Polymer-derived Co/Ni–SiOC(N) ceramic electrocatalysts for oxygen reduction reaction in fuel cells†
Abstract
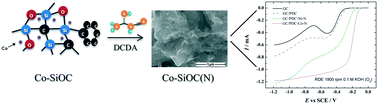
Cobalt/nickel-containing SiOC-based porous ceramic electrocatalysts were prepared by pyrolysis of poly(methyl silsesquioxane) and poly(methyl phenyl silsesquioxane) as preceramic precursors combined with graphite and Co/Ni metal salts at 1000 °C in an atmosphere of nitrogen. Subsequently, the Co/Ni–SiOC materials were N-doped using dicyandiamide (DCDA) as a nitrogen source and pyrolysed at 800 °C in an inert atmosphere. The structural properties and composition of the catalysts were characterised by scanning electron microscopy (SEM), X-ray diffraction, X-ray photoelectron spectroscopy (XPS) and N2 adsorption analysis. The evaluation of the polymer-derived SiOC(N) ceramic electrocatalysts as a new class of catalysts for the oxygen reaction reduction (ORR) was carried out by the rotating disk electrode (RDE) method under acidic, neutral and alkaline conditions. The O2 reduction studies revealed that the N-doped materials exhibited enhanced ORR performance, confirming the positive influence of the nitrogen functionalities introduced into the catalysts. The Co-containing N-doped SiOC catalyst exhibited significantly higher ORR activity compared with the studied materials along with the highest electron transfer number in all the studied solutions. Long-term ORR performance testing indicated that the durability of this catalyst was superior as compared to that of commercial Pt/C. These observations suggest that the Co-containing N-doped SiOC catalyst is a promising cathode material for fuel cells (FCs) and microbial FC devices.


 Please wait while we load your content...
Please wait while we load your content...