Interplay of nucleophilic catalysis with proton transfer in the nitrile reductase QueF from Escherichia coli†
Abstract
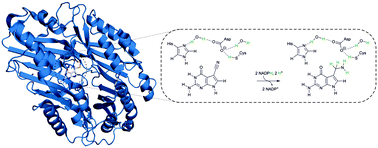
Enzymatic transformations of the nitrile group are important in biology as well as in synthetic chemistry. The enzyme QueF catalyses the conversion of 7-cyano-7-deazaguanine (preQ0) to 7-aminomethyl-7-deazaguanine (preQ1), a unique approach towards biological four-electron reduction of a nitrile to an amine. The catalytic reaction involves a QueF–preQ0 thioimidate adduct that is converted to preQ1 in two NADPH dependent reduction steps via an imine intermediate. The QueF active site comprises a cysteine nucleophile flanked by an aspartic acid and additionally contains a histidine. Here, we used mutagenesis of E. coli QueF (C190A, C190S, D197A, D197H, and H229A) to study the functional interplay between these enzyme residues in covalent catalysis. Substitution of Cys190 or Asp197 annihilates preQ0 covalent binding and largely disrupts the nitrile-to-amine reductase activity. The H229A variant readily forms the thioimidate adduct and is 24-fold less active for preQ0 reduction than wild-type ecQueF (kcat = 7.2 min−1). Using isothermal titration calorimetry, we show that the non-covalent step of preQ0 binding involves proton uptake mediated by Asp197 with His229 as the likely protonated group. Catalytic proton transfer from the Cys190 thiol via Asp197 to the nitrile nitrogen promotes the covalent intermediate. We suggest that protonated (charged) His229 facilitates the polarization of the substrate nitrile for nucleophilic attack on carbon by Cys190, and through proton relay via Asp197, it could provide the proton for re-protonating Cys190 during the formation of the imine intermediate.


 Please wait while we load your content...
Please wait while we load your content...