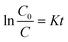Hollow BiVO4/Bi2S3 cruciate heterostructures with enhanced visible-light photoactivity†
Chen
Zhuang
cf,
Lanqin
Tang
 *abef,
Zhentao
Yu
cf,
Tianxiao
Peng
d,
Yongcai
Zhang
d,
Liang
Li
ef,
Yong
Zhou
*abef,
Zhentao
Yu
cf,
Tianxiao
Peng
d,
Yongcai
Zhang
d,
Liang
Li
ef,
Yong
Zhou
 *aef and
Zhigang
Zou
acef
*aef and
Zhigang
Zou
acef
aNational Laboratory of Solid State Microstructures, Department of Physics, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, P. R. China. E-mail: lanqin_tang@163.com; zhouyong1999@nju.edu.cn
bCollege of Chemistry and Chemical Engineering, Yancheng Institute of Technology, Yancheng 22401, P. R. China
cDepartment of Materials Science and Engineering, Nanjing University, Nanjing 210093, P. R. China
dCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, P. R. China
eKey Laboratory of Modern Acoustics, MOE, Institute of Acoustics, Department of Physics, Nanjing University, Nanjing 210093, P. R. China
fEco-materials and Renewable Energy Research Center (ERERC), Nanjing University, Nanjing 210093, P. R. China
First published on 5th December 2018
Abstract
Novel BiVO4/Bi2S3 cruciformities with hollow heterostructures were synthesized by an anion exchange method. The cruciate BiVO4 acted as a template for the formation of special BiVO4/Bi2S3 heterostructures. The proportion of Bi2S3 in the heterostructures could be simply controlled by adjusting the amount of Na2S during the reaction with BiVO4 cruciate flakes. The as-prepared BiVO4/Bi2S3 heterostructures exhibit superior photo-responsive range and enhanced visible-light photoreduction activity for Cr6+ ions. A possible enhanced photoreduction mechanism is proposed.
Introduction
Monoclinic bismuth vanadate (m-BiVO4) with a band gap of 2.4 eV has been widely used for visible-light photocatalytic evolution of O2 and organic matter degradation.1–4 As a consequence of serious recombination of photogenerated electrons and holes, its specific applications still suffer from poor quantum yield.5,6 Therefore, a series of BiVO4-based heterostructures are designed to improve the separation of photoinduced carriers owing to the well-matched energy levels between BiVO4 and semiconductors.7–10Ion exchange is a novel approach for the synthesis of photocatalysts with heterostructures through an ion exchange reaction at the interface of two semiconductors.11–15 Based on the Kirkendall effect, the movement of atoms is from one semiconductor with a lower diffusion coefficient into the other one with a higher diffusion coefficient.16–18 Hollow structures can be eventually generated because of the different diffusion rates of the two components.19–24
Orthorhombic bismuth sulfide (Bi2S3) is a direct semiconductor with a band gap of 1.3 eV, and has received increasing attention owing to its potential applications in many fields such as electrochemical hydrogen storage,25 hydrogen sensors,26 X-ray computed tomography imaging,27 biomolecule detection,28 and lithium ion batteries.29 Due to its narrow band gap and large absorption coefficient, Bi2S3 can be applied as an efficient sensitizer to extend light absorption of semiconductors into the visible-light region.30
Herein, unique hollow BiVO4/Bi2S3 cruciate heterostructures are constructed via a simple anion exchange method assisted by hydrothermal treatment of BiVO4 cruciate flakes in Na2S aqueous solution. The cruciate BiVO4 plays an important template role in the formation of novel BiVO4/Bi2S3 hollow heterostructures. The hollow structure of the special cruciate unities also enlarges the area for electron transfer. The proportion of BiVO4 to Bi2S3 can be easily adjusted by controlling the amount of Na2S during the anion exchange process. Purposeful spatial isolation of photogenerated electrons and holes from the heterojunction interface area significantly increases the survivability of the electrons crossing therein. The optimized visible-light absorption range of the heterostructures by loading an appropriate amount of Bi2S3 is the prerequisite to obtain high photoreduction efficiency for Cr6+ ions.
Experimental section
Synthesis of the NH4V4O10 nanoribbon
All the reagents were analytical pure and used as received without further purification. Typically, 0.315 g of BiCl3 and 0.117 g of NH4VO3 were dissolved in 50 mL of deionized water (DI, the corresponding suspension is abbreviated as A). Then 1.0 mL of 1.0 mol L−1 ethanolamine aqueous solution was dropped into A under magnetic stirring. After static settlement for 30 min, solution B was obtained and then transferred into a 100 mL Teflon-lined stainless-steel autoclave at 160 °C for 24 h. After the autoclave was cooled to room temperature, a dark green sponge was collected, and washed with ethanol and DI water more than 3 times.Synthesis of BiVO4 cruciate flakes
BiVO4 is prepared with the assistance of hydrothermal treatment using NH4V4O10 as the source of vanadium. Typically, 0.096 g of the above as-prepared NH4V4O10 nanoribbon and 0.315 g of BiCl3 were dissolved in 50 mL of DI water under magnetic stirring for 24 h, and the dark green solution C was obtained. Then, solution C was transferred into a 100 mL Teflon-lined stainless-steel autoclave at 200 °C for 1 h. Finally, yellow powder was separated by centrifugation, washed with ethanol and DI water more than 3 times, and dried in a lyophilizer for 2 days.Synthesis of BiVO4/Bi2S3 cruciate heterostructures
0.015 g of BiVO4 cruciate flakes were dissolved in 50 mL of DI water. After a certain amount of Na2S·9H2O aqueous solution was added dropwise to the suspension above, the resulting mixture was transferred into a 100 mL Teflon-lined stainless-steel autoclave at 150 °C for 5 h. By controlling the volume of the 0.024 M Na2S aqueous solution during the anion exchange process and the calculated weight ratio of BiVO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B2S3, R is selected as 5.6, 2.8, 0.8 and 0.4, and the corresponding samples were designated as S-1, S-2, S-3 and S-4, respectively.
B2S3, R is selected as 5.6, 2.8, 0.8 and 0.4, and the corresponding samples were designated as S-1, S-2, S-3 and S-4, respectively.
Materials characterization
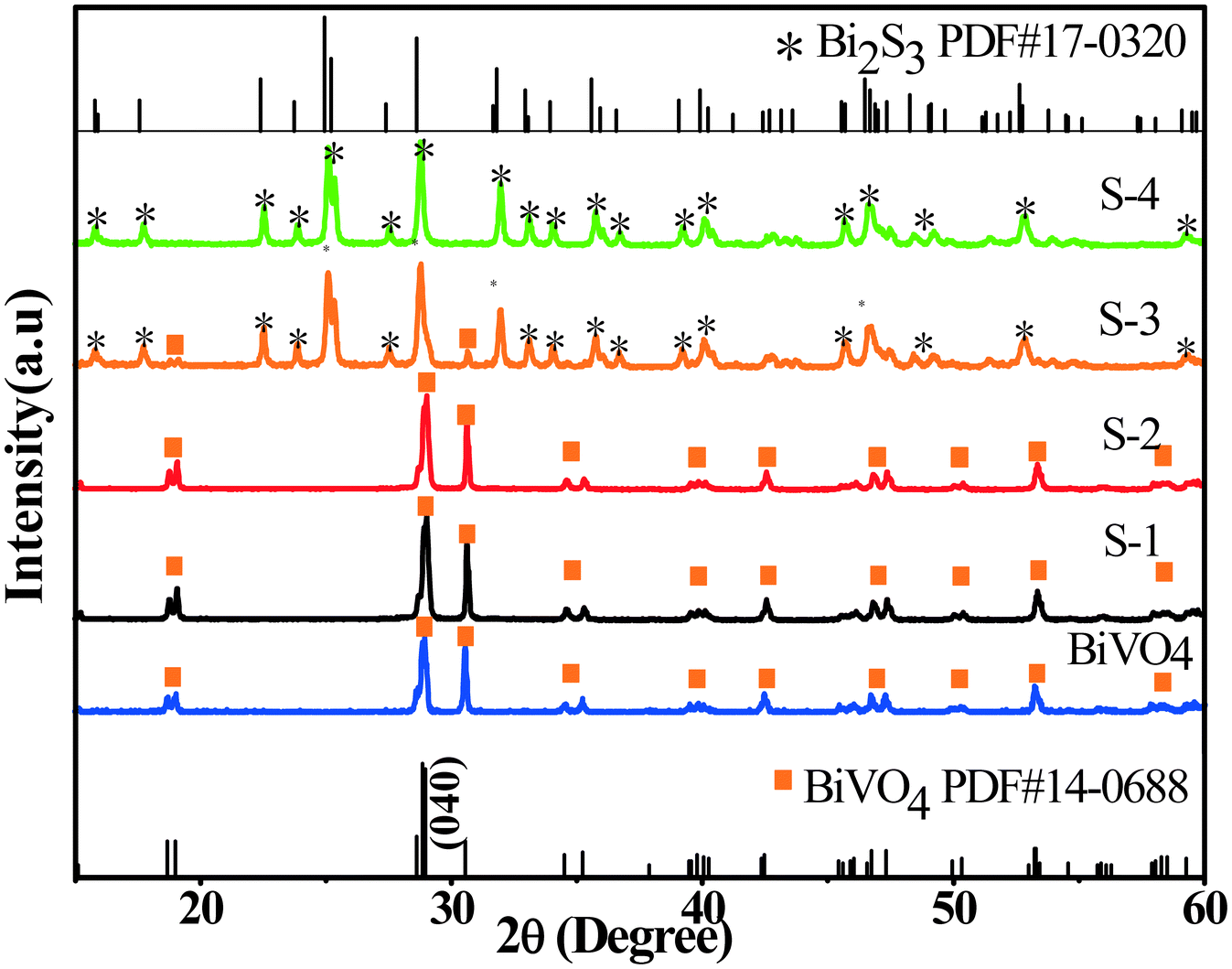
The crystallographic phases of the prepared samples were analyzed by powder X-ray diffraction (XRD, D8 ADVANCE, Bruker) at room temperature. The XRD curves were described with a sweep rate of 10° min−1 using Cu-Kα radiation at 40 kV and 40 mA. The morphologies and structures were characterized by field emission scanning electron microscopy (FE-SEM) on an XL30 ESEM FEG scanning electron microscope conducted at 15.0 kV. The transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were taken on a JEM 200CX TEM apparatus. Energy dispersive X-ray spectroscopy (EDX) and high-angle annular dark field (HAADF) images were also obtained with a spectroscope attached to the HRTEM. The diffuse reflectance graphs were obtained over the range of 300–800 nm with a UV-vis spectrophotometer (UV-2550, Shimadzu) and transformed to the absorption spectrum on the basis of the Kubelka–Munk relationship. The chemical states were analysed by X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Fisher Scientific) and were calibrated using the reference binding energy of C 1s at 284.6 eV.Photoelectrochemical measurement
The photoelectrochemical response was measured using an electrochemical workstation (CHI-630D, Shanghai Chenhua) in a three-electrode cell under AM 1.5G illumination (standard 100 mW cm−2) cast by an Oriel 92251A-1000 sunlight simulator and was calibrated using the standard reference of a Newport silicon solar cell. The as-prepared BiVO4 or BiVO4/Bi2S3 cruciate heterostructures were coated onto a FTO glass with an area of 1 × 1 cm2 and employed as the working electrode. The BiVO4 and BiVO4/Bi2S3 electrodes were then annealed at 450 °C and 350 °C for 30 min, respectively. A Pt foil served as the counter electrode, Ag/AgCl in saturated KCl as the reference electrode, and 0.02 M Na2SO4 aqueous solution as the working electrolyte. The active area of the BiVO4 sample was fixed to 0.28 cm2 by using a black mask. The photocurrent of the sample was measured from the back side (electrolyte–substrate interface). A cyclic voltammetry method was adopted with a scan rate of 10 mV s−1.Measurement of photocatalytic activity
The photocatalytic activity of the as-prepared BiVO4 and BiVO4/Bi2S3 cruciate heterostructures was evaluated by photocatalytic reduction of Cr6+ irradiated with a visible-light source (λ > 420 nm). Before the photocatalytic test, 300 mL of 10 mg L−1 K2Cr2O7 aqueous solution (pH 5.5) and 100 mg BiVO4 or BiVO4/Bi2S3 cruciate heterostructures were mixed and magnetically stirred for 60 min in the dark to achieve Cr6+ adsorption equilibrium. 4.0 mL of the suspension was taken out of the reactor every 30 min and separated by centrifugation. The Cr6+ concentration was measured by a colorimetric method with diphenylcarbazide.31Results and discussion
BiVO4 cruciate flakes are prepared by hydrothermal treatment of NH4V4O10 (Fig. S1, see the ESI†). The crystallographic structure of BiVO4 can be confirmed by XRD analysis (Fig. 1). All the diffraction peaks can be indexed to the monoclinic phase of BiVO4 with lattice constants of a = 5.195, b = 11.70 and c = 5.092 (JCPDS card no. 14-0688). A series of BiVO4/Bi2S3 cruciate heterostructures are prepared by a facile anion exchange method using BiVO4 cruciate flakes as the precursor and Na2S as the sulfidation reagent (Fig. S1†). For samples S-1–S-4, the diffraction peaks can be well assigned to the monoclinic phase of BiVO4, as well as the orthorhombic phase of Bi2S3 (JCPDS card no. 17-0320, a = 11.14, b = 11.30 and c = 3.981), confirming the formation of BiVO4/Bi2S3 heterostructures. With the increasing amount of Na2S, the diffraction intensity of the (040) plane of BiVO4 deceases, while the intensity of that of Bi2S3 becomes stronger, indicating the increasing content of Bi2S3 in the BiVO4/Bi2S3 heterostructures.Fig. S2† shows the high-resolution XPS spectra of BiVO4 and S-3. The peaks with binding energies of 163.8 eV and 158.6 eV are attributed to Bi 4f7/2 and Bi 4f5/2 in BiVO4 (Fig. S2a2†). The protruding part in Fig. S2a1† (161.3 eV) located between Bi 4f5/2 and Bi 4f7/2 could be assigned to S 2p.32,33 According to Fig. S2b,† the peaks with binding energies of 529.6 eV and 531.7 eV are attributed to O 1s in BiVO4 and BiVO4/Bi2S3, respectively. The V 2p peaks with binding energies of 523.5 eV and 516.3 eV for V 2p are in good agreement with those in a previous report (Fig. S2c†).34 The appearance of Bi, S, O and V elements in XPS confirms the formation of Bi2S3 on the BiVO4 flakes. Relative intensities of V 2p in the BiVO4 flakes are obviously stronger than those in the BiVO4/Bi2S3 heterostructures, indicating a decrease in the amount of BiVO4 in BiVO4/Bi2S3 due to the anion exchange reaction.35
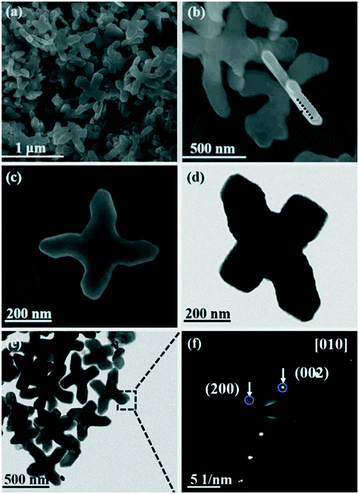
As shown in Fig. 2, the structure and morphology of the as-obtained BiVO4 are characterized by FE-SEM and TEM. The FE-SEM images show that the BiVO4 cruciformities used as precursors have a flake-like structure with a diameter of 600–900 nm and a thickness of 80 nm (Fig. 2a and b). The high-resolution FE-SEM images further suggest the smooth top and bottom surfaces of these cruciate flakes (Fig. 2c). From the selected area electron diffraction (SAED) results, the patterns can be assigned to the (200) and (002) planes of the monoclinic BiVO4 phase (Fig. 2f). Thus, the surface of the BiVO4 flakes can be confirmed as the [010] plane.
Sample S-3 of the as-prepared BiVO4/Bi2S3 heterostructures is characterized in detail to study the formation process of the special hollow structures. The size and shape of the cruciate BiVO4 flakes are well duplicated and the precursors are converted into hollow heterostructures (Fig. 3). Some broken ones clearly confirm the hollow cruciate feature of the BiVO4/Bi2S3 heterostructures (Fig. 3b and c), which can enable the full use of visible light and the illumination from the entries and cavities through multiple reflections. The surface of the heterostructures becomes much rougher compared with that of the BiVO4 flakes (Fig. 3d). With the increasing amount of Na2S, the surfaces of obtained BiVO4/Bi2S3 heterostructures become much rougher (Fig. S3†). The detailed structure of the as-prepared BiVO4/Bi2S3 heterostructures is analyzed by TEM observation (Fig. 4). It is anticipated that the initial anion exchange reaction with the assistance of hydrothermal treatment generates a thin Bi2S3 layer around the surface of the BiVO4 flakes. A further anion exchange reaction between BiVO4 and S2− ions leads to the evolution of solid cruciformities into hollow heterostructures. The slower inward diffusion rate of S2− anions than the outward diffusion rate of Bi3+ cations leads to the evacuation of Bi3+ in the inner region.16–18 As a result, the surface components of BiVO4/Bi2S3 are mainly Bi2S3 while the residual BiVO4 is inside.23
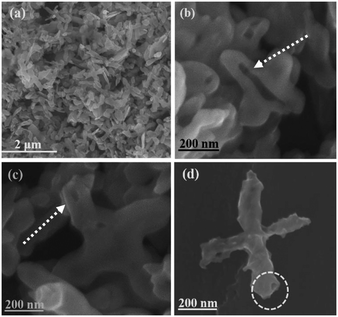 | ||
| Fig. 3 (a)–(d) FE-SEM images of S-3. (b) and (c) indicate BiVO4/Bi2S3 with hollow structures. The circle in (d) shows the entry of the tube. | ||
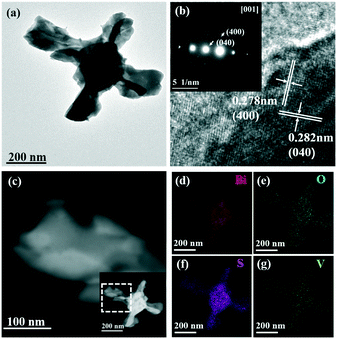
The TEM images shown in Fig. 4 further indicate the hollow structure of the BiVO4/Bi2S3 heterostructures. Lattice fringes with a d-spacing of 0.278 nm and 0.282 nm are assigned to the (400) and (040) planes of the orthorhombic Bi2S3 phase, respectively, which confirms the formation of Bi2S3 on the surface of BiVO4 (Fig. 4b). The SAED result shown in the inset of Fig. 4b indicates the top surface of the BiVO4/Bi2S3 flake as the [001] plane. The HAADF images clearly show the curling of flakes into tubular structures (Fig. 4c). Elemental mapping analysis of the BiVO4/Bi2S3 heterostructure confirms the homogeneous distribution of Bi, V, O and S elements. According to the results shown in Fig. S3,† the S/V atomic ratio keeps increasing as the amount of Na2S increases, which is consistent with the XRD result shown in Fig. 1. Furthermore, the series of BiVO4/Bi2S3 heterostructures exhibit a similar cross-like shape except for the surface roughness.
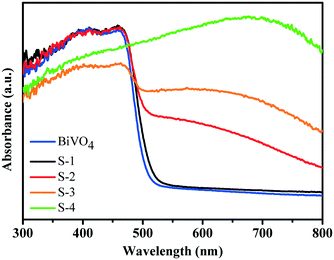
By UV-visible diffuse-reflectance spectroscopy, BiVO4 cruciate flakes and a series of BiVO4/Bi2S3 heterostructures are investigated (Fig. 5). The BiVO4 cruciate flakes exhibit a weak visible-light absorption range of ∼525 nm because of their intrinsic bandgap. When R is 5.6, S-1 exhibits nearly the same absorption band as that of BiVO4. On further increasing the amount of Bi2S3, the obtained BiVO4/Bi2S3 heterostructures exhibit an obvious red shift into the visible region. A broad visible-light absorption band at ∼800 nm is detected in S-4, when the weight ratio of V to S is decreased to 0.4. This indicates a possible electronic coupling effect between BiVO4 and Bi2S3. The color change from yellow to black also indicates the broadening visible light absorption edge of the series of BiVO4/Bi2S3 heterostructures (Fig. S1†). Electron–hole pairs can therefore be greatly generated by BiVO4/Bi2S3 heterostructures under visible-light irradiation compared to BiVO4 alone.
Fig. 6 shows the photocatalytic reduction of Cr6+ with BiVO4 and hollow BiVO4/Bi2S3 cruciate heterostructures under visible-light irradiation. With the increasing weight ratio of S to V, the photocatalytic activity of BiVO4/Bi2S3 heterostructures is significantly improved. S-3 with an R of 0.8 has the highest photocatalytic activity, and Cr6+ can be completely degraded within 120 min. Further loading B2S3 contrarily decreases the photoreduction activity of S-4, probably due to excess Bi2S3 blocking the charge transport.36 The CB and VB for BiVO4 are estimated to be 0.35 and 2.71 eV and 0.12 and 1.42 eV for Bi2S3.37 The combination of BiVO4 and Bi2S3 will form an ideal heterostructure with rapid photoinduced charge separation and decreased chance of recombination of electron–hole pairs by the synergetic effect.32,33,36–38 The band alignment of the elegant heterostructure facilitates the photogenerated electrons and holes in BiVO4 and Bi2S3, respectively, and results in a spatial charge separation for long survival (Fig. S4†).
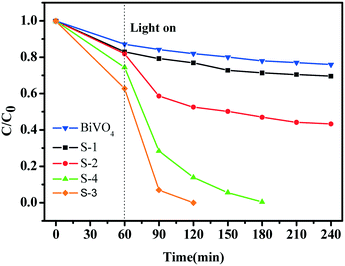 | ||
| Fig. 6 Photocatalytic reduction of Cr6+ with BiVO4 and the series of BiVO4/Bi2S3 heterostructures under visible-light irradiation. | ||
A kinetic model is also discussed to better understand the enhanced photoreduction activity of the series of BiVO4/Bi2S3 heterostructures. When the initial concentration of the target is relatively lower, the Langmuir–Hinshelwood (L–H) model can be expressed as:39
The apparent first-order constant is determined to be 0.00141 (BiVO4), 0.00185 (S-1), 0.0036 (S-2), 0.02804 (S-3) and 0.02647 (S-4). It is clear that the reaction rate of S-3 has increased by ∼20 times compared with BiVO4 alone, indicating the exact role of the BiVO4/Bi2S3 heterostructures. Furthermore, the heterostructures of S-3 with a broader visible-light absorption range have higher photocatalytic performance than S-1 and S-2, implying that the enhanced photoreduction activity for Cr6+ is also subject to the broadened visible-light absorption range. Meanwhile, the balance between the amount of B2S3 and the increased visible-light absorption range should be considered, as the excess Bi2S3 could block the transportation of the photo-induced charge.36
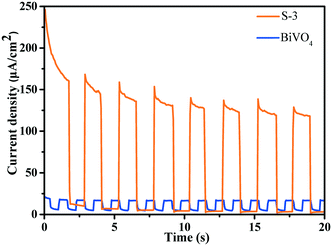
According to the results of the transient photocurrent response, S-3 shows highly promoted current generation, which is approximately 6.0 times higher than that of BiVO4 (Fig. 7). The prominently enhanced photocurrent intensity of the BiVO4/Bi2S3 heterostructures further proves the efficient separation of the photogenerated electrons and holes and the lower recombination probability under visible-light irradiation.40
Conclusions
In summary, unique BiVO4/Bi2S3 cruciate hollow heterostructures are successfully constructed via an anion exchange reaction with BiVO4 used as the precursors. The hollow BiVO4/Bi2S3 cruciate heterostructures exhibit excellent photocurrent response and photocatalytic activity for reduction of Cr6+ under visible-light illumination. The work here provides a new design and further exploration of novel heterostructures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by 973 Programs (No. 2014CB239302 and 2013CB632404), the NSF of China (No. 21773114, 21603183 and 21473091), the NSF of Jiangsu Province (No. BK2012015 and BK20130425), the Jiangsu Postdoctoral Science Foundation (1601062B) and the National Laboratory of Solid State Microstructures (M31044).Notes and references
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- S. Kohtani, M. Koshiko, A. Kudo, K. Tokumura, Y. Ishigaki, A. Toriba, K. Hayakawa and R. Nakagaki, Appl. Catal., B, 2003, 46, 573–586 CrossRef CAS.
- W. J. Jo, J. W. Jang, K. J. Kong, H. J. Kang, J. Y. Kim, H. Jun, K. P. S. Parmar and J. S. Lee, Angew. Chem., Int. Ed., 2012, 51, 3147–3151 CrossRef CAS PubMed.
- M. Zhou, H. B. Wu, J. Bao, L. Liang, X. W. Lou and Y. Xie, Angew. Chem., Int. Ed., 2013, 52, 8579–8583 CrossRef CAS PubMed.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 RSC.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- X. Zhang, Y. Gong, X. Dong, X. Zhang, C. Ma and F. Shi, Mater. Chem. Phys., 2012, 136, 472–476 CrossRef CAS.
- D. J. Martin, P. J. T. Reardon, S. J. A. Moniz and J. Tang, J. Am. Chem. Soc., 2014, 136, 12568–12571 CrossRef CAS PubMed.
- H. L. Guo, H. Du, Y. F. Jiang, N. Jiang, C. C. Shen, X. Zhou, Y. N. Liu and A. W. Xu, J. Phys. Chem. C, 2017, 121, 107–114 CrossRef CAS.
- S. Balachandran and M. Swaminathan, Dalton Trans., 2013, 42, 5338–5347 RSC.
- D. H. Son, S. M. Hughes, Y. Yin and A. Paul Alivisatos, Science, 2004, 306, 1009–1012 CrossRef CAS PubMed.
- J. B. Rivest and P. K. Jain, Chem. Soc. Rev., 2013, 42, 89–96 RSC.
- C. M. Li, Y. Xu, W. G. Tu, G. Chen and R. Xu, Green Chem., 2017, 19, 882–899 RSC.
- M. V. Kovalenko, D. V. Talapin, M. A. Loi, F. Cordella, G. Hesser, M. I. Bodnarchuk and W. Heiss, Angew. Chem., Int. Ed., 2008, 47, 3029–3033 CrossRef CAS PubMed.
- S. Xiong and H. C. Zeng, Angew. Chem., Int. Ed., 2012, 51, 949–952 CrossRef CAS PubMed.
- K. N. Tu and U. Gosele, Appl. Phys. Lett., 2005, 86, 3 CrossRef.
- L. Dloczik and R. Könenkamp, Nano Lett., 2003, 3, 651–653 CrossRef CAS.
- R. D. Robinson, B. Sadtler, D. O. Demchenko, C. K. Erdonmez, L. W. Wang and A. P. Alivisatos, Science, 2007, 317, 355–358 CrossRef CAS PubMed.
- Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711–714 CrossRef CAS PubMed.
- H. Cao, X. Qian, C. Wang, X. Ma, J. Yin and Z. Zhu, J. Am. Chem. Soc., 2005, 127, 16024–16025 CrossRef CAS PubMed.
- H. Cheng, B. Huang, Y. Liu, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 9729–9731 RSC.
- M. A. Bhosale, S. C. Karekar and B. M. Bhanage, ChemistrySelect, 2016, 1, 6297–6307 CrossRef CAS.
- J. Park, H. Zheng, Y. W. Jun and A. P. Alivisatos, J. Am. Chem. Soc., 2009, 131, 13943–13945 CrossRef CAS PubMed.
- J. Gao, Q. Li, H. Zhao, L. Li, C. Liu, Q. Gong and L. Qi, Chem. Mater., 2008, 20, 6263–6269 CrossRef CAS.
- B. Zhang, X. Ye, W. Hou, Y. Zhao and Y. Xie, J. Phys. Chem. B, 2006, 110, 8978–8985 CrossRef CAS PubMed.
- K. Yao, W. W. Gong, Y. F. Hu, X. L. Liang, Q. Chen and L. M. Peng, J. Phys. Chem. C, 2008, 112, 8721–8724 CrossRef CAS.
- K. Ai, Y. Liu, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Mater., 2011, 23, 4886–4891 CrossRef CAS PubMed.
- L. Cademartiri, F. Scotognella, P. G. O'Brien, B. V. Lotsch, J. Thomson, S. Petrov, N. P. Kherani and G. A. Ozin, Nano Lett., 2009, 9, 1482–1486 CrossRef CAS PubMed.
- Z. Zhang, C. Zhou, H. Lu, M. Jia, Y. Lai and J. Li, Mater. Lett., 2013, 91, 100–102 CrossRef CAS.
- Y. Bessekhouad, D. Robert and J. V. Weber, J. Photochem. Photobiol., A, 2004, 163, 569–580 CrossRef CAS.
- T. X. Wang, S. H. Xu and F. X. Yang, Mater. Lett., 2012, 83, 46–48 CrossRef CAS.
- W. Wang, X. Wang, C. Zhou, B. Du, J. Cai, G. Feng and R. Zhang, J. Phys. Chem. C, 2017, 121, 19104–19111 CrossRef CAS.
- D. Zhao, W. Wang, W. Zong, S. Xiong, Q. Zhang, F. Ji and X. Xu, Materials, 2017, 10(8), 891 CrossRef PubMed.
- N. Myung, S. Ham, S. Choi, Y. Chae, W. G. Kim, Y. J. Jeon, K. J. Paeng, W. Chanmanee, N. R. de Tacconi and K. Rajeshwar, J. Phys. Chem. C, 2011, 115, 7793–7800 CrossRef CAS.
- Z. Zhou, Y. Li, K. Lv, X. Wu, Q. Li and J. Luo, Mater. Sci. Semicond. Process., 2018, 75, 334–341 CrossRef CAS.
- X. Gao, H. B. Wu, L. Zheng, Y. Zhong, Y. Hu and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 5917–5921 CrossRef CAS.
- D. K. Ma, M. L. Guan, S. S. Liu, Y. Q. Zhang, C. W. Zhang, Y. X. He and S. M. Huang, Dalton Trans., 2012, 41, 5581–5586 RSC.
- J. Wang, J. Jin, X. Wang, S. Yang, Y. Zhao, Y. Wu, S. Dong, J. Sun and J. Sun, J. Colloid Interface Sci., 2017, 505, 805–815 CrossRef CAS PubMed.
- J. Rong, T. Zhang, F. Qiu, X. Rong, X. Zhu and X. Zhang, J. Alloys Compd., 2016, 685, 812–819 CrossRef CAS.
- Q. P. Luo, X. Y. Yu, B. X. Lei, H. Y. Chen, D. B. Kuang and C. Y. Su, J. Phys. Chem. C, 2012, 116, 8111–8117 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cy01899e |
| This journal is © The Royal Society of Chemistry 2019 |