Oliveira and L. A. Lerin, Enzymatic esterification for the synthesis of butyl stearate and ethyl stearate, Biocatal. Agric. Biotechnol., 2018, 16, 373–377 CrossRef.
- S. Gholivand, O. Lasekan, C. P. Tan, F. Abas and L. S. Wei, Optimization of enzymatic esterification of dihydrocaffeic acid with hexanol in ionic liquid using response surface methodology, Chem. Cent. J., 2017, 11, 44 CrossRef PubMed.
- P. dos Santos, C. A. Rezende and J. Martínez, Activity of immobilized lipase from Candida antarctica (Lipozyme 435) and its performance on the esterification of oleic acid in supercritical carbon dioxide, J. Supercrit. Fluids, 2016, 107, 170–178 CrossRef CAS.
- A. Galgali, S. D. Gawas and V. K. Rathod, Ultrasound assisted synthesis of citronellol laurate by using Novozym 435, Catal. Today, 2018, 309, 133–139 CrossRef CAS.
- C. Jiang, Y. Lu, Z. Li, C. Li and R. Yan, Enzymatic Synthesis of L-Ascorbyl Fatty Acid Esters Under Ultrasonic Irradiation and Comparison of Their Antioxidant Activity and Stability, J. Food Sci., 2016, 81, C1370–1377 CrossRef CAS PubMed.
- M. Balen, G. R. Gomes, J. M. Kratz, C. M. O. Simões, A. Valério and D. de Oliveira, Enzymatic synthesis of ascorbyl ester derived from linoleic acid, Bioprocess Biosyst. Eng., 2017, 40, 265–270 CrossRef CAS PubMed.
- K. V. Bhavsar and G. D. Yadav, Microwave assisted solvent-free synthesis of n-butyl propionate by immobilized lipase as catalyst, Biocatal. Agric. Biotechnol., 2018, 14, 264–269 CrossRef.
- K. V. Bhavsar and G. D. Yadav, Process intensification by microwave irradiation in immobilized-lipase catalysis in solvent-free synthesis of ethyl valerate, Mol. Catal., 2018, 461, 34–39 CrossRef CAS.
- M. Zare, M.-T. Golmakani and M. Niakousari, Lipase synthesis of isoamyl acetate using different acyl donors: Comparison of novel esterification techniques, LWT--Food Sci. Technol., 2019, 101, 214–219 CrossRef CAS.
- A. G. A. Sá, A. C. de Meneses, L. A. Lerin, P. H. H. de Araújo, C. Sayer and D. de Oliveira, Biocatalysis of aromatic benzyl-propionate ester by different immobilized lipases, Bioprocess Biosyst. Eng., 2018, 41, 585–591 CrossRef PubMed.
- C. Sbardelotto, S. Piazza, N. Nyari, R. Dallago, D. Oliveira and V. Oliveira, et al., Optimization of solvent-free geranyl butanoate production using Novozyme 435 and homemade polyurethane immobilized Novozyme NZL-102-lyo-Hq as catalysts, Quim. Nova, 2018, 41, 905–911 CAS.
- H. M. Salvi, M. P. Kamble and G. D. Yadav, Synthesis of Geraniol Esters in a Continuous-Flow Packed-Bed Reactor of Immobilized Lipase: Optimization of Process Parameters and Kinetic Modeling, Appl. Biochem. Biotechnol., 2018, 184, 630–643 CrossRef CAS PubMed.
- M. Koutinas, C. Yiangou, N. M. Osório, K. Ioannou, A. Canet and F. Valero, et al., Application of commercial and non-commercial immobilized lipases for biocatalytic production of ethyl lactate in organic solvents, Bioresour. Technol., 2018, 247, 496–503 CrossRef CAS PubMed.
- Y.-Z. Qin, M.-H. Zong, W.-Y. Lou and N. Li, Biocatalytic upgrading of 5-hydroxymethylfurfural (HMF) with levulinic acid to HMF levulinate in biomass-derived solvents, ACS Sustainable Chem. Eng., 2016, 4, 4050–4054 CrossRef CAS.
- E. Altuntepe, V. N. Emel'yanenko, M. Forster-Rotgers, G. Sadowski, S. P. Verevkin and C. Held, Thermodynamics of enzyme-catalyzed esterifications: II Levulinic acid esterification with short-chain alcohols, Appl. Microbiol. Biotechnol., 2017, 101, 7509–7521 CrossRef CAS PubMed.
- K. V. Bhavsar and G. D. Yadav, n-Butyl levulinate synthesis using lipase catalysis: comparison of batch reactor versus continuous flow packed bed tubular microreactor, J. Flow Chem., 2018, 8, 97–105 CrossRef.
- M. Serrano-Arnaldos, M. F. Máximo-Martín, M. C. Montiel-Morte, S. Ortega-Requena, E. Gómez-Gómez and J. Bastida-Rodríguez, Solvent-free enzymatic production of high quality cetyl esters, Bioprocess Biosyst. Eng., 2016, 39, 641–649 CrossRef CAS PubMed.
- M. Serrano-Arnaldos, S. Ortega-Requena, M. C. Montiel, F. Máximo, J. Bastida and M. D. Murcia, Preliminary economic assessment: a valuable tool to establish biocatalytic process feasibility with an in-lab immobilized lipase, J. Chem. Technol. Biotechnol., 2019, 94, 409–417 CrossRef CAS.
- P. dos Santos, G. L. Zabot, M. A. A. Meireles, M. A. Mazutti and J. Martínez, Synthesis of eugenyl acetate by enzymatic reactions in supercritical carbon dioxide, Biochem. Eng. J., 2016, 114, 1–9 CrossRef CAS.
- L. Chen, W. Li and J. Xin, Enzymatic synthesis in nonaqueous medium and free radical scavenging ability of ferulyl oleins, Zhongguo Liangyou Xuebao, 2016, 31, 120–125 Search PubMed.
- C. Ülger and S. Takaç, Kinetics of lipase-catalysed methyl gallate production in the presence of deep eutectic solvent, Biocatal. Biotransform., 2017, 35, 407–416 CrossRef.
- A. Jahangiri, A. H. Møller, M. Danielsen, B. Madsen, B. Joernsgaard and S. Vaerbak, et al., Hydrophilization of bixin by lipase-catalyzed transesterification with sorbitol, Food Chem., 2018, 268, 203–209 CrossRef CAS PubMed.
- S. H. Hwang, Z. Wang and S. S. Lim, Chemo-enzymatic synthesis of vinyl and L-ascorbyl phenolates and their inhibitory effects on advanced glycation end products, Food Chem., 2017, 214, 726–735 CrossRef CAS PubMed.
- T. Egger, L. S. Egger and G. Fieg, Scale and causes of catalyst activity loss in enzymatic catalyzed reactive distillation, Chem. Eng. Sci., 2018, 178, 324–334 CrossRef CAS.
- W. Liu and F. Duan, Lipase-catalyzed transesterification of epoxidized soybean oil to prepare epoxy methyl esters, Grasas Aceites, 2018, 69, 247 CrossRef.
- A. M. Hidalgo, A. Sánchez, J. L. Gómez, E. Gómez, M. Gómez and M. D. Murcia, Kinetic Study of the Enzymatic Synthesis of 2-Phenylethyl Acetate in Discontinuous Tank Reactor, Ind. Eng. Chem. Res., 2018, 57, 11280–11287 CrossRef CAS.
- M. A. Arthur-Santiago, R. M. Oliart-Ros, M. G. Sánchez-Otero and G. Valerio-Alfaro, Mechanochemo-enzymatic synthesis of aromatic aldehyde oxime esters, Nat. Prod. Commun., 2018, 13, 875–878 CrossRef.
- A. Papadaki, A. Mallouchos, M.-N. Efthymiou, C. Gardeli, N. Kopsahelis and E. C. G. Aguieiras, et al., Production of wax esters via microbial oil synthesis from food industry waste and by-product streams, Bioresour. Technol., 2017, 245, 274–282 CrossRef CAS PubMed.
- M. Sayed, Y. Gaber, A. Bornadel and S.-H. Pyo, Multi-steps green process for synthesis of six-membered functional cyclic carbonate from trimethylolpropane by lipase catalyzed methacrylation and carbonation, and thermal cyclization, Biotechnol. Prog., 2016, 32, 83–88 CrossRef CAS PubMed.
- A. Bornadel, M. Ismail, M. Sayed, R. Hatti-Kaul and S.-H. Pyo, Six-membered cyclic carbonates from trimethylolpropane: Lipase-mediated synthesis in a flow reactor and in silico evaluation of the reaction, Biotechnol. Prog., 2017, 33, 375–382 CrossRef CAS PubMed.
- P. D. Tomke and V. K. Rathod, Enzyme as biocatalyst for synthesis of octyl ethanoate using acoustic cavitation: Optimization and kinetic study, Biocatal. Agric. Biotechnol., 2016, 7, 145–153 CrossRef.
- H. Kim and C. Park, Enzymatic synthesis of phenethyl ester from phenethyl alcohol with acyl donors, Enzyme Microb. Technol., 2017, 100, 37–44 CrossRef CAS PubMed.
- J. Xin, L. Sun, S. Chen, Y. Wang and C. Xia, Synthesis of L-Ascorbyl Flurbiprofenate by Lipase-Catalyzed Esterification and Transesterification Reactions, BioMed Res. Int., 2017, 2017, 5751262 Search PubMed.
- C. Cui, Z. Zhang, Q. Zeng and B. Chen, Insight into the synthesis of isosorbide diester plasticizer using immobilized lipases, RSC Adv., 2016, 6, 108180–108186 RSC.
- R. Naoum, J. P. Séguin, J. F. Trant, M. B. Frampton, T. Hudlický and P. M. Zelisko, A chemoenzymatic route to chiral siloxanes, Tetrahedron, 2016, 72, 4027–4031 CrossRef CAS.
- P. Ramesh, R. Anjibabu, Y. N. Reddy, M. Yedukondalu, T. N. Reddy and B. Kummari, et al., Chemoenzymatic Synthesis of the HMG-CoA Reductase Inhibitor Rosuvastatin and Natural Styryl Lactone Cryptomoscatone E1, Asian J. Org. Chem., 2017, 6, 984–987 CrossRef CAS.
- T. D. S. Fonseca, L. D. Lima, M. D. C. F. de Oliveira, T. L. G. de Lemos, D. Zampieri and F. Molinari, et al., Chemoenzymatic Synthesis of Luliconazole Mediated by Lipases, Eur. J. Org. Chem., 2018, 2018, 2110–2116 CrossRef CAS.
- S. M. Gupta, M. P. Kamble and G. D. Yadav, Insight into microwave assisted enzyme catalysis in process intensification of reaction and selectivity: Kinetic resolution of (R,S)-flurbiprofen with alcohols, Mol. Catal., 2017, 440, 50–56 CrossRef CAS.
- E. Łukowska-Chojnacka, A. Kowalkowska and A. Napiórkowska, Lipase-catalyzed kinetic resolution of novel antitubercular benzoxazole derivatives, Chirality, 2018, 30, 457–468 CrossRef PubMed.
- Y.-R. Yeh, Y.-J. Tzeng and S.-W. Tsai, Quantitative Improvements and Insights into CALB-Catalyzed Resolution of trans- and cis-2-Phenylcyclopropyl Azolides, ChemistrySelect, 2018, 3, 5353–5360 CrossRef CAS.
- D. Listunov, E. Joly, C. Duhayon, N. Saffon-Merceron, I. Fabing and Y. Génisson, et al., Methinylogation Approach in Chiral Pharmacophore Design: from Alkynyl- to Allenyl-carbinol Warheads against Tumor Cells, ChemMedChem, 2018, 13, 1711–1722 CrossRef CAS PubMed.
- A. Iemhoff, J. Sherwood, C. R. McElroy and A. J. Hunt, Towards sustainable kinetic resolution, a combination of bio-catalysis, flow chemistry and bio-based solvents, Green Chem., 2018, 20, 136–140 RSC.
- J. Zhang, R. Kolluri, S. G. Alvarez, M. M. Irving, R. Singh and M. A. J. Duncton, Enantioselective synthesis of an octahydroindolizine (indolizidine) alcohol using an enzymatic resolution, Org. Biomol. Chem., 2017, 15, 2953–2961 RSC.
- Y. Zhang, F. Cheng, H. Yan, J. Zheng and Z. Wang, The enzymatic resolution of 1-(4-chlorophenyl)ethylamine by Novozym 435 to prepare a novel triazolopyrimidine herbicide, Chirality, 2018, 30, 1225–1232 CrossRef CAS PubMed.
- H. N. Hoang, Y. Nagashima, S. Mori, H. Kagechika and T. Matsuda, CO2-expanded bio-based liquids as novel solvents for enantioselective biocatalysis, Tetrahedron, 2017, 73, 2984–2989 CrossRef CAS.
- C. Sanfilippo, A. A. Paternò and A. Patti, Resolution of racemic amines via lipase-catalyzed benzoylation: Chemoenzymatic synthesis of the pharmacologically active isomers of labetalol, Mol. Catal., 2018, 449, 79–84 CrossRef CAS.
- D. B. Magadum and G. D. Yadav, Enantioselective resolution of (R,S)-A-methyl-4-pyridinemethanol using immobilized biocatalyst: Optimization and kinetic modeling, Biochem. Eng. J., 2017, 122, 152–158 CrossRef CAS.
- A. S. Malyemez, E. Bayraktar and U. Mehmetoǧlu, Optimization of process parameters and kinetic modeling for the enantioselective kinetic resolution of (R,S)-2-pentanol, Turk Biyokim. Derg., 2017, 42, 600–608 CAS.
- M. P. Kamble and G. D. Yadav, Kinetic Resolution of (R,S)-α-Tetralol by Immobilized Candida antarctica Lipase B: Comparison of Packed-Bed over Stirred-Tank Batch Bioreactor, Ind. Eng. Chem. Res., 2017, 56, 1750–1757 CrossRef CAS.
- I. Kmecz, Z. Varga and E. Székely, One pot kinetic resolution and product separation with corn germ oil and supercritical carbon dioxide, J. Supercrit. Fluids, 2018, 141, 218–223 CrossRef CAS.
- D. J. Strub, A. Garbos and S. Lochyñski, Synthesis, lipase catalyzed kinetic resolution, and determination of the absolute configuration of enantiomers of the Morita-Baylis-Hillman adduct 3-hydroxy-2-methylenebutanenitrile, ARKIVOC, 2016, 2017, 313–323 Search PubMed.
- S. Serra and D. De Simeis, Two Complementary Synthetic Approaches to the Enantiomeric Forms of the Chiral Building Block (2,6,6-Trimethyltetrahydro-2H-pyran-2-yl)methanol: Application to the Stereospecific Preparation of the Natural Flavor Linaloyl Oxide, Catalysts, 2018, 8, 362 Search PubMed.
- C. Jiang and G. Cheng, Synergistic Effect of Pd/C and Novozyme 435 on the Dynamic Kinetic Resolution of 1,1,1-trifluoroisopropylamine, Chem. Eng. Commun., 2016, 203, 1222–1226 CrossRef CAS.
- Y. N. Reddy, T. N. Kumari, P. Thota, P. Jyothi and A. K. Gupta, Chemoenzymatic total synthesis of cryptocaryalactone natural products, Tetrahedron Lett., 2018, 59, 160–162 CrossRef CAS.
- Y. Shimotori, M. Hoshi, Y. Osawa and T. Miyakoshi, Synthesis of various β-D-glucopyranosyl and β-Dxylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities, Heterocycl. Commun., 2017, 23, 213–223 CAS.
- S.-G. Zhu, J.-X. Huang, G.-M. Zhang, S.-X. Chen and F.-L. Zhang, Development of a Practical Enzymatic Synthesis of Isosorbide-2-acetate, Org. Process Res. Dev., 2018, 22, 1548–1552 CrossRef CAS.
- A. Sembayeva, B. Berhane and J. A. Carr, Lipase-mediated regioselective modifications of macrolactonic sophorolipids, Tetrahedron, 2017, 73, 1873–1880 CrossRef CAS.
- Z. Findrik, G. Megyeri, L. Gubicza, K. Bélafi-Bakó, N. Nemestóthy and M. Sudar, Lipase catalyzed synthesis of glucose palmitate in ionic liquid, J. Cleaner Prod., 2016, 112, 1106–1111 CrossRef CAS.
- E. Abdulmalek, N. F. Hamidon and M. B. Abdul Rahman, Optimization and characterization of lipase catalysed synthesis of xylose caproate ester in organic solvents, J. Mol. Catal. B: Enzym., 2016, 132, 1–4 CrossRef CAS.
- K.-H. Zhao, Y.-Z. Cai, X.-S. Lin, J. Xiong, P. Halling and Z. Yang, Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents, Molecules, 2016, 21, 1294 CrossRef PubMed.
- X. Zhang, C. Yang, J. Li, Q. Meng, H. Raza and L. Zhang, Enzymatic synthesis of mannitol dioctanoate and its utilisation in the preparation of structured edible oil, Int. J. Food Sci. Technol., 2016, 51, 588–594 CrossRef CAS.
- Y. Sun, L. Liu, Z. Luo and R. Yan, Studies on synthesis and antioxidant activity of isoorientin-6′′-oleate and isovitexin-6′′-oleate, Zhongguo Shipin Xuebao, 2016, 16, 47–53 Search PubMed.
- N. Rana, M. Kumar, V. Khatri, J. Maity and A. K. Prasad, Enzymatic separation of epimeric 4-C-hydroxymethylated furanosugars: Synthesis of bicyclic nucleosides, Beilstein J. Org. Chem., 2017, 13, 2078–2086 CrossRef CAS PubMed.
- D. An, F. Liang and D. Feng, Synthesis and investigation of surface active properties of green glucosyl esters, J. Dispersion Sci. Technol., 2019, 40(3), 332–337 CrossRef CAS.
- R. Kumar, M. Kumar, A. Singh, N. Singh, J. Maity and A. K. Prasad, Synthesis of novel C-4′-spiro-oxetano-α-L-ribonucleosides, Carbohydr. Res., 2017, 445, 88–92 CrossRef CAS PubMed.
- C. Q. Sun, K. D. Johnson, H. Wong and L. Y. Foo, Biotransformation of Flavonoid Conjugates with Fatty Acids and Evaluations of Their Functionalities, Front. Pharmacol., 2017, 8, 759 CrossRef PubMed.
- W. Yang, M. Kortesniemi, B. Yang and J. Zheng, Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives, J. Agric. Food Chem., 2018, 66, 2909–2916 CrossRef CAS PubMed.
- E. Heřmánková-Vavříková, A. Křenková, L. Petrásková, C. S. Chambers, J. Zápal and M. Kuzma, et al., Synthesis and Antiradical Activity of Isoquercitrin Esters with Aromatic Acids and Their Homologues, Int. J. Mol. Sci., 2017, 18, 1074 CrossRef PubMed.
- E. Vavříková, F. Langschwager, L. Jezova-Kalachova, A. Křenková, B. Mikulová and M. Kuzma, et al., Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties, Int. J. Mol. Sci., 2016, 17, 899 CrossRef PubMed.
- H. P. Ziaullah and V. Rupasinghe, Sonochemical enzyme-catalyzed regioselective acylation of flavonoid glycosides, Bioorg. Chem., 2016, 65, 17–25 CrossRef CAS PubMed.
- E. Vavříková, V. Křen, L. Jezova-Kalachova, M. Biler, B. Chantemargue and M. Pyszková, et al., Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization, Eur. J. Med. Chem., 2017, 127, 263–274 CrossRef PubMed.
- M.-Y. Liang, Y. Chen, M. G. Banwell, Y. Wang and P. Lan, Enzymatic Preparation of a Homologous Series of Long-Chain 6-O-Acylglucose Esters and Their Evaluation as Emulsifiers, J. Agric. Food Chem., 2018, 66, 3949–3956 CrossRef CAS PubMed.
- S. Serra and D. De Simeis, A study on the lipase-catalysed acylation of 6,7-dihydroxy-linalool, Nat. Prod. Commun., 2016, 11, 1217–1220 CrossRef PubMed.
- T. Méline, M. Muzard, M. Deleu, H. Rakotoarivonina, R. Plantier-Royon and C. Rémond, D-Xylose and L-arabinose laurate esters: Enzymatic synthesis, characterization and physico-chemical properties, Enzyme Microb. Technol., 2018, 112, 14–21 CrossRef PubMed.
- L. F. Chávez-Flores, H. I. Beltran, D. Arrieta-Baez and D. Reyes-Duarte, Regioselective synthesis of lactulose esters by Candida antarctica and Thermomyces lanuginosus lipases, Catalysts, 2017, 7, 263 CrossRef.
- J. A. Lujan-Montelongo, H. L. Mendoza-Figueroa, C. Silva-Cuevas, A. C. Sánchez-Chávez, L. A. Polindara-García and S. Oliveros-Cruz, et al., Highly regioselective enzymatic synthesis of lutein-3-monoesters, Tetrahedron Lett., 2018, 59, 4096–4101 CrossRef CAS.
- L. Casas-Godoy, J. Arrizon, D. Arrieta-Baez, F. J. Plou and G. Sandoval, Synthesis and emulsifying properties of carbohydrate fatty acid esters produced from Agave tequilana fructans by enzymatic acylation, Food Chem., 2016, 204, 437–443 CrossRef CAS PubMed.
- M. F. P. Ribeiro, K. C. Pais, B. S. M. de Jesus, R. Fernandez-Lafuente, D. M. G. Freire and E. A. Manoel, et al., Lipase Regioselective O-Acetylations of a myo-Inositol Derivative: Efficient Desymmetrization of 1,3-Di-O-benzyl- myo -inositol, Eur. J. Org. Chem., 2018, 2018, 386–391 CrossRef CAS.
- K. Muthusamy, V. Sridharan, C. U. Maheswari and S. Nagarajan, Lipase catalyzed synthesis of fluorescent glycolipids: Gelation studies and graphene incorporated self-assembled sheet formation for semiconductor applications, Green Chem., 2016, 18, 3722–3731 RSC.
- C. Xu, H. Zhang, J. Shi, M. Zheng, X. Xiang and F. Huang, et al., Ultrasound irradiation promoted enzymatic alcoholysis for synthesis of monoglyceryl phenolic acids in a solvent-free system, Ultrason. Sonochem., 2018, 41, 120–126 CrossRef CAS PubMed.
- S. Sun and B. Hu, A novel method for the synthesis of glyceryl monocaffeate by the enzymatic transesterification and kinetic analysis, Food Chem., 2017, 214, 192–198 CrossRef CAS PubMed.
- A. E. Polloni, E. A. Rebelatto, J. G. Veneral, D. de Oliveira, J. V. Oliveira and P. H. H. Araújo, et al., Enzymatic ring opening polymerization of ω-pentadecalactone in different solvents in a variable-volume view reactor, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 1219–1227 CrossRef CAS.
- A. E. Polloni, J. G. Veneral, E. A. Rebelatto, D. de Oliveira, J. V. Oliveira and P. H. H. Araújo, et al., Enzymatic ring opening polymerization of ω-pentadecalactone using supercritical carbon dioxide, J. Supercrit. Fluids, 2017, 119, 221–228 CrossRef CAS.
- Y. J. Wong, S. K. Arumugasamy and J. Jewaratnam, Performance comparison of feedforward neural network training algorithms in modeling for synthesis of polycaprolactone via biopolymerization, Clean Technol. Environ. Policy, 2018, 20, 1971–1986 CrossRef CAS.
- S. W. Duchiron, E. Pollet, S. Givry and L. Avérous, Enzymatic synthesis of poly(ε-caprolactone-co-ε-thiocaprolactone), Eur. Polym. J., 2017, 87, 147–158 CrossRef CAS.
- R. L. Pflughaupt, S. A. Hopkins, P. M. Wright and A. P. Dove, Synthesis of poly(ω-pentadecalactone)-b-poly(acrylate) diblock copolymers via a combination of enzymatic ring-opening and RAFT polymerization techniques, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 3326–3335 CrossRef CAS.
- N. U. Kaya, A. Onen and Y. Guvenilir, Photopolymerization of acrylates by enzymatically synthesized PCL based macrophotoinitiator, eXPRESS Polym. Lett., 2017, 11, 493–503 CrossRef CAS.
- A. E. Polloni, V. Chiaradia, E. M. Figura, J. P. de Paoli, D. de Oliveira and J. V. de Oliveira, et al., Polyesters from Macrolactones Using Commercial Lipase NS 88011 and Novozym 435 as Biocatalysts, Appl. Biochem. Biotechnol., 2017, 1–14 Search PubMed.
- J. Yuan, Y. Dai, Y. Yu, P. Wang, Q. Wang and X. Fan, Biocatalytic synthesis of poly(ε-caprolactone) using modified lipase in ionic liquid media, Eng. Life Sci., 2016, 16, 371–378 CrossRef CAS.
- Y. Jiang, D. Maniar, A. J. J. Woortman and K. Loos, Enzymatic synthesis of 2,5-furandicarboxylic acid-based semi-aromatic polyamides: Enzymatic polymerization kinetics, effect of diamine chain length and thermal properties, RSC Adv., 2016, 6, 67941–67953 RSC.
- D. Maniar, K. F. Hohmann, Y. Jiang, A. J. J. Woortman, J. Van Dijken and K. Loos, Enzymatic Polymerization of Dimethyl 2,5-Furandicarboxylate and Heteroatom Diamines, ACS Omega, 2018, 3, 7077–7085 CrossRef CAS PubMed.
- B. Peng, C.-Y. Xiong, Y. Huang, J.-N. Hu, X.-M. Zhu and Z.-Y. Deng, Enzymatic Synthesis of Polyglycerol Fatty Acid Esters and Their Application as Emulsion Stabilizers, J. Agric. Food Chem., 2018, 66, 8104–8113 CrossRef CAS PubMed.
- V. Taresco, R. G. Creasey, J. Kennon, G. Mantovani, C. Alexander and J. C. Burley, et al., Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis, Polymer, 2016, 89, 41–49 CrossRef CAS.
- V. Khatri, S. Bhatia, K. Achazi, S. Deep, E. Kohli and S. K. Sharma, et al., Lipase-mediated synthesis of sugar-PEG-based amphiphiles for encapsulation and stabilization of indocyanine green, RSC Adv., 2017, 7, 37534–37541 RSC.
- G. B. Perin and M. I. Felisberti, Enzymatic synthesis and structural characterization of methacryloyl-D-fructose- and methacryloyl-D-glucose-based monomers and poly(methacryloyl-D-fructose)-based hydrogels, J. Chem. Technol. Biotechnol., 2018, 93, 1694–1704 CrossRef CAS.
- J. A. P. da Silva, N. S. M. Cardozo and C. L. Petzhold, Enzymatic synthesis of andiroba oil based polyol for the production of flexible polyurethane foams, Ind. Crops Prod., 2018, 113, 55–63 CrossRef CAS.
- D. Bresolin, V. Mazurek, A. Valério, C. Sayer, P. H. H. de Araújo and D. de Oliveira, Poly(urea-urethane) nanoparticles using mono- and diacylglycerol from glycerolysis of castor oil as biopolyol and stabilizer, Eur. Polym. J., 2018, 108, 529–535 CrossRef CAS.
- D. Bresolin, A. S. Estrella, J. R. P. da Silva, A. Valério, C. Sayer and P. H. H. de Araújo, et al., Synthesis of a green polyurethane foam from a biopolyol obtained by enzymatic glycerolysis and its use for immobilization of lipase N. S.-40116, Bioprocess Biosyst. Eng., 2019, 42, 213–222 CrossRef CAS PubMed.
- L. Goujard, P.-J. Roumanet, B. Barea, Y. Raoul, F. Ziarelli and J. Le Petit, et al., Evaluation of the Effect of Chemical or Enzymatic Synthesis Methods on Biodegradability of Polyesters, J. Polym. Environ., 2016, 24, 64–71 CrossRef CAS.
- L. Liang, J. Long and G. Li, Lipase-catalyzed synthesis of hyperbranched polyester improved by autocatalytic prepolymerization process, J. Appl. Polym. Sci., 2019, 136, 47221 CrossRef.
- X. He, G. Wu, L. Xu, J. Yan and Y. Yan, Lipase-Catalyzed Synthesis, Properties Characterization, and Application of Bio-Based Dimer Acid Cyclocarbonate, Polymer, 2018, 10, 262 CAS.
- Y. Liang, Y. Zhang, Y. Hu, B. Xia, X. Lin and Q. Wu, Lipase-catalyzed synthesis of chiral poly(ester amide)s with an alternating sequence of hydroxy acid and l/d-aspartate units, Polym. Chem., 2018, 9, 1412–1420 RSC.
- X. Bao, L. Niu, Y. Wang, Z. Zeng, X. Lai and Z. Jia, et al., Properties and Structure of Myristate Acid Starch Esters Prepared by Lipase, Zhongguo Liangyou Xuebao, 2017, 32, 52–57 Search PubMed.
- X. Bao, L. Niu, Y. Wang, J. Chen, J. Yu and L. Fang, et al., Solvent-free system enzymatic synthesis of myristate acid starch ester and properties, Zhongguo Liangyou Xuebao, 2017, 32, 29–33 Search PubMed.
- J. Yuan, Z. Xiang, X. Fan, Q. Wang, J. Yao and W. Tang, et al., Preparation of hydrophobic starch through lipase-catalyzed reaction in ionic liquids, Shipin Yu Shengwu Jishu Xuebao, 2017, 36, 1152–1156 Search PubMed.
- D. Li, X. Zhang and Y. Tian, Ionic liquids as novel solvents for biosynthesis of octenyl succinic anhydride-modified waxy maize starch, Int. J. Biol. Macromol., 2016, 86, 119–125 CrossRef CAS PubMed.
- S. Weinberger, A. Pellis, J. Comerford, T. Farmer and G. Guebitz, Efficient Physisorption of Candida antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis, Catalysts, 2018, 8, 369 CrossRef.
- W. Huang, N. Zhu, Y. Liu, J. Wang, J. Zhong and Q. Sun, et al., A novel microfluidic enzyme-organocatalysis combination strategy for ring-opening copolymerizations of lactone, lactide and cyclic carbonate, Chem. Eng. J., 2019, 356, 592–597 CrossRef CAS.
- C.-T. Li, M. Zhang, Y.-X. Weng and J.-X. Qin, Influence of ether linkage on the enzymatic degradation of PBS copolymers: Comparative study on poly (butylene succinate-co-diethylene glycol succinate) and poly (butylene succinate-co-butylene diglycolic acid), Int. J. Biol. Macromol., 2018, 118, 347–356 CrossRef CAS PubMed.
- S. Sun, G. Wang and P. Wang, A cleaner approach for biodegradable lubricants production by enzymatic glycerolysis of castor oil and kinetic analysis, J. Cleaner Prod., 2018, 188, 530–535 CrossRef CAS.
- E. D. C. Cavalcanti, É. C. G. Aguieiras, P. R. da Silva, J. G. Duarte, E. P. Cipolatti and R. Fernandez-Lafuente, et al., Improved production of biolubricants from soybean oil and different polyols via esterification reaction catalyzed by immobilized lipase from Candida rugosa, Fuel, 2018, 215, 705–713 CrossRef CAS.
- H. Tirunagari, L. Kuna, B. Shalini and K. Thenkrishnan, Ammonolysis of (5S)-N-(tert-butoxycarbonyl)-5-(methoxycarbonyl)-2-pyrroline with immobilized Candida antarctica lipase B (CALB) in a packed bed reactor, Process Biochem., 2018, 65, 109–114 CrossRef CAS.
- A. M. Testera, M. Santos, A. Girotti, F. J. Arias, J. M. Báñez and M. Alonso, et al., A novel lipase-catalyzed method for preparing ELR-based bioconjugates, Int. J. Biol. Macromol., 2019, 121, 752–759 CrossRef CAS PubMed.
- R. Ménard, S. Caillol and F. Allais, Ferulic acid-based renewable esters and amides-containing epoxy thermosets from wheat bran and beetroot pulp: Chemo-enzymatic synthesis and thermo-mechanical properties characterization, Ind. Crops Prod., 2017, 95, 83–95 CrossRef.
- Y. Chen, Y. Wang, Q. Jin, X. Wang, J. Hu and X. Wang, Preparation of arachidonoyl ethanolamide by enzymatic amidation of arachidonic acid purified from microbial oil, Process Biochem., 2018, 66, 120–125 CrossRef CAS.
- X. Tian, S. Zhang and L. Zheng, First Novozym 435 lipase-catalyzed Morita-Baylis-Hillman reaction in the presence of amides, Enzyme Microb. Technol., 2016, 84, 32–40 CrossRef CAS PubMed.
- L. Zhang, F. Li, C. Wang, L. Zheng, Z. Wang and R. Zhao, et al., Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage, Catalysts, 2017, 7, 115 CrossRef.
- Z. Wang, X. Chen, C. Wang, L. Zhang, F. Li and W. Zhang, et al., A mild and efficient dakin reaction mediated by lipase, Green Chem. Lett. Rev., 2017, 10, 269–273 CrossRef CAS.
- W. E. Ladner and G. M. Whitesides, Lipase-catalyzed hydrolysis as a route to esters of chiral epoxy alcohols, J. Am. Chem. Soc., 1984, 106, 7250–7251 CrossRef CAS.
- A. S. Bajwa, S. Sathaye, V. M. Kulkarni and A. V. Patwardhan, Chemoenzymatic epoxidation of Karanja oil: An alternative to chemical epoxidation?, Asia-Pac. J. Chem. Eng., 2016, 11, 314–322 CrossRef CAS.
- B. M. Abdullah, R. M. Yusop, J. Salimon, D. Derawi and W. A. Ahmed, Epoxidation synthesis of linoleic acid for renewable energy applications, Malays. J. Anal. Sci., 2016, 20, 131–141 CrossRef.
- W. Liu, J. Chen, R. Liu and Y. Bi, Revisiting the Enzymatic Epoxidation of Vegetable Oils by Perfatty Acid: Perbutyric Acid Effect on the Oil with Low Acid Value, J. Am. Oil Chem. Soc., 2016, 93, 1479–1486 CrossRef CAS.
- J. Chen, J. Zhou, W. Liu, Y. Bi and D. Peng, Enzymatic epoxidation of soybean oil in the presence of perbutyric acid, Chem. Pap., 2017, 71, 2139–2144 CrossRef CAS.
- M. S. Bhalerao, V. M. Kulkarni and A. V. Patwardhan, Ultrasound-assisted chemoenzymatic epoxidation of soybean oil by using lipase as biocatalyst, Ultrason. Sonochem., 2018, 40, 912–920 CrossRef CAS.
- G. V. Waghmare and V. K. Rathod, Ultrasound assisted enzyme catalyzed hydrolysis of waste cooking oil under solvent free condition, Ultrason. Sonochem., 2016, 32, 60–67 CrossRef CAS PubMed.
- K. A. Omar, M. E. Gounga, R. Liu, E. Mlyuka and X. Wang, Effects of microbial lipases on hydrolyzed milk fat at different time intervals in flavour development and oxidative stability, J. Food Sci. Technol., 2016, 53, 1035–1046 CrossRef CAS.
- D. Derawi, N. A. Z. Azman and M. F. Jumadi, Preliminary study on production of monoacylglycerol and diacylglycerol of virgin coconut oil via enzymatic glycerolysis using lipase Candida antarctica (Novozym 435), Malays. J. Anal. Sci., 2017, 21, 37–45 CrossRef.
- K. A. Omar, M. E. Gounga, R. Liu, W. Mwinyi, W. Aboshora and A. H. Ramadhan, et al., Triacylglycerol composition, melting and crystallization profiles of lipase catalysed anhydrous milk fats hydrolysed, Int. J. Food Prop., 2017, 20, 1230–1245 CAS.
- A. A. Odaneth, R. N. Vadgama, A. D. Bhat and A. M. Lali, Tuning Lipase Reaction for Production of Fatty Acids from Oil, Appl. Biochem. Biotechnol., 2016, 180, 504–515 CrossRef CAS PubMed.
- K. A. Omar, M. E. Gounga, R. Liu, W. Aboshora, N. Q. M. Al-Hajj and Q. Jin, et al., Influence of lipase under ultrasonic microwave assisted extraction on changes of triacylglycerol distribution and melting profiles during lipolysis of milk fat, RSC Adv., 2016, 6, 100857–100865 RSC.
- V. Kasche, Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products, Enzyme Microb. Technol., 1986, 8, 4–16 CrossRef CAS.
- M. Corzo-Martínez, L. Vázquez, P. Arranz-Martínez, N. Menéndez, G. Reglero and C. F. Torres, Production of a bioactive lipid-based delivery system from ratfish liver oil by enzymatic glycerolysis, Food Bioprod. Process., 2016, 100, 311–322 CrossRef.
- P. Arranz-Martínez, M. Corzo-Martínez, L. Vázquez, G. Reglero and C. F. Torres, Lipase catalyzed glycerolysis of ratfish liver oil at stirred tank basket reactor: A kinetic approach, Process Biochem., 2018, 64, 38–45 CrossRef.
- D. Li, W. Wang, X. Qin, X. Li, B. Yang and Y. Wang, A Novel Process for the Synthesis of Highly Pure n-3 Polyunsaturated Fatty Acid (PUFA)-Enriched Triglycerides by Combined Transesterification and Ethanolysis, J. Agric. Food Chem., 2016, 64, 6533–6538 CrossRef CAS PubMed.
- N. Zhang, X. Yang, J. Fu, Q. Chen, Z. Song and Y. Wang, Production of diacylglycerol-enriched oil by glycerolysis of soybean oil using a bubble column reactor in a solvent-free system, J. Oleo Sci., 2016, 65, 207–216 CrossRef CAS PubMed.
- Á. G. Solaesa, M. T. Sanz, R. Melgosa and S. Beltrán, Substrates emulsification process to improve lipase-catalyzed sardine oil glycerolysis in different systems. Evaluation of lipid oxidation of the reaction products, Food Res. Int., 2017, 100, 572–578 CrossRef PubMed.
- N. N. A. Jamlus, J. Salimon and D. Derawi, Enzymatic glycerolysis of methyl laurate utilizing Candida antarctica lipase B, Malays. J. Anal. Sci., 2016, 20, 1365–1372 CrossRef.
- Y. Zhang, X. Wang, D. Xie, S. Zou, Q. Jin and X. Wang, Synthesis and concentration of 2-monoacylglycerols rich in polyunsaturated fatty acids, Food Chem., 2018, 250, 60–66 CrossRef CAS PubMed.
- T. Liu, Y. Zhao, T. Wang, Y. Qu, T. Li and D. Yu, Studies on the Synthesis of Low-Energy SLS-Type Structure Lipid by Two Enzymatic Steps, Zhongguo Shipin Xuebao, 2017, 17, 75–83 Search PubMed.
- Y. Zhang, X. Wang, S. Zou, D. Xie, Q. Jin and X. Wang, Synthesis of 2-docosahexaenoylglycerol by enzymatic ethanolysis, Bioresour. Technol., 2018, 251, 334–340 CrossRef CAS PubMed.
- Á. G. Solaesa, M. T. Sanz, M. Falkeborg, S. Beltrán and Z. Guo, Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation, Food Chem., 2016, 190, 960–967 CrossRef PubMed.
- H.-J. Lee, M. Haq, P. S. Saravana, Y.-N. Cho and B.-S. Chun, Omega-3 fatty acids concentrate production by enzyme-catalyzed ethanolysis of supercritical CO2 extracted oyster oil, Biotechnol. Bioprocess Eng., 2017, 22, 518–528 CrossRef CAS.
- L. Vázquez, N. González, G. Reglero and C. Torres, Solvent-Free Lipase-Catalyzed Synthesis of Diacylgycerols as Low-Calorie Food Ingredients, Front. Bioeng. Biotechnol., 2016, 4, 6 Search PubMed.
- X. Yan, X. Zhao, G. Ma, L. Dai, W. Du and D. Liu, Enzymatic ethanolysis of fish oil for selective concentration of polyunsaturated fatty acids (PUFAs) with flexible production of corresponding glycerides and ethyl esters, J. Chem. Technol. Biotechnol., 2018, 93, 2399–2405 CrossRef CAS.
- M. Haq, P. Pendleton and B.-S. Chun, Utilization of Atlantic Salmon By-product Oil for Omega-3 Fatty Acids Rich 2-Monoacylglycerol Production: Optimization of Enzymatic Reaction Parameters, Waste Biomass Valoriz. DOI:10.1007/s12649-018-0392-9 , in press.
- X. Diao, H. Guan, B. Kong and X. Zhao, Preparation of diacylglycerol from lard by enzymatic glycerolysis and its compositional characteristics, Korean J. Food Sci. Anim. Resour., 2017, 37, 813–822 Search PubMed.
- Z. Zhang, F. Liu, X. Ma, H. Huang and Y. Wang, Two-Stage Enzymatic Preparation of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) Enriched Fish Oil Triacylglycerols, J. Agric. Food Chem., 2018, 66, 218–227 CrossRef CAS PubMed.
- S. B. More, J. T. Waghmare and P. R. Gogate, Ultrasound pretreatment as a novel approach for intensification of lipase catalyzed esterification of tricaprylin, Ultrason. Sonochem., 2017, 36, 253–261 CrossRef CAS PubMed.
- R. Morales-Medina, M. Munio, A. Guadix and E. M. Guadix, Development of an up-grading process to produce MLM structured lipids from sardine discards, Food Chem., 2017, 228, 634–642 CrossRef CAS PubMed.
- S.-S. Chen, S.-Z. Luo, Z. Zheng, Y.-Y. Zhao, M. Pang and S.-T. Jiang, Enzymatic lipophilization of epicatechin with free fatty acids and its effect on antioxidative capacity in crude camellia seed oil, J. Sci. Food Agric., 2017, 97, 868–874 CrossRef CAS PubMed.
- A. A. Esperón-Rojas, R. Baeza-Jiménez, C. Cano-Sarmiento and H. S. García, Structured mono- and diacylglycerols with a high content of medium chain fatty acids, J. Oleo Sci., 2017, 66, 991–996 CrossRef PubMed.
- M. Liu, J. Fu, Y. Teng, Z. Zhang, N. Zhang and Y. Wang, Fast Production of Diacylglycerol in a Solvent Free System via Lipase Catalyzed Esterification Using a Bubble Column Reactor, J. Am. Oil Chem. Soc., 2016, 93, 637–648 CrossRef CAS.
- D. Li, W. Wang, L. Zhang, N. Liu, M. Faiza and C. P. Tan, et al., Synthesis of C. L.A-Rich Lysophosphatidylcholine by Immobilized M. A.S1-H108A-Catalyzed Esterification: Effects of the Parameters and Monitoring of the Reaction Process, Eur. J. Lipid Sci. Technol., 2018, 120, 1700529 CrossRef.
- S. B. More, J. S. Waghmare, P. R. Gogate and S. N. Naik, Improved synthesis of medium chain triacylglycerol catalyzed by lipase based on use of supercritical carbon dioxide pretreatment, Chem. Eng. J., 2018, 334, 1977–1987 CrossRef CAS.
- N. H. Kim, H. Kim, N. Choi, Y. Kim, B. H. Kim and I.-H. Kim, Production of stearidonic acid-rich triacylglycerol via a two-step enzymatic esterification, Food Chem., 2019, 270, 332–337 CrossRef CAS PubMed.
- D. Li, W. Wang, X. Li, R. Durrani, B. Yang and Y. Wang, Preparation of Highly Pure n-3 PUFA-Enriched Triacylglycerols by Two-Step Enzymatic Reactions Combined with Molecular Distillation, J. Am. Oil Chem. Soc., 2017, 94, 225–233 CrossRef CAS.
- H. Woo, J. Kim, I.-H. Kim, H.-D. Choi, I.-W. Choi and B. H. Kim, Substrate selectivity of Novozym 435 in the esterification of glycerol with an equimolar mixture of linoleic, conjugated linoleic, and pinolenic acids, Eur. J. Lipid Sci. Technol., 2016, 118, 928–937 CrossRef CAS.
- I. Aissa, N. Kharrat, F. Aloui, M. Sellami, M. Bouaziz and Y. Gargouri, Valorization of antioxidants extracted from olive mill wastewater, Biotechnol. Appl. Biochem., 2017, 64, 579–589 CrossRef CAS PubMed.
- H. R. Reyes and C. G. Hill, Kinetic modeling of interesterification reactions catalyzed by immobilized lipase, Biotechnol. Bioeng., 1994, 43, 171–182 CrossRef CAS PubMed.
- D. Kowalska and E. Gruczynska, The physico-chemical properties and oxidative stabilities of enzymatically interesterified lard and rapeseed oil blends containing 35 and 25% of lard, Riv. Ital. Sostanze Grasse, 2016, 93, 11–20 CAS.
- M. Marín-Suárez, R. Morales-Medina, E. M. Guadix and A. Guadix, A Simple Enzymatic Process to Produce Functional Lipids From Vegetable and Fish Oil Mixtures, Eur. J. Lipid Sci. Technol., 2017, 119, 1700233 CrossRef.
- Y. Li, J. Zhao, X. Xie, Z. Zhang, N. Zhang and Y. Wang, A low trans margarine fat analog to beef tallow for healthier formulations: Optimization of enzymatic interesterification using soybean oil and fully hydrogenated palm oil, Food Chem., 2018, 255, 405–413 CrossRef CAS PubMed.
- S. A. Korma, X. Zou, A. H. Ali, S. M. Abed, Q. Jin and X. Wang, Preparation of structured lipids enriched with medium- and long-chain triacylglycerols by enzymatic interesterification for infant formula, Food Bioprod. Process., 2018, 107, 121–130 CrossRef CAS.
- Y. S. Prasad, B. Saritha, A. Tamizhanban, K. Lalitha, S. Kabilan and C. U. Maheswari, et al., Enzymatic synthesis and self-assembly of glycolipids: robust self-healing and wound closure performance of assembled soft materials, RSC Adv., 2018, 8, 37136–37145 RSC.
- J. Lu, Q. Jin, X. Wang and X. Wang, Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system, Process Biochem., 2017, 54, 89–95 CrossRef CAS.
- S. A. Willett and C. C. Akoh, Application of Taguchi Method in the Enzymatic Modification of Menhaden Oil to Incorporate Capric Acid, J. Am. Oil Chem. Soc., 2018, 95, 299–311 CrossRef CAS.
- D. L. Compton, J. R. Goodell, M. A. Berhow, J. A. Kenar, S. C. Cermak and K. O. Evans, Feruloylated Products from Coconut Oil and Shea Butter, J. Am. Oil Chem. Soc., 2017, 94, 397–411 CrossRef CAS.
- T.-W. Zhu, H.-T. Weng, X. Zhang, H. Wu and B. Li, Mechanistic insight into the relationship between triacylglycerol and crystallization of lipase-catalyzed interesterified blend of palm stearin and vegetable oil, Food Chem., 2018, 260, 306–316 CrossRef CAS PubMed.
- C. A. Álvarez and C. C. Akoh, Enzymatic Synthesis of High sn-2 DHA and ARA Modified Oils for the Formulation of Infant Formula Fat Analogues, J. Am. Oil Chem. Soc., 2016, 93, 383–395 CrossRef.
- S. B. More, P. Gogate, J. Waghmare and S. N. Naik, Intensified synthesis of structured lipids from oleic acid rich moringa oil in the presence of supercritical CO2, Food Bioprod. Process., 2018, 112, 86–95 CrossRef CAS.
- X. Cao, W. Wei, X. Zhang and F. Feng, Analysis of Regioselectivity of Lipases Using Acidolysis of Lauric Acid and Camellia Oil, Zhongguo Liangyou Xuebao, 2017, 32, 74–80 Search PubMed.
- Y. He, C. Qiu, Z. Guo, J. Huang, M. Wang and B. Chen, Production of new human milk fat substitutes by enzymatic acidolysis of microalgae oils from Nannochloropsis oculata and Isochrysis galbana, Bioresour. Technol., 2017, 238, 129–138 CrossRef CAS PubMed.
- S. Sun, P. Wang and S. Zhu, Enzymatic incorporation of caffeoyl into castor oil to prepare the novel castor oil-based caffeoyl structured lipids, J. Biotechnol., 2017, 249, 66–72 CrossRef CAS PubMed.
- Z. Zhang, X. Ma, H. Huang, G. Li and Y. Wang, Enzymatic Production of Highly Unsaturated Monoacyglycerols and Diacylglycerols and Their Emulsifying Effects on the Storage Stability of a Palm Oil Based Shortening System, J. Am. Oil Chem. Soc., 2017, 94, 1175–1188 CrossRef CAS.
- C. M. Verdasco-Martín, E. Garcia-Verdugo, R. Porcar, R. Fernandez-Lafuente and C. Otero, Selective synthesis of partial glycerides of conjugated linoleic acids via modulation of the catalytic properties of lipases by immobilization on different supports, Food Chem., 2018, 245, 39–46 CrossRef PubMed.
- N. Bassan, R. H. Rodrigues, R. Monti, C. Tecelão, S. Ferreira-Dias and A. V. Paula, Enzymatic modification of grapeseed (Vitis vinifera L.) oil aiming to obtain dietary triacylglycerols in a batch reactor, LWT--Food Sci. Technol., 2019, 99, 600–606 CrossRef CAS.
- B. Norjannah, H. C. Ong and H. H. Masjuki, Effects of methanol and enzyme pretreatment to Ceiba pentandra biodiesel production, Energy Sources, Part A, 2017, 39, 1548–1555 CrossRef CAS.
- D. A. Sánchez, G. M. Tonetto and M. L. Ferreira, An insight on acyl migration in solvent-free ethanolysis of model triglycerides using Novozym 435, J. Biotechnol., 2016, 220, 92–99 CrossRef PubMed.
- Y. Endo, T. Hatanaka, K. Maeda, K. Arafune, T. Yamamoto and K. Itoh, et al., Use of ethanol with triolein for fatty acid ethyl ester as biodiesel fuel in a Novozym®435 fixed-bed reactor, Biomass Bioenergy, 2018, 108, 433–438 CrossRef CAS.
- K. Maeda, H. Kuramochi, K. Arafune, K. Itoh and T. Yamamoto, Transesterification of triolein and methanol by Novozym 435 with dimethyl ether, J. Chem. Eng. Jpn., 2017, 50, 924–928 CrossRef CAS.
- S. K. Karmee, Enzymatic Biodiesel Production from Manilkara Zapota (L.) Seed Oil, Waste Biomass Valoriz., 2018, 9, 725–730 CrossRef CAS.
- F. Strniša, M. Bajić, P. Panjan, I. Plazl, A. M. Sesay and P. Žnidaršič-Plazl, Characterization of an enzymatic packed-bed microreactor: Experiments and modeling, Chem. Eng. J., 2018, 350, 541–550 CrossRef.
- M. W. Mumtaz, H. Mukhtar, U. A. Dilawer, S. M. Hussain, M. Hussain and M. Iqbal, et al., Biocatalytic transesterification of Eruca sativa oil for the production of biodiesel, Biocatal. Agric. Biotechnol., 2016, 5, 162–167 CrossRef.
- R. Manurung, S. Wage and T. Taslim, Optimization of enzymatic process in biodiesel production for green process development, J. Eng. Appl. Sci., 2017, 12, 2485–2491 Search PubMed.
- W. R. R. Fidalgo, A. Ceron, L. Freitas, J. C. Santos and H. F. de Castro, A fluidized bed reactor as an approach to enzymatic biodiesel production in a process with simultaneous glycerol removal, J. Ind. Eng. Chem., 2016, 38, 217–223 CrossRef CAS.
- B. Infanzón, S. Cesarini, J. Martínez, F. I. J. Pastor and P. Diaz, Alternative Oils Tested as Feedstocks for Enzymatic FAMEs Synthesis: Toward a More Sustainable Process, Biotechnol. Prog., 2017, 33, 1209–1217 CrossRef PubMed.
- G. Ma, L. Dai, D. Liu and W. Du, Lipase-Mediated Selective Methanolysis of Fish Oil for Biodiesel Production and Polyunsaturated Fatty Acid Enrichment, Energy Fuels, 2018, 32, 7630–7635 CrossRef CAS.
- J. M. Encinar, J. F. González, N. Sánchez and S. Nogales-Delgado, Sunflower oil transesterification with methanol using immobilized lipase enzymes, Bioprocess Biosyst. Eng., 2019, 42, 157–166 CrossRef CAS PubMed.
- I. A. Nehdi, H. M. Sbihi, L. E. Blidi, U. Rashid, C. P. Tan and S. I. Al-Resayes, Biodiesel production from Citrillus colocynthis oil using enzymatic based catalytic reaction and characterization studies, Protein Pept. Lett., 2018, 25, 164–170 CrossRef CAS PubMed.
- H. C. Nguyen, S.-H. Liang, T. T. Doan, C.-H. Su and P.-C. Yang, Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology, Energy Convers. Manage., 2017, 145, 335–342 CrossRef CAS.
- H. C. Nguyen, S.-H. Liang, S.-S. Chen, C.-H. Su, J.-H. Lin and C.-C. Chien, Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology, Energy Convers. Manage., 2018, 158, 168–175 CrossRef CAS.
- J. Chen, X. Lu, Y. Nie and J. Ji, Production of biodiesel through transesterification of soybean oil catalyzed by immobilized lipase, Zhongguo Liangyou Xuebao, 2016, 31, 57–60 Search PubMed.
- S. K. Karmee, W. Swanepoel and S. Marx, Biofuel production from spent coffee grounds via lipase catalysis, Energy Sources, Part A, 2018, 40, 294–300 CrossRef CAS.
- E. Navarro López, A. Robles Medina, P. A. González Moreno, L. Esteban Cerdán and E. Molina Grima, Extraction of microalgal lipids and the influence of polar lipids on biodiesel production by lipase-catalyzed transesterification, Bioresour. Technol., 2016, 216, 904–913 CrossRef PubMed.
- E. Navarro López, A. Robles Medina, L. Esteban Cerdán, P. A. González Moreno, M. D. Macías Sánchez and E. Molina Grima, Fatty acid methyl ester production from wet microalgal biomass by lipase-catalyzed direct transesterification, Biomass Bioenergy, 2016, 93, 6–12 CrossRef.
- K. H. Kim, O. K. Lee, C. H. Kim, J.-W. Seo, B.-R. Oh and E. Y. Lee, Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass, Bioresour. Technol., 2016, 211, 472–477 CrossRef CAS PubMed.
- D. C. Panadare and V. K. Rathod, Microwave assisted enzymatic synthesis of biodiesel with waste cooking oil and dimethyl carbonate, J. Mol. Catal. B: Enzym., 2016, 133, S518–S524 CrossRef.
- K. H. Kim and E. Y. Lee, Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate, Energies, 2017, 11, 82 CrossRef.
- M. Hajar and F. Vahabzadeh, Production of a biodiesel additive in a stirred basket reactor using immobilized lipase: Kinetic and mass transfer analysis, Korean J. Chem. Eng., 2016, 33, 1220–1231 CrossRef CAS.
- Y. Li, H. Wang, J. Lu, A. Chu, L. Zhang and Z. Ding, et al., Preparation of immobilized lipase by modified polyacrylonitrile hollow membrane using nitrile-click chemistry, Bioresour. Technol., 2019, 274, 9–17 CrossRef CAS PubMed.
- C. M. T. Santin, S. Michelin, R. P. Scherer, A. Valério, M. D. Luccio and D. Oliveira, et al., Comparison of macauba and soybean oils as substrates for the enzymatic biodiesel production in ultrasound-assisted system, Ultrason. Sonochem., 2017, 35, 525–528 CrossRef CAS PubMed.
- M. C. P. Zenevicz, A. Jacques, M. J. A. Silva, A. Furigo, V. Oliveira and D. de Oliveira, Study of a reactor model for enzymatic reactions in continuous mode coupled to an ultrasound bath for esters production, Bioprocess Biosyst. Eng., 2018, 41, 1589–1597 CrossRef CAS PubMed.
- L. T. A. Souza, A. A. Mendes and Castro H. F. de, Selection of Lipases for the Synthesis of Biodiesel from Jatropha Oil and the Potential of Microwave Irradiation to Enhance the Reaction Rate, BioMed Res. Int., 2016, 2016, 1404567 Search PubMed.
- J. Qin, X. Zou, S. Lv, Q. Jin and X. Wang, Influence of ionic liquids on lipase activity and stability in alcoholysis reactions, RSC Adv., 2016, 6, 87703–87709 RSC.
- G. Bauer, S. Lima, J. Chenevard, M. Sugnaux and F. Fischer, Biodiesel via in situ wet microalgae biotransformation: Zwitter-type ionic liquid supported extraction and transesterification, ACS Sustainable Chem. Eng., 2017, 5, 1931–1937 CrossRef CAS.
- M. Nordblad, A. K. Pedersen, A. Rancke-Madsen and J. M. Woodley, Enzymatic pretreatment of low-grade oils for biodiesel production, Biotechnol. Bioeng., 2016, 113, 754–760 CrossRef CAS PubMed.
- T. M. Mata, I. Trovisco, A. Pinto, E. Matos, A. A. Martins and N. S. Caetano, Enzymatic esterification for acidity reduction of poultry fat, Chem. Eng. Trans., 2017, 57, 2005–2010 Search PubMed.
- D. A. Teixeira, C. R. da Motta, C. M. S. Ribeiro and A. M. de Castro, A rapid enzyme-catalyzed pretreatment of the acidic oil of macauba (Acrocomia aculeata) for chemoenzymatic biodiesel production, Process Biochem., 2017, 53, 188–193 CrossRef CAS.
- X. Wang, X. Wang and T. Wang, An effective method for reducing free fatty acid content of high-acid rice bran oil by enzymatic amidation, J. Ind. Eng. Chem., 2017, 48, 119–124 CrossRef CAS.
- J. Ryu, N. Choi, H. Kim, B. H. Kim, H.-R. Kim and I.-H. Kim, Synthesis of fatty acid methyl esters using mixed enzyme in a packed bed reactor, J. Oleo Sci., 2018, 67, 321–326 CrossRef CAS PubMed.
- K. Pedro, J. Parreira, I. Correia, C. Henriques and M. Langone, Enzymatic biodiesel synthesis from acid oil using a lipase mixture, Quim. Nova, 2017, 41, 284–291 Search PubMed.
- N. Choi, J.-S. Lee, J. Kwak, J. Lee and I.-H. Kim, Production of biodiesel from acid oil via a two-step enzymatic transesterification, J. Oleo Sci., 2016, 65, 913–921 CrossRef CAS PubMed.
- C. José, R. D. Bonetto, L. A. Gambaro, M. P. Guauque Torres, M. L. Foresti and M. L. Ferreira, et al., Investigation of the causes of deactivation-degradation of the commercial biocatalyst Novozym 435 in ethanol and ethanol-aqueous media, J. Mol. Catal. B: Enzym., 2017, 71, 95–107 CrossRef.
- C. José and L. E. Briand, Deactivation of Novozym® 435 during the esterification of ibuprofen with ethanol: Evidences of the detrimental effect of the alcohol, React. Kinet., Mech. Catal., 2010, 99, 17–22 Search PubMed.
- L. Hilterhaus, O. Thum and A. Liese, Reactor concept for lipase-catalyzed solvent-free conversion of highly viscous reactants forming two-phase systems, Org. Process Res. Dev., 2008, 12, 618–625 CrossRef CAS.
- I. Petry, A. Ganesan, A. Pitt, B. D. Moore and P. J. Halling, Proteomic methods applied to the analysis of immobilized biocatalysts, Biotechnol. Bioeng., 2006, 95, 984–991 CrossRef CAS PubMed.
- J. C. S. dos Santos, N. Rueda, L. R. B. Gonçalves and R. Fernandez-Lafuente, Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment, Enzyme Microb. Technol., 2015, 77, 1–7 CrossRef PubMed.
- B. Schyrr, S. Pasche, G. Voirin, C. Weder, Y. C. Simon and E. J. Foster, Biosensors based on porous cellulose nanocrystal-poly(vinyl alcohol) scaffolds, ACS Appl. Mater. Interfaces, 2014, 6, 12674–12683 CrossRef CAS PubMed.
- P. Saunders and J. Brask, Improved Immobilization Supports for Candida Antarctica Lipase B, ed. Loos K, in Biocatalysis in Polymer Chemistry Weinheim, Germany, Wiley-V. C.H Verlag GmbH & Co. K. G.aA, 2010, pp. 65–82 Search PubMed.
- B. Chen, E. M. Miller, L. Miller, J. J. Maikner and R. A. Gross, Effects of macroporous resin size on Candida antarctica lipase B adsorption, fraction of active molecules, and catalytic activity for polyester synthesis, Langmuir, 2007, 23, 1381–1387 CrossRef CAS PubMed.
- P. Nicolás, V. L. Lassalle and M. L. Ferreira, About the role of typical spacer/crosslinker on the design of efficient magnetic biocatalysts based on nanosized magnetite, J. Mol. Catal. B: Enzym., 2015, 122, 296–304 CrossRef.
- J. Sun, Y. Jiang, L. Zhou and J. Gao, Immobilization of Candida antarctica lipase B by adsorption in organic medium, New Biotechnol., 2010, 27, 53–58 CrossRef CAS PubMed.
- H. Zhao and Z. Song, Migration of reactive trace compounds from Novozym® 435 into organic solvents and ionic liquids, Biochem. Eng. J., 2010, 49, 113–118 CrossRef CAS.
- A. Idris and A. Bukhari, Immobilized Candida antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis, Biotechnol. Adv., 2012, 30, 550–563 CrossRef CAS PubMed.
- M. L. Foresti, M. Galle, M. L. Ferreira and L. E. Briand, Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: experimental and molecular modeling aspects, J. Chem. Technol. Biotechnol., 2009, 84, 1461–1473 CrossRef CAS.
- M. V. Toledo, C. José, S. E. Collins, R. D. Bonetto, M. L. Ferreira and L. E. Briand, Esterification of R/S-ketoprofen with 2-propanol as reactant and solvent catalyzed by Novozym® 435 at selected conditions, J. Mol. Catal. B: Enzym., 2012, 83, 108–119 CrossRef CAS.
- M. V. Toledo, C. José, S. E. Collins, M. L. Ferreira and L. E. Briand, Towards a green enantiomeric esterification of R/S-ketoprofen: A theoretical and experimental investigation, J. Mol. Catal. B: Enzym., 2015, 118, 52–61 CrossRef CAS.
- M. L. Foresti, G. Valle, R. Bonetto, M. L. Ferreira and L. E. Briand, FTIR, SEM and fractal dimension characterization of lipase B from Candida antarctica immobilized onto titania at selected conditions, Appl. Surf. Sci., 2010, 256, 1624–1635 CrossRef CAS.
- J. C. S. dos Santos, O. Barbosa, C. Ortiz, A. Berenguer-Murcia, R. C. Rodrigues and R. Fernandez-Lafuente, Importance of the Support Properties for Immobilization or Purification of Enzymes, ChemCatChem, 2015, 7, 2413–2432 CrossRef CAS.
- K. T. Kunas and E. T. Papoutsakis, Damage mechanisms of suspended animal cells in agitated bioreactors with and without bubble entrainment, Biotechnol. Bioeng., 1990, 36, 476–483 CrossRef CAS PubMed.
- W.-L. Liu, S.-H. Lo, B. Singco, C.-C. Yang, H.-Y. Huang and C.-H. Lin, Novel trypsin–FITC@MOF bioreactor efficiently catalyzes protein digestion, J. Mater. Chem. B, 2013, 1, 928–932 RSC.
- R. L. Giordano, R. C. Giordano and C. L. Cooney, Performance of a continuous Taylor–Couette–Poiseuille vortex flow enzymic reactor with suspended particles, Process Biochem., 2000, 35, 1093–1101 CrossRef CAS.
- A. Marty, V. Dossat and J.-S. Condoret, Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: Influence of the production of hydrophilic compounds, Biotechnol. Bioeng., 1997, 56, 232–237 CrossRef CAS PubMed.
- P. Pires-Cabral, E. Dubreucq, M. M. R. Da Fonseca and S. Ferreira-Dias, Partitioning of water in organic systems with lipase immobilized in polyurethane foams, Biochem. Eng. J., 2005, 26, 29–37 CrossRef CAS.
- W. Chulalaksananukul, J. S. Condoret, P. Delorme and R. M. Willemot, Kinetic study of esterification by immobilized lipase in n-hexane, FEBS Lett., 1990, 276, 181–184 CrossRef CAS PubMed.
- S. Colombié, R. J. Tweddell, J.-S. Condoret and A. Marty, Water activity control: A way to improve the efficiency of continuous lipase esterification, Biotechnol. Bioeng., 1998, 60, 362–368 CrossRef.
- P. Mensah and G. Carta, Adsorptive control of water in esterification with immobilized enzymes. Continuous operation in a periodic counter-current reactor, Biotechnol. Bioeng., 1999, 66, 137–146 CrossRef CAS PubMed.
- N. Ognjanovic, D. Bezbradica and Z. Knezevic-Jugovic, Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability, Bioresour. Technol., 2009, 100, 5146–5154 CrossRef CAS PubMed.
- V. Dossat, D. Combes and A. Marty, Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: Influence of the glycerol production, Enzyme Microb. Technol., 1999, 25, 194–200 CrossRef CAS.
- M. Y. Ríos, E. Salazar and H. F. Olivo, Baeyer–Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea–hydrogen peroxide in ethyl acetate, Green Chem., 2007, 9, 459–462 RSC.
- K. Hernandez, A. Berenguer-Murcia, R. C. Rodrigues and R. Fernandez-Lafuente, Hydrogen Peroxide in Biocatalysis. A Dangerous Liaison, Curr. Org. Chem., 2012, 16, 2652–2672 CrossRef CAS.
- U. Törnvall, C. M. Fürst, R. Hatti-Kaul and M. Hedström, Mass spectrometric analysis of peptides from an immobilized lipase: focus on oxidative modifications, Rapid Commun. Mass Spectrom., 2009, 23, 2959–2964 CrossRef PubMed.
- U. Törnvall, M. Hedström, K. Schillén and R. Hatti-Kaul, Structural, functional and chemical changes in Pseudozyma antarctica lipase B on exposure to hydrogen peroxide, Biochimie, 2010, 92, 1867–1875 CrossRef PubMed.
- U. Törnvall, C. Orellana-Coca, R. Hatti-Kaul and D. Adlercreutz, Stability of immobilized Candida antarctica lipase B during chemo-enzymatic epoxidation of fatty acids, Enzyme Microb. Technol., 2007, 40, 447–451 CrossRef.
- K. Hernandez and R. Fernandez-Lafuente, Lipase B from Candida antarctica immobilized on octadecyl Sepabeads: A very stable biocatalyst in the presence of hydrogen peroxide, Process Biochem., 2011, 46, 873–878 CrossRef CAS.
- O. Barbosa, R. Torres, C. Ortiz and R. Fernandez-Lafuente, The slow-down of the CALB immobilization rate permits to control the inter and intra molecular modification produced by glutaraldehyde, Process Biochem., 2012, 47, 766–774 CrossRef CAS.
- C. Pizarro, M. C. Brañes, A. Markovits, G. Fernández-Lorente, J. M. Guisán and R. Chamy, et al., Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis, J. Mol. Catal. B: Enzym., 2012, 78, 111–118 CrossRef CAS.
- G. Fernandez-Lorente, L. Betancor, A. V. Carrascosa, J. M. Palomo and J. M. Guisan, Modulation of the selectivity of immobilized lipases by chemical and physical modifications: Release of omega-3 fatty acids from fish oil, J. Am. Oil Chem. Soc., 2012, 89, 97–102 CrossRef CAS.
- G. Fernandez-Lorente, C. Pizarro, D. López-Vela, L. Betancor, A. V. Carrascosa and B. Pessela, et al., Hydrolysis of fish oil by lipases immobilized inside porous supports, J. Am. Oil Chem. Soc., 2011, 88, 819–826 CrossRef CAS.
- M. Villalba, C. M. Verdasco-Martín, J. C. S. dos Santos, R. Fernandez-Lafuente and C. Otero, Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction, Enzyme Microb. Technol., 2016, 90, 35–44 CrossRef CAS PubMed.
- Z. Cabrera, G. Fernandez-Lorente, R. Fernandez-Lafuente, J. M. Palomo and J. M. Guisan, Enhancement of Novozym-435 catalytic properties by physical or chemical modification, Process Biochem., 2009, 44, 226–231 CrossRef CAS.
- C. M. Verdasco-Martín, M. Villalba, J. C. S. dos Santos, M. Tobajas, R. Fernandez-Lafuente and C. Otero, Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil, Biochem. Eng. J., 2016, 111, 75–86 CrossRef.
- P. Nicolás, V. Lassalle and M. L. Ferreira, Immobilization of C. A.L. B. on lysine-modified magnetic nanoparticles: influence of the immobilization protocol, Bioprocess Biosyst. Eng., 2018, 41, 171–184 CrossRef PubMed.
- E. Abaházi, Z. Boros and L. Poppe, Additives enhancing the catalytic properties of lipase from Burkholderia cepacia immobilized on mixed-function-grafted mesoporous silica gel, Molecules, 2014, 19, 9818–9837 CrossRef PubMed.
- L. O. Wiemann, R. Nieguth, M. Eckstein, M. Naumann, O. Thum and M. B. Ansorge-Schumacher, Composite Particles of Novozyme 435 and Silicone: Advancing Technical Applicability of Macroporous Enzyme Carriers, ChemCatChem, 2009, 1, 455–462 CrossRef CAS.
- C. Forsyth and S. V. Patwardhan, Controlling performance of lipase immobilised on bioinspired silica, J. Mater. Chem. B, 2013, 1, 1164–1174 RSC.
- S. Bhattacharya, A. Drews, E. Lyagin, M. Kraume and M. B. Ansorge-Schumacher, Efficient Chemo-Enzymatic Epoxidation Using silcoat-Novozym®435: Characterizing the Multiphase System, Chem. Eng. Technol., 2012, 35, 1448–1455 CrossRef CAS.
- R. Nieguth, M. Eckstein, L. O. Wiemann, O. Thum and M. B. Ansorge-Schumacher, Enabling Industrial Biocatalytic Processes by Application of Silicone-Coated Enzyme Preparations, Adv. Synth. Catal., 2011, 353, 2522–2528 CrossRef CAS.
- R. Nieguth, L. O. Wiemann, M. Eckstein, O. Thum, D. Poncelet and M. B. Ansorge-Schumacher, Scalable preparation of silCoat -biocatalysts by use of a fluidized-bed reactor, Eng. Life Sci., 2017, 17, 613–619 CrossRef CAS.
- J. Lee, J. Kim, H. Jia, M. I. Kim, J. H. Kwak and S. Jin, et al., Simple synthesis of hierarchically ordered mesocellular mesoporous silica materials hosting crosslinked enzyme aggregates, Small, 2005, 1, 744–753 CrossRef CAS PubMed.
- I. K. Moon, J. Kim, J. Lee, H. Jia, B. N. Hyon and K. Y. Jong, et al., Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: A simple and effective method for enzyme stabilization, Biotechnol. Bioeng., 2007, 96, 210–218 CrossRef PubMed.
- M. Wang, W. Qi, Q. Yu, R. Su and Z. He, Cross-linking enzyme aggregates in the macropores of silica gel: A practical and efficient method for enzyme stabilization, Biochem. Eng. J., 2010, 52, 168–174 CrossRef CAS.
- L. Betancor, F. López-Gallego, A. Hidalgo, M. Fuentes, O. Podrasky and G. Kuncova, et al., Advantages of the pre-immobilization of enzymes on porous supports for their entrapment in sol-gels, Biomacromolecules, 2005, 6, 1027–1030 CrossRef CAS PubMed.
- L. Wilson, A. Illanes, B. C. C. Pessela, O. Abian, R. Fernández-Lafuente and J. M. Guisán, Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media, Biotechnol. Bioeng., 2004, 86, 558–562 CrossRef PubMed.
- P. Selvakumar and P. Sivashanmugam, Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production, Energy Convers. Manage., 2019, 179, 141–151 CrossRef CAS.
- J. Noro, R. L. Reis, A. Cavaco-Paulo and C. Silva, Ultrasound-assisted biosynthesis of novel methotrexate-conjugates, Ultrason. Sonochem., 2018, 48, 51–56 CrossRef CAS PubMed.
- N. R. Khan, S. D. Gawas and V. K. Rathod, Enzyme-catalysed production of n-butyl palmitate using ultrasound-assisted esterification of palmitic acid in a solvent-free system, Bioprocess Biosyst. Eng., 2018, 41, 1621–1634 CrossRef CAS PubMed.
- N. Paludo, J. S. Alves, C. Altmann, M. A. Z. Ayub, R. Fernandez-Lafuente and R. C. Rodrigues, The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase, Ultrason. Sonochem., 2015, 22, 89–94 CrossRef CAS PubMed.
- L. P. Fallavena, F. H. F. Antunes, J. S. Alves, N. Paludo, M. A. Z. Ayub and R. Fernandez-Lafuente, et al., Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei, RSC Adv., 2014, 4, 8675–8681 RSC.
- J. S. Alves, C. Garcia-Galan, M. F. M. Schein, A. M. A. Silva, O. Barbosa and M. M. A. Z. Ayub, et al., Combined Effects of Ultrasound and Immobilization Protocol on Butyl Acetate Synthesis Catalyzed by C. A.L. B, Molecules, 2014, 19, 9562–9576 CrossRef PubMed.
- S. C. Kim, Y. H. Kim, H. Lee, D. Y. Yoon and B. K. Song, Lipase-catalyzed synthesis of glycerol carbonate from renewable glycerol and dimethyl carbonate through transesterification, J. Mol. Catal. B: Enzym., 2007, 49, 75–78 CrossRef CAS.
- A.-F. Hsu, K. Jones, T. A. Foglia and W. N. Marmer, Immobilized lipase-catalysed production of alkyl esters of restaurant grease as biodiesel, Biotechnol. Appl. Biochem., 2002, 36, 181–186 CrossRef CAS PubMed.
- F. Chamouleau, D. Coulon, M. Girardin and M. Ghoul, Influence of water activity and water content on sugar esters lipase-catalyzed synthesis in organic media, J. Mol. Catal. B: Enzym., 2001, 11, 949–954 CrossRef CAS.
- S. Šabeder, M. Habulin and Z. Knez, Lipase-catalyzed synthesis of fatty acid fructose esters, J. Food Eng., 2006, 77, 880–886 CrossRef.
- A. B. Martins, J. L. R. Friedrich, J. C. Cavalheiro, C. Garcia-Galan, O. Barbosa and M. A. Z. Ayub, et al., Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads, Bioresour. Technol., 2013, 134, 417–422 CrossRef CAS PubMed.
- J. L. R. Friedrich, F. P. Peña, C. Garcia-Galan, R. Fernandez-Lafuente, M. A. Z. Ayub and R. C. Rodrigues, Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica, J. Chem. Technol. Biotechnol., 2013, 88, 1089–1095 CrossRef CAS.
- V. G. Tacias-Pascacio, B. Torrestiana-Sánchez, L. Dal Magro, J. J. Virgen-Ortíz, F. J. Suárez-Ruíz and R. C. Rodrigues, et al., Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization, Renewable Energy, 2019, 135, 1–9 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |