Open Access Article
Open Access ArticleMain group mechanochemistry: from curiosity to established protocols†
Davin
Tan
and
Felipe
García
 *
*
School of Physical and Mathematical Sciences, Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, 637371, Singapore. E-mail: fgarcia@ntu.edu.sg
First published on 26th February 2019
Abstract
In the last few decades, mechanochemistry has become rapidly established as a powerful tool enabling environmentally-benign and sustainable chemical syntheses. Not only have these techniques been demonstrated as viable alternatives to traditional solution-based syntheses, but they have also received attention for their ability to enable new reactivity and “unlocking” novel compounds inaccessible by conventional methods. Reflecting the rising popularity of mechanochemistry, many excellent reviews highlighting its benefits have recently been published. Whilst the scope of most of these focuses on organic chemistry, transition-metal catalysis, porous framework materials, coordination compounds and supramolecular synthesis, few have addressed the use of mechanochemical ball milling for the synthesis of compounds containing s- and p-block elements. This tutorial review turns the spotlight towards mechanochemical research in the field of inorganic main group chemistry, highlighting significant advantages that solid-state inorganic reactions often possess, and the potential for these to drive the development of greener methodologies within the modern main group arena.
Key learning points(1) Mechanochemistry and its historical background.(2) Tools and parameters affecting mechanochemical syntheses. (3) Solid and liquid additives in mechanochemistry. (4) In situ vs. ex situ reaction monitoring. (5) Key examples of mechanochemically synthesised main group compounds and complexes. |
1. Introduction
Traditional solution-based methodologies have dominated in most synthetic research laboratories and industrial manufacturing protocols since modern chemistry was formulated in the 17th century whereas, despite its long history, the use of mechanochemical techniques in chemical synthesis has only experienced a vibrant renaissance over the last few decades.The primary driving force for the current expansion of solid-state methodologies is the pressing need for cleaner, safer and more sustainable chemical transformations – particularly since raw materials are becoming ever scarcer. Moreover, the large amount of waste created by the generalised use of solvents throughout synthetic processes negatively affects both the environment and public health.
One strategy to address the need for cleaner, greener and more sustainable synthetic methods is to drastically minimise, or completely remove, solvent usage throughout synthetic routes, from laboratory set-ups to large-scale manufacturing. In this respect, solid-state mechanochemical methodologies offer great promise due to their ability to achieve solvent-free, or nearly solvent-free, synthetic routes to complex molecules.
1.1 Main group mechanochemistry
The synthesis of new and novel main group materials, compounds and frameworks is fundamental to the advancement of chemistry as a discipline, which, in turn, propels new developments within neighbouring fields, such as physical, materials, engineering and biomedical sciences.For instance, main group Grignard and Wittig reagents have deeply influenced the field of organic synthesis, leading to key advancements in the chemical and pharmaceutical industries. Moreover, recent examples of main group species containing very low oxidation states, coordination numbers or unique bonding motifs have begun to populate the new generation of mainstream chemistry textbooks.
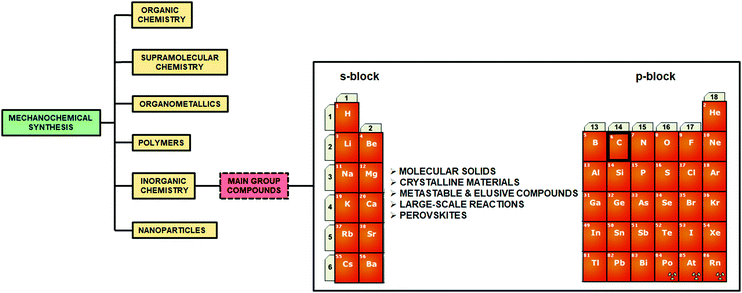
While interest in the fundamental and applied aspects of main group chemistry continues to grow, the development and application of environmentally benign synthetic methodologies represents a burgeoning, yet still relatively niche field of research. This is particularly true in the case of mechanochemistry, despite sustained efforts towards its use in organic synthesis, catalysis and transformation reactions, about which several comprehensive reviews have recently been published.1–5 Within the context of inorganic mechanochemistry, several reviews have focused on the formation of transition metal complexes, organometallic compounds, oxides, alloys and nano-materials (Fig. 1).6–13 However, despite the many examples of both classical and modern mechanochemical routes to compounds comprising s- and p-block elements, these have not yet been comprehensively addressed.11
This review serves as a stepping-stone to new efforts towards mechanochemical methods within the main group arena, focusing on novel, rare and important compounds or materials that have been enabled by mechanochemistry. We also set out to demonstrate that mechanochemistry is a viable and often powerful tool for main group synthesis, particularly in an age where the need to develop more environmentally-benign chemical syntheses is greater than ever.
1.2 What is mechanochemistry?
Mechanochemical reaction is defined as: a chemical reaction that is induced by the direct absorption of mechanical energy. The mechanical energy can be imparted into the system by different modes of mechanical action (i.e., impact, compression, shearing, stretching, grinding, etc.). This mechanical action can result in particle size reduction, creation of active sites for chemical reactivity – or the generation of fresh active surfaces – for other particles to contact, coalesce and react.1–151.3 Overview and history of mechanochemistry
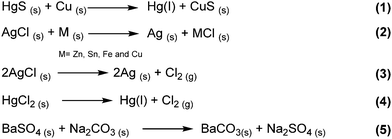
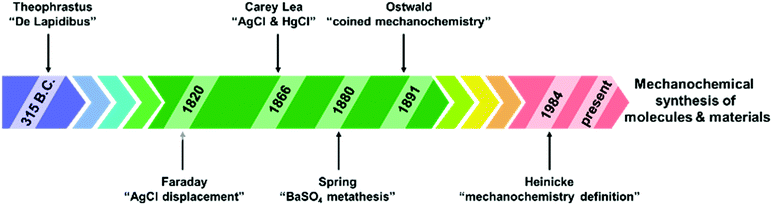
Early historical beginnings of mechanochemistry find their roots in primitive Stone Age technologies (e.g., to process building materials such as clay, or the grinding of seeds and wheat). Within this context, a piece of equipment synonymous with both alchemy and modern mechanochemistry that has appeared consistently throughout the history of mankind is the mortar and pestle.4,5,10,13,16The mortar and pestle is a universal tool found across many cultures worldwide and in most modern laboratories. Traditionally, the mortar and pestle has been used to pound and grind solids together as observed in food ingredients, medicines and materials. According to Takacs,12 who described the chronological development of mechanochemistry, one of the earliest written records of a mechanochemical reaction dates back to 315 BC in the book De Lapidibus (On Stones) by Theophrastus of Eresus. Described was a method to obtain quicksilver (i.e., mercury) by grinding cinnabar – a bright scarlet to brick-red form of mercury(II) sulfide (HgS) – using a copper or bronze mortar and pestle. This process involved the reduction of HgS by the material of the mortar and pestle, to form elemental Hg and cupric sulfide (CuS) (Scheme 1, eqn (1)). Takacs also described the addition of small amounts of vinegar to serve as a lubricant and to accelerate the reaction. The use of a liquid additive to the solid-state grinding process anticipates the modern technique of liquid-assisted grinding (LAG) – which will be discussed in later sections of this review. Inadvertently, the first written record of mechanochemistry may also be the first written evidence of an inorganic chemical reaction, highlighting historical ties between inorganic and mechanochemistry.
From ca. 300 BC to the 18th century, records of mechanochemical grinding to induce chemical reactions are negligible compared to its use for transformations associated with metal mining, alloying, and metallurgical operations, as well as to process grains, minerals, building materials, etc.7,12 During this period, it is plausible that some mechanochemical reactions occurred while manual grinding of two or more solids together. However, without any written records, it is difficult to determine whether any sort of systematic mechanochemistry or research in the area ever occurred.
In the 19th century, several milestones in mechanochemistry were reported.7,12 Similar to Theophrastus's reaction, Faraday described the ‘‘dry way’’ of reducing silver chloride (AgCl) by grinding with zinc, tin, iron, and copper in a mortar (Scheme 1, eqn (2)). This sparked the beginning of systematic scientific studies on mechanochemistry, and several organic and inorganic transformations induced by manual grinding followed. Another major contributor to the early systematic development of mechanochemistry was Carey Lea. He observed that heating mercuric chloride (HgCl2) and silver chloride (AgCl) led to phase transformations (i.e., sublimation and melting, respectively) without decomposition. However, at room temperature both solids rapidly decomposed upon grinding (Scheme 1, eqn (3) and (4)). His observations not only illustrated that mechanical action could induce chemical changes, but also that these changes may be unique from those produced by thermally-induced reactions. This work is often highlighted as one of the first, and perhaps the most significant, demonstrations of the differences between thermochemical and mechanochemical processes, earning Lea the mantle of “the father of mechanochemistry”.12
In 1880, Spring studied the salt metathesis reaction between barium sulfate (BaSO4) and sodium carbonate (Na2CO3) induced by repeated compression and pulverization of their solids. Importantly, this reaction is an early example of a process difficult to achieve in aqueous solution, due to the poor solubility of BaSO4 – but readily accomplished by manual grinding in the solid state. This work underscored the benefit of mechanochemistry for chemical transformations involving poorly soluble compounds, which is further echoed by more modern examples discussed later in this text. Interestingly, this study potentially represents the first example of mechanochemical transformations of main group compounds.11,12 However, the term mechanochemistry was not officially coined until 1891, when Ostwald included it in his terminology of chemical science, along with photochemistry, thermochemistry, electrochemistry and other types of chemical transformations. Later in 1984, Heinicke formulated the current and widely established definition of mechanochemistry (Fig. 2).
1.4 Tools, symbols and techniques
In the same way that thermal, sonochemical, microwave and photochemical reactions are often designated by generally accepted symbols, a symbol comprising three circles arranged in a triangular fashion was recently proposed by Hanusa et al. to denote a mechanochemical reaction (Scheme 2).6 This symbol not only applies to the use of mechanical mills, but also to other related techniques (e.g., manual grinding) and allows mechanochemical reactions to be readily distinguished from their solution counterparts.
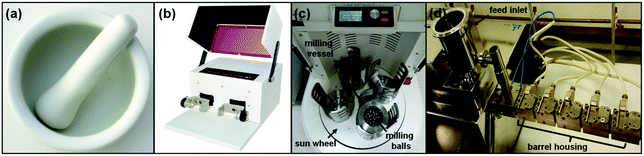 | ||
| Fig. 3 Pictures of (a) mortar and pestle, (b) electric shaker mill, (c) planetary mill and (d) twin-screw extruder. Reproduced from ref. 18 with permission from the RSC, copyright 2007. | ||
In the 20th century with the arrival of automation, a paradigm shift in the development of mechanochemical tools began to transform how laboratory-scale solid state reactions were conducted.1–4 Nowadays, mechanochemical reactions are preferentially conducted using automated ball mills, a basic type of grinder, comprising a closed reaction vessel (jar) charged with ball bearings. They carry out a similar function to grinding with a mortar and pestle, but in a reproducible manner. In addition, mechanical automation, unlike manual hand grinding, allows for longer reaction times (i.e., several hours) which are sometimes required to achieve very small particle sizes and amorphization of reagents.1,4–6,9,12
There are two main types of ball mills: shaker or vibration mills, and planetary mills (Fig. 3b and c, respectively). In shaker mills, the reaction jar is rapidly oscillated from side to side causing the enclosed ball bearing/s to shear and grind the reagents together (Fig. 4a). On the other hand, in planetary mills, the reaction vessels are spun at high speed, counter-rotatory to the main spinning “sun wheel” (thus, the term planetary) which results in the balls grinding the solids within the jars (Fig. 4b).1,5,6,10,19
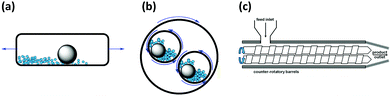 | ||
| Fig. 4 Schematic representation illustrating the mode of motion of the cross-section of a (a) shaker and (b) planetary mill, and (c) twin-screw extruder. | ||
In addition to ball milling, twin-screw extrusion (TSE) is a recent addition to the family of mechanochemical tools (Fig. 3d).17 In this setup solid reagents are ground together by a pair of counter-rotatory screws as the mixture is transported along the extrusion path through a barrel (Fig. 4c). Different sections or zones of the screw can be readily exchanged to enable distinct mechanical actions (i.e., simple mixing, conveying or grinding). The reagent feed-rate, screw and barrel lengths, and screw rotating speed can be adjusted to optimize reaction conditions. As the mechanochemical reaction occurs in a continuous process, TSE can be considered as a solid-state equivalent to solution-based flow-reactors.13
Another method to control a ball milling reaction is by changing the operational vibrational frequency or rotation speed of the mills.1,5,11 This allows the energy input into the reaction system to be controlled, thus enabling optimal manipulation and reproducibility over reaction conditions. Mack et al. have shown that similar to solution-based reactions, ball milling energetics in mechanochemical reactions are linked to the Arrhenius’ equation. Adjusting the frequency or speed of milling directly affect the motion of the ball bearings, which in turn influence reaction kinetics and reaction outcomes.6,14
Ball-to-reagent-ratio (BRR) is another important variable to consider in ball milling reactions,1–4 and is defined as the total mass of the ball bearings in the media divided by the total mass of the reagents in the reaction. The BRR is an experimental parameter which has been shown to influence reaction kinetics and can be varied to fine-tune and optimize a mechanochemical reaction.
In early reports of mechanochemistry, the volumes of liquid additives incorporated into mechanochemical reactions were poorly described and/or quantified – with experimental protocols vaguely describing from “adding one or two drops of solvent” to “a few drops of liquid”. Furthermore, the terminologies used to describe such techniques were also inconsistent, varying from “kneading”, “solvent-drop grinding” and “solvent-assisted grinding” to describe analogous processes.3,7,12,15
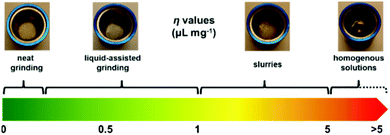
Recently, the term liquid-assisted grinding (LAG) was proposed to unify and describe mechanochemical reactions in which a defined volume of liquid is added.16 A LAG parameter (η) – defined as the ratio of liquid (in μL) to the combined weights of solid reactants (in mg) – was introduced. This parameter permits a systematic cross comparison of the various grinding techniques such as neat grinding (i.e., η = 0) or LAG (0 < η ≤ 1). Higher η values correspond to the formation of slurries and homogenously dissolved reactions (Fig. 6).3,4
Using the LAG concept, other grinding techniques have emerged, such as ion- and liquid-assisted grinding (ILAG), vapour-assisted grinding (VAG) and polymer-assisted grinding (POLAG).1,3,12,16 Similarly, these techniques involve the addition of sub-stoichiometric amounts of additives to the grinding process, as a means to afford better control over chemical reactivity and selectivity.1–3,11
In some cases, the addition of inert milling auxiliaries (e.g., NaCl salts), can be used as a means of “diluting” the mechanochemical action and influencing reaction kinetics.1,5,8,11,12,16 These milling auxiliaries are sometimes added in stoichiometric amounts (1–5 equivalents) to assist the breakdown of aggregated clumps of solids. This is particularly useful in preventing formation of sticky or viscous reaction mixtures which may hinder the adequate motion of the ball bearing(s) during the milling process. With the addition of the milling auxiliary, a more “powder-like” milling environment can be generated, improving the overall mixing and homogeneity of the reagents, and hence, promoting reactivity.1
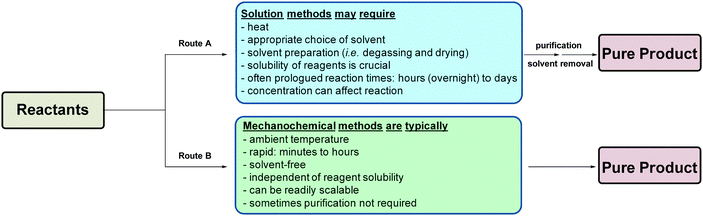 | ||
| Scheme 3 Variables associated with solvent-based and mechanochemical methodologies via route A and route B, respectively. | ||
In many solvent-based inorganic syntheses, the solvent quality can adversely affect the reaction outcome. Hence, solvent-free mechanochemical reactions not only circumvent any possible solubility issue, but avoid the need for thorough solvent conditioning (e.g., distilling, degassing and drying) – which is often labour, time and energy-intensive.
Another advantage of mechanochemistry over traditional solution-based methodologies is its ability to enable the use of reagents that are typically assumed to be inert or highly insoluble (e.g., carbonates, oxides etc.).2,4–7,11
However, it is important to note at this point, that mechanochemistry is not a panacea to all synthetic problems, for solution-based methods offer certain advantages over solid-state synthesis. For instance, control over reaction kinetics, aid in dispersing heat in exothermic reactions, and creating homogenous reaction mixtures.4,5,7,11 Hence, certain compounds and product distributions can only be achieved by the used of conventional solution-based methods.2,5–7,11
In order to understand how mechanochemical reactions proceed, a variety of solid-state characterization techniques are typically employed. These techniques are used either individually or in combination to monitor and assess product formation throughout.3,4,7,8,12
The characterization of crystalline powders relies heavily on powder X-ray diffraction (PXRD), a powerful technique that allows quick, qualitative analyses of reaction mixtures by a straightforward comparison of the reagent and product diffraction patterns (diffractograms).3,4,7,8,15,17 Comparison of these diffractograms is generally done in conjunction with previously obtained diffraction data deposited in international databases such as the Cambridge Structural Database (CSD).
An important advantage of PXRD is its ability to determine the structures of reaction products – or even intermediates – without the need to grow diffraction-quality single crystals. This can be extremely useful with dealing with “material-like” compounds, examples of which are discussed later. Furthermore, PXRD allows for the structural characterization of compounds that are either sensitive or unobtainable in solution and cannot be characterized using solvent-dependent analytical techniques.4,7,16
However, PXRD alone cannot entirely rationalise mechanochemical reactions, as it is only adequately sensitive to transformations of highly crystalline materials. Prolonged grinding often results in complete or partial amorphization, leading to a loss of diffraction signal or reduction in overall quality of the data obtained, respectively.16,17
Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy are two spectroscopic techniques independent of compound crystallinity that directly measure the relative energies (stretching frequencies) of chemical bonds.3,9,15,17 Both FTIR and Raman spectroscopy qualitatively assess changes in covalent bonding interactions, either by bond scission or bond formation, as characteristic FTIR and Raman bands can be readily identified. Whilst Raman spectroscopy requires a specialized Raman laser probe, FTIR spectroscopy can be found in most laboratories and is thus more readily accessible.17
Further characterisation methods such as solution and solid-state nuclear magnetic resonance spectroscopy (ssNMR), ultra-violet and visible light spectroscopy (UV-vis) and Mössbauer spectroscopy can also be used to monitor and analyse mechanochemical reactions. Examples illustrating the use of these techniques will be highlighted in later sections.
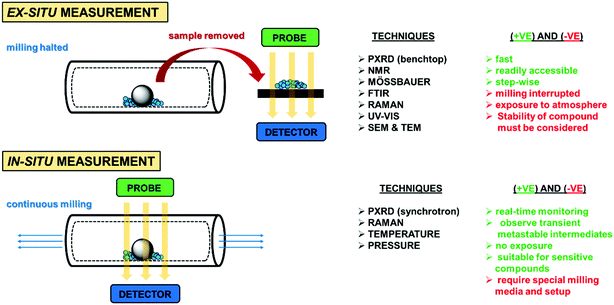
In general, these measurements are performed ex situ or in a stepwise manner (Fig. 7). Ex situ measurements have the advantage of simplicity and ease of implementation but require intermittently stopping the grinding or milling process and sampling the jar content for analysis. This frequent interruption and exposure of the reaction mixture to the open atmosphere may affect the reaction outcomes. Furthermore, these measurements rely on the assumption that the reaction ceases once milling is stopped, which is not always the case. Hence, these issues should be considered and accommodated for to prevent inconsistencies or data misinterpretation.16,17
Recently, extensive efforts have been made to develop platforms to conduct in situ measurements. In this case, the reaction mixture can be characterised without any interruption of milling process, allowing for real-time monitoring and characterization of the products and transient metastable phases formed. Furthermore, in situ techniques enable accurate kinetic measurements to be obtained, providing better insights into the mechanism of the mechanochemical process. Whereas in situ PXRD measurements require access to penetrative synchrotron X-ray radiation source, in situ Raman measurements can be performed using more readily available benchtop Raman spectrometers.17 Apart from diffraction and spectroscopic techniques, in situ temperature and pressure monitoring can also be conducted using customised jars with inbuilt temperature and barometric sensors.3,9,17
Despite these recent developments, the in situ monitoring of mechanochemical reactions is still limited, and the development of commercially available integrated systems comprising milling and monitoring capabilities remains a pressing need.
2. From molecules to materials: s-block
For ease of reading, we have organized this review according to progression across the periodic table: starting from s-block hydrides, alkaline (Group 1) and alkaline earth metals (Group 2), to the p-block elements boron (Group 13), carbon (Group 14), pnictogens, chalcogens and halogen (Group 13–17, respectively). To the best of our knowledge, the use of mechanochemistry to functionalize or react with noble gases (Group 18) remains unexplored. We have further separated each section into the areas of molecular compounds, materials, and composite nanoparticles.2.1 Molecular species of the s-block
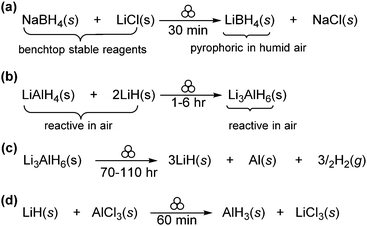
In this section, we will explore the use of mechanochemistry for the synthesis of s-block molecular compounds, beginning with hydrides and alkaline metals (Group 1) and subsequently considering alkaline-earth metals (Group 2).Beyond their use as reducing agents, hydride-based compounds have also been explored as candidates for high capacity energy storage materials for fuel-cell applications. In particular, alanates (e.g., NaAlH4 and LiAlH4) and borohydrides, have been proposed as high capacity hydrogen storage materials. Reversible reactions between different Li–Al-hydride species are of particular interest due to their high theoretical capacity for hydrogen storage (e.g., Li3AlH6 → LiH and LiAlH4 → Li3AlH6 5.5 and 7.9 wt%, respectively). Pecharsky et al. studied the solid-state mechanochemical transformations of aluminium hydrides under inert atmosphere conditions (Scheme 4b),19 finding that stoichiometric milling of LiAlH4 with LiH for 1 h readily afforded their desired Li3AlH6 product. However, due to the catalytic effect of iron present in the stainless-steel milling media, Li3AlH6 decomposed to LiH, elemental Al, and H2 gas when milled for extended periods (i.e., 110 hours). The catalytic role of iron was further confirmed by the increased rate of decomposition of Li3AlH6 in the presence of iron powder or iron salts, reinforcing the importance of selecting appropriate milling media.
Further expanding on the idea of mechanochemical salt metathesis, they also reported the neat mechanochemical synthesis of alanes from LiH and AlCl3 under ambient conditions (Scheme 4d).20 Traditionally, these species require the use of donor solvents (i.e., diethyl ether) for their syntheses, leading to the formation of solvate adducts. The application of mechanochemistry bypasses unstable intermediates – formed in solution – that readily decompose into elemental Al and hydrogen, enabling the quantitative formation of adduct-free alane species.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, yielded the desired potassium tris-bis(trimethyl-silyl)propylberyllate complex in almost quantitative yields in only 15 minutes. In solution, comparable yields were only obtained after a 16 hour reflux in diethyl ether.
1 ratio, yielded the desired potassium tris-bis(trimethyl-silyl)propylberyllate complex in almost quantitative yields in only 15 minutes. In solution, comparable yields were only obtained after a 16 hour reflux in diethyl ether.
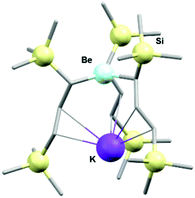 | ||
| Fig. 8 Molecular structure of the potassium tris-bis(trimethylsilyl)propyl-beryllate as determined by X-ray diffraction crystallography. Hydrogen atoms are omitted for clarity. | ||
Another example within the organometallic family is the synthesis of a strontium complex that can act as a chemical vapor deposition (CVD) precursor to strontium-based semiconductors.22 In solution, Sr(Cp′)2 (Cp′= n-propyltetramethylcyclopentadiene) is typically synthesised via a salt metathesis reaction between alkali metal cyclopentadienides (i.e., KCp′) and metal halides (e.g., SrI2). However, this reaction is hindered by the low solubility of strontium iodide in solvents compatible with the air- and water-sensitive nature of reagents and products (i.e., Cp′ salts). Furthermore, typical polar solvents such as tetrahydrofuran (THF) and dimethyl ether (DME) form stable adducts with the SrCp2′ product, ultimately compromising the integrity of the semiconductor obtained through CVD.
Both these issues of solubility and the formation of stable adducts can be circumvented via LAG in ether in a custom-made attrition glass reactor fitted with a PTFE paddle and stainless-steel balls (Fig. 9).22 In this way, the product was obtained in high yields within a few hours (88% isolated yield) and was readily scalable (up to 500 g); although the steel-on-glass reactor used showed signs of erosion upon prolonged milling.
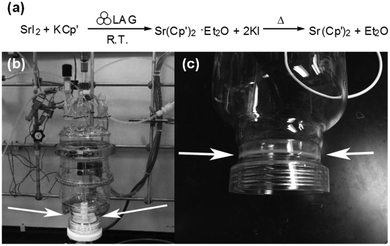 | ||
| Fig. 9 (a) Synthesis of the Sr(Cp′)2 organometallic complex by LAG and thermal removal of the Et2O solvate. (b) Picture of the custom mechanochemical reactor assembly is based on commercially available large-scale glassware. (c) The arrows show gradual wear and tear from the milling media on the sides of the glass reactor, yet no metal contaminants were found in the product. Reproduced from ref. 22 with permission from the RSC, copyright 2014. | ||
This work not only serves to demonstrate the compatibility of mechanochemistry with manufacturing air-sensitive semiconductor precursors in large scales, but again reiterates the increasingly pressing need to develop better and more appropriate mechanochemical tools and set-ups for synthetic purposes.
2.2 Materials containing s-block elements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios – affording a mixture of different adducts and their respective salt by-products. In addition, four new metastable borohydride compounds were observed and characterised using in situ PXRD. Two possessing both possessing framework structures – α-Cd(BH4)2 and β-Cd(BH4)2 – and the potassium salts of [{Cd(BH4)3}n]n− and [Cd(BH4)4]2−, which display polymeric and isolated complex anions, respectively (Fig. 10).
1 molar ratios – affording a mixture of different adducts and their respective salt by-products. In addition, four new metastable borohydride compounds were observed and characterised using in situ PXRD. Two possessing both possessing framework structures – α-Cd(BH4)2 and β-Cd(BH4)2 – and the potassium salts of [{Cd(BH4)3}n]n− and [Cd(BH4)4]2−, which display polymeric and isolated complex anions, respectively (Fig. 10).
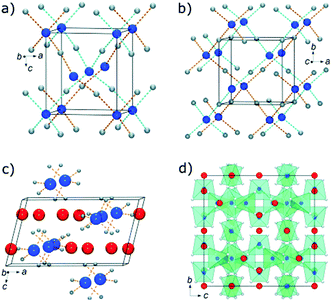 | ||
| Fig. 10 Crystal structures of (a) α-Cd(BH4)2, (b) β-Cd(BH4)2, (c) K2Cd-(BH4)4, and (d) KCd(BH4)3. Hydrogen atoms are omitted for clarity. The following colouring scheme is used: Cd (blue), K (red), B (light grey). Reproduced from ref. 23 with permission from Willey, copyright 2012. | ||
In addition to solid–solid mechanochemical reactions, heterogeneous solid–gas reactions are also possible. Cuevas and et al. performed the ball-milling reduction of solid Li3N by elemental H2 in a reaction comprised of two main steps. First, lithium imide was formed, followed by the subsequent formation of lithium amide, with LiH formed as a byproduct in both (Fig. 11).24 A combination of in situ pressure and temperature measurements with ex situ PXRD studies provided insights into the reaction mechanism. Temperature measurements revealed that the reaction equilibrates at 49 °C within 30 minutes of milling, accompanied by a fast absorption of hydrogen by Li3N to form Li2NH. Upon prolonged milling (up to 2 hours), further hydrogenation of Li2NH occurred producing LiNH2. Interestingly, despite the reaction scheme being similar to the previously described thermally driven process, the formation of an intermediate Li4NH phase is not observed during the mechanochemical process.24
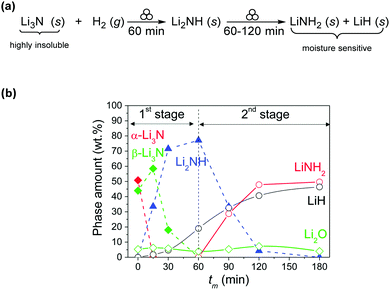 | ||
| Fig. 11 (a) Solid-gas reaction for the step-wise mechanochemical reduction of Li3N by H2. (b) Evolution of phase amounts during ball milling of Li3N powder under hydrogen gas. Reproduced from ref. 24 with permission from the RSC, copyright 2015. | ||
Another research area gaining momentum is use of mechanochemistry in the synthesis of Li-ion materials. For such applications, low temperature pure-phase synthesis without compromising electrochemical properties is greatly desired. Tarascon et al. reported the mechanochemical synthesis of Li–Mn–O spinels for lithium battery anodes (Scheme 5a).25 Milling a stoichiometric mixture of Li2O and MnO2 for 8 hours afforded a spinel-type material, whose electrochemical performance rivalled that of LiMn2O4 ceramic materials obtained at elevated temperatures.
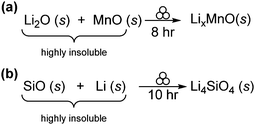 | ||
| Scheme 5 (a) Synthesis of LixMnO spinels for ion batteries. (b) Synthesis of composite Li4SiO4 materials for lithium ion-batteries. | ||
Similarly, Yang et al. reported the mechanochemical reduction of SiO with Li metal under inert atmosphere conditions, to form Li4SiO4 after 10 hours of milling (Scheme 5b). Compared with pure SiO and pure silicon as anode materials, the prepared composite demonstrated larger capacity and superior cyclability. These functional enhancements were attributed to the uniform distribution of nanoparticles obtained throughout the mechanochemical process.26 These two examples highlight that Li-ion based electrodes for battery materials with enhanced properties are readily obtained using mechanochemical methods.
Mechanochemistry hereby provides a soft synthetic route to highly pure isostructural Ba1−xSrxLiF3 species avoiding the need for high temperature by ball milling stoichiometric mixtures of BaF2, SrF2 and LiF together in a zirconia milling media (i.e., milling jars and milling balls). Ex situ PXRD and ultrafast 19F ssNMR spectroscopy revealed a series of different local environments for the F− anions – a quaternary fluoride – which rapidly decompose under elevated temperatures. This quaternary fluoride phase is only isolatable under mechanochemical treatment and is inaccessible by any other synthetic methods.
Related work by Scholtz et al. demonstrated that Group 2 mixed metal ternary fluoride composites, MgF2–MF2 (M = Ca, Sr, B) – e.g., SrMgF4 and BaMgF4 (Fig. 12), can be readily obtained by mechanochemistry. Ball milling fluorine-free precursors (i.e., carbonate, acetate or hydroxide salts) with ammonium fluoride in a planetary ball mill for 4 hrs afforded the desired ternary fluoride products without significant phase impurities.28 Here, mechanochemistry bypassed the use of toxic and highly reactive metal fluoride species for their synthesis.
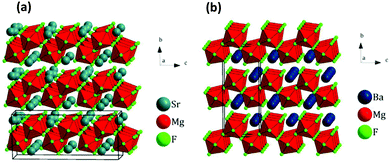 | ||
| Fig. 12 Crystal structures of (a) SrMgF4, (P21) and (b) BaMgF4, (Cmc21). Unit cells are indicated by black lines. Mg is in the middle of the red octahedra. Reproduced from ref. 28 with permission from Elsevier, copyright 2015. | ||
In both previous cases, mechanochemistry provides good stoichiometric control to generate well-defined and highly pure products with enhanced ionic conductivity in a convenient one-step mechanochemical preparation techniques.
3. p-Block compounds: from the “air-sensitive” boron, carbon and pnictogen groups, to stable chalcogens and halogens
In the previous section, the benefits of mechanochemistry for the synthesis of s-block compounds have been articulated. The merits of mechanochemistry extend to the formation and reactions of Groups 13 to 17 compounds.3.1 Molecular synthesis and transformations
With regards to heavier Group 13 compounds, indium(III) complexes are of particular interest since they have relatively low toxicities, are highly water-stable and have been shown to be capable of acting as catalysts for organic reactions. Furthermore, recent reports have indicated that in the presence of appropriate ligands, these species can undergo metal-to-ligand charge transfer and exhibit long-lived excited states – and thus potentially function as light photosensitizers.
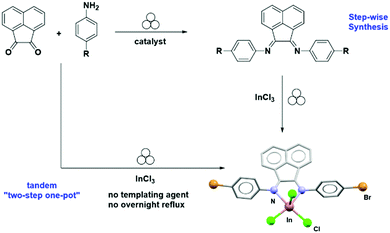
In this aspect, a two-step mechanochemical synthesis of indium(III) bis(imino)acenaphthene (BIAN) complexes by neat grinding in a vibrational mill, has been recently reported.30 In this case, the first step involved an acid catalysed condensation reaction between acenaphthenequinone with functionalised anilines in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio, in the presence of acetic acid or trifluoroacetic acid to afford the desired BIAN ligand. In contrast, conventional solution methods often require the use of templating agent (i.e., ZnCl2) and overnight reflux in toluene to obtain these BIAN ligands.
2 stoichiometric ratio, in the presence of acetic acid or trifluoroacetic acid to afford the desired BIAN ligand. In contrast, conventional solution methods often require the use of templating agent (i.e., ZnCl2) and overnight reflux in toluene to obtain these BIAN ligands.
Mechanochemical synthesis readily affords the BIAN compounds, bypassing the use of toxic transition metal as templates. Subsequently, milling the ligand with InCl3 produced the desired indium complexes in good yields (Fig. 14). Notably, reactions performed at 180 °C in the same vessel without milling led to decomposition over a 4 hour time course, indicating that the reaction required mechanical energy to proceed, rather than residual heat produced during the milling process.30
The authors also demonstrated a rare example of a multistep mechanochemical synthesis of In–BIAN complexes performed in a one-pot two-step manner – without ligand isolation, unlike previously reported examples.
With regards to germanium compounds, mechanochemical methodologies have been employed as a more benign and mild method to access organo-germanium compounds through the processing of germanium ores or germanium metal – avoiding the use of metal chlorides.
Standard industrial processing of metal ores requires the extensive use of chlorine-containing compounds (i.e. gaseous chlorine or concentrated hydrochloric acid) for the extraction and refinement of metals and metal oxides into metal chloride salts. Such harsh chemical treatment has many environmental and health risks – in terms of storage, transportation and exposure. Moreover, the large-scale manufacturing of these inorganic metal chloride salts is highly energetically intensive, and their downstream manipulation involves stringent regulation as these salts are corrosive and often moisture-sensitive (i.e., they easily revert to the metal oxide when in contact with water).
Glavinovic et al. have recently used mechanochemistry to process germanium and germanium oxide into organogermane compounds by treatment with redox active organic compounds such as o-quinones and catechols, respectively (Scheme 6).31 Under LAG conditions, milling of germanium oxide with two equivalents of 3,5-di-tert-butylcatechol and a monodentate ligand (e.g., pyridine) afforded a bench-top stable Ge(IV) bis-catecholate complex in 3 hours. Similarly, the desired product can be formed by grinding germanium powder with o-quinones (used as a two-electron oxidizing agent). Further reaction of the Ge(IV) biscatecholate complex with Grignard reagents generate a series of organo-germane GeR4 species.
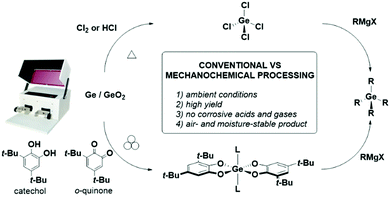 | ||
| Scheme 6 Comparison of the mechanochemical and solution methods to achieve organo-germane compounds. | ||
This chlorine-free mechanochemical protocol circumvents the formation of corrosive and moisture-sensitive GeCl4 – the conventional entry point to the synthesis of a diverse range of organo-germane species – and presents an environmentally benign route to Ge(IV) compounds from raw germanium metal and its ores.
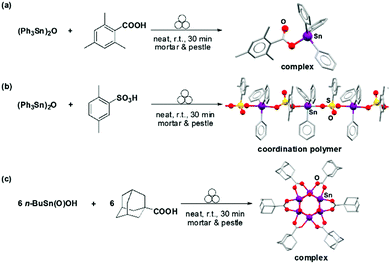
On descending the group, Chandrasekhar et al. prepared a series of organotin Sn(IV) complexes, clusters, and coordination polymers (Fig. 15) via mortar and pestle grinding. These species are attractive targets as they display a large structural diversity and have found applications in catalysis.32
Traditional syntheses of organotin cages and clusters require the use of harsh reaction conditions (i.e., high-boiling solvents under refluxing conditions) and several synthetic steps. Moreover, these species are generally difficult to synthesise and often require the use of high dilution methods or a Dean–Stark apparatus for the removal of water formed during the condensation reactions.
However, these species can be readily obtained by manually grinding organic protic acids (e.g., carboxylic, phosphinic and sulfonic acids), with organotin solid precursors for 20–60 minutes at ambient temperatures in a solventless manner (Fig. 15) affording the desired products in near quantitative yields.
More recently, the power of mechanochemistry to access otherwise inaccessible species has been demonstrated in organotin chemistry. Ball milling potassium trisallyl anion A− (see Fig. 13) and SnCl2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for 5 minutes leads to the formation of a tris(allyl) stannate polymer (i.e., [SnA′3K]∞), whereas longer grinding (ca. 15 minutes), or the use of a 3
1 ratio for 5 minutes leads to the formation of a tris(allyl) stannate polymer (i.e., [SnA′3K]∞), whereas longer grinding (ca. 15 minutes), or the use of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of reagents, produces sterically crowded tetra(allyl)tin species [SnA′4]. The latter quickly decomposes (<1 hour) in solution. In stark contrast, they are stable for several months in the solid state due to intramolecular London dispersion forces. Notably, none of these species are attainable from solution-based methods.33
1 ratio of reagents, produces sterically crowded tetra(allyl)tin species [SnA′4]. The latter quickly decomposes (<1 hour) in solution. In stark contrast, they are stable for several months in the solid state due to intramolecular London dispersion forces. Notably, none of these species are attainable from solution-based methods.33
Seminal work by Pecharsky and Balema demonstrated the first mechanochemical synthesis of phosphorus ylides by grinding phosphines with organo-bromides in the presence of excess K2CO3 under inert atmosphere, and subsequent Wittig reactions to form olefins in a one-pot two-step manner (Scheme 7).34 The same year, they also demonstrated the first mechanochemical synthesis of phosphonium salts by neat grinding of triphenylphosphine with alkyl bromides.35
 | ||
| Scheme 7 One-pot Wittig reaction via in situ formation of the phosphorus ylide by mechanochemistry as reported by Pecharsky. | ||
Ball milling has been successfully used for the synthesis of a series of chalcogenide (sulfide and selenide) oxidised tertiary phosphines, aminophosphines and bisphosphines in quantitative yields, significantly simplifying the isolation of highly pure products compared to solution-based methods. Mechanochemistry offers a readily scalable, atom economic (100%) method with ideal E-factors (E = 0) to access these versatile families of phosphorous based compounds.36
Inorganic ring structures comprising main group element backbones have also been synthesised mechanochemically. Cyclodiphosphazanes – cyclic P2N2 rings containing alternating trivalent phosphorus and nitrogen atoms – are topologically diverse species possessing unique chemical versatility that are notoriously arduous to synthesise and isolate due to their air- and moisture-sensitivity, and the incompatibility of their halogenated precursors with protic solvents.
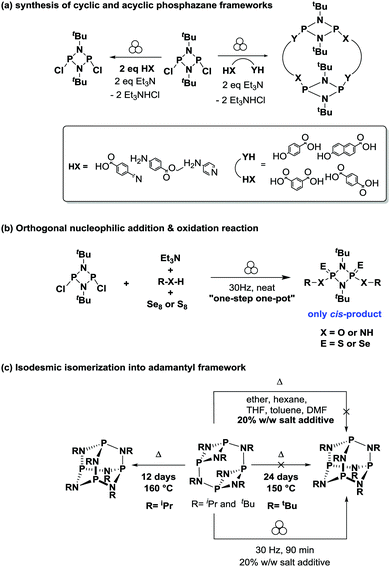
García et al. have demonstrated that mechanochemical routes to cyclophosphazane species can circumvent solvent compatibility issues, simplify the tedious processes associated with the use of strict anhydrous solvents, and minimise unwanted side-products (Scheme 8).
For instance, reacting dichlorocyclodiphosph(III)azanes with various N- and O-type nucleophiles to form a series of cyclic and acyclic phosphazane species (Scheme 8a);37 as well as the one-pot orthogonal nucleophilic addition and chalcogenic oxidation reaction to form substituted cyclodiphosph(V)azanes in the ball mill (Scheme 8b).38 These multi-component reactions selectively form the cis-oxidized products in high yields and effectively truncate the two-step reaction, into a “one-step one-pot” manner. This is in stark contrast to solution methods, where oxidation reactions typically result in a mixture of cis/trans isomers.
Furthermore, the steric demand of the nitrogen substituent controls the stability and accessibility of different cyclophosphazane isomers that can interconvert under suitable experimental conditions. A textbook example is the adamantoid P4(NR)6 frameworks, which can be obtained by isomerization of their less thermodynamically-stable macrocyclic [{P(μ-NR)}2(μ-NR)]2 counterparts upon heating. However, its tert-butyl (tBu) analogue does not isomerise even after prolonged heating – which has been rationalised over the last few decades by its highly sterically-encumbered nature.
Milling the tert-butyl bearing macrocycle for just 90 minutes under ILAG conditions readily yielded the adamantoid isomer, which is in stark contrast to previous efforts involving prolonged heating (24 days at 150 °C) or under reflux in a range of solvents with identical amounts of salt additive (Scheme 8c).39
These developments in main group element frameworks illustrate the impact that switching from a solution to mechanochemical environment can have on established chemical syntheses.
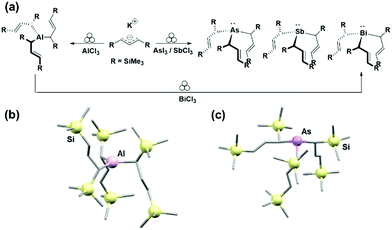
With reference to heavier elements within the group, Hanusa et al. extended the family of tris-allyl complexes and expanded their scope to Group 15 elements. The group mechanochemically synthesised As-, Sb- and Bi-tris(trimethylsilyl)allyl organometallic complexes in a one-step manner, directly or by using their previously obtained Al complex as a precursor in a two-step synthetic route (Fig. 13).29
The mechanochemical route offered several other advantages. Firstly, excess oxone could be readily removed from the reaction mixture due to its water-soluble nature, facilitating purification of the desired product. Furthermore, stereoselectivity could be controlled by simply changing the metal halide source, favouring the formation of one isomer over the other (i.e., cis vs. trans). Lastly, metal halide salts are less toxic than standard halogenation reagents (e.g., Br2, pyridinium bromide, etc.).40
The same authors also reported a five-component, “one-pot” sequential telescoping synthesis of an organometallic TMEDA triscarbonyl-fluoro Re(I) complex, starting from Re2(CO)10 (Fig. 16b).41 Their synthetic route comprised several orthogonal steps namely, oxidation using oxone, iodination using NaI, halide exchange using AgF, and decarbonylative chelation using TMEDA as a bidentate ligand (Fig. 16b).
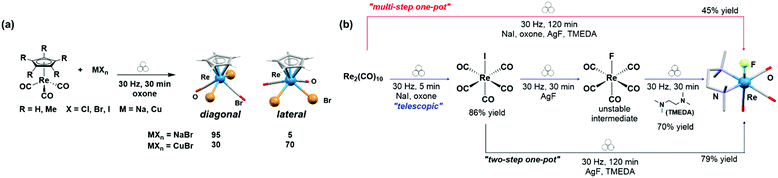 | ||
| Fig. 16 (a) Mechanochemical bromination of organometallic rhenium complexes using oxone and NaBr or CuBr.40 (b) Multistep mechanochemical transformation of Re2(CO)10 to mono-rhenium complexes. Hydrogen atoms are omitted for clarity.41 | ||
These two examples in combination with the complexes described in previous sections, illustrate the use of mechanochemistry as a powerful and straightforward methodology for the synthesis organometallic compounds.
3.2 Materials containing p-block elements
Orlovskaya et al. reported the mechanochemical synthesis of a novel hexagonal OsB2 phase by milling elemental Os and B under an inert atmosphere, using tungsten carbide milling media.42 The most commonly studied OsB2 is the thermodynamically stable orthorhombic form, which is generally produced by high temperature sintering and annealing. On the other hand, mechanochemical synthesis exclusively produced the new hexagonal phase at ambient temperature, without requiring thermal treatment (Fig. 17).
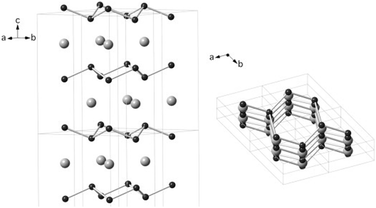 | ||
| Fig. 17 Crystal structure of hexagonal OsB2. Osmium atoms are the larger gray spheres, and boron atoms are the smaller black spheres. Reproduced from ref. 42 with permission from Elsevier, copyright 2014. | ||
The milling reaction was conducted over 20 hours, with interruptions every 30 minutes to allow the reaction to equilibrate to room temperature. Ex situ PXRD monitoring demonstrated that initial amorphization of the Os and B metals was accompanied by formation of hexagonal OsB2, which crystallises upon prolonged grinding (12 hours). The formation of the metastable hexagonal OsB2 phase was attributed to the stress and strain induced by high energy ball milling.
Amidoboranes are a class of nitrogen and boron containing compounds typically obtained via deprotonation of the aminoborane N atom with alkali or alkaline earth metal bases. Within this family, mixed metal amidoboranes, have attracted attention as hydrogen storage materials with “tuneable” properties. Their dehydrogenation properties can be modulated by varying the type of metal incorporated.
These species can be readily prepared in a quantitative manner by ball milling.43 However, the monitoring and optimisation of these reactions can be challenging and generally disruptive since milling has to be stopped and the vessel frequently opened for sampling. This process is therefore especially tedious when working with air- and moisture-sensitive compounds.
Addressing this challenge, Biliškov et al. recently reported the real-time in situ monitoring of the synthesis of two bimetallic amidoboranes M2Mg(NH2BH3)4 (M = Li and Na) (Fig. 18).43 These species were prepared by grinding NH3BH3, MgH2 and MH (M = Li or Na) in a transparent jar on a shaker mill equipped with a Raman probe. Their in situ measurements revealed a two-step mechanism, in which the metal hydride reacts with the NH3BH3 to form a MNH2BH3·NH3BH3 intermediate that further reacts with MgH2 to afford the M2Mg(NH2BH3)4 product. This work further highlights the importance of in situ measurements to understand reaction mechanisms and kinetics for solid-state hydrogen storage materials.
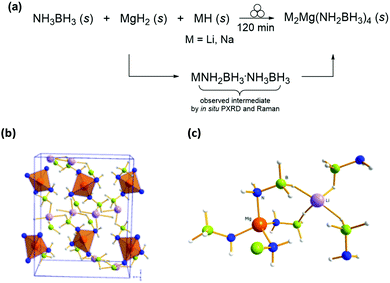 | ||
| Fig. 18 (a) Mechanochemical formation of mixed metal amido-boranes. (b) Crystal packing and (c) bonding environment of DLMAB. Each Mg2+ is bound to four N– forming a tetrahedron with NH2BH3– groups, and each Li+ is octahedrally coordinated through six hydride H atoms of NH2BH3– moieties. Reproduced from ref. 43 with permission from Willey, copyright 2017. | ||
Descending the group, although there are many known gallium-based molecular species and materials, the most widely studied compound is gallium nitride (GaN). This is largely owing to its unique semiconducting properties that have been exploited in several technological applications, including as light-emitting diodes and blue laser devices. Commonly, bulk GaN powders are obtained by heating Ga2O3 under a NH3 gas atmosphere at high temperatures and pressures.
In 2008, Kano et al. reported a direct multi-gram synthesis of GaN from Ga2O3 and Li3N under NH3 atmosphere in a planetary mill using a customized zirconia jar (Scheme 9).44 A major advantage of their method is that the reaction completes within 2 hours and does not require high reaction temperatures and pressures. Interestingly, during the milling process, two reactions occurred simultaneously – a homogenous solid–solid salt metathesis reaction between Ga2O3 and Li3N, and a heterogenous solid–gas reaction between Ga2O3 and NH3 – both yielding the desired GaN product.
 | ||
| Scheme 9 (a) Mechanochemical reaction between Ga2O3 with Li3N and NH3, to form GaN. (b) Illustration of ZrO2 reactor with Ga2O3 and Li3N sample mixture within the overpot and gas flow arrangement. Reproduced from ref. 44 with permission from Elsevier, copyright 2008. | ||
Lewiński et al. demonstrated the ball milling synthesis of a hybrid inorganic–organic CH3NH3PbI3 (MAPbI3) perovskite material that was implemented in photovoltaic devices.45 Grinding CH3NH3I with PbI2 in a mortar and pestle in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio resulted in incomplete conversion, however, the desired perovskite was formed in a shaker mill within 30 minutes, as evidenced by ex situ PXRD studies. In contrast to solvothermal methods which require a 5% molar excess of CH3NH3I to enable complete conversion to the perovskite, mechanochemistry allows for exact stoichiometric synthesis of perovskites. The perovskite was used in a simple solar cell device via a one-step deposition method, and its power conversion efficiency compared with their solvothermally synthesised counterparts.
1 stoichiometric ratio resulted in incomplete conversion, however, the desired perovskite was formed in a shaker mill within 30 minutes, as evidenced by ex situ PXRD studies. In contrast to solvothermal methods which require a 5% molar excess of CH3NH3I to enable complete conversion to the perovskite, mechanochemistry allows for exact stoichiometric synthesis of perovskites. The perovskite was used in a simple solar cell device via a one-step deposition method, and its power conversion efficiency compared with their solvothermally synthesised counterparts.
Evaluation of their photovoltaic properties revealed that, although the photon to electron conversion efficiency of the mechanochemically synthesised perovskite was slightly lower (i.e., 1 mA cm−2 lower current density), its open circuit voltage was higher (100 mV, with a 4% higher fill factor), resulting in better device performance overall.45
In addition, comparison of their current–voltage forward and backward sweep showed no hysteresis for the mechanochemically synthesised material, suggesting a lower presence of defects. The exact stoichiometric control avoided the creation of Pb ion vacancies, which can act as electron traps, resulting in a hysteresis-free device with enhanced performance. Direct access to perovskite-based photovoltaic devices illustrates the potential of mechanochemistry for the fabrication of solar cells with enhanced performance (Fig. 19).
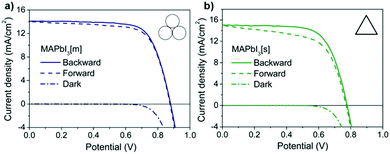 | ||
| Fig. 19 Current–voltage measurements of the MAPbI3 perovskite devices prepared synthesised (a) mechanochemically and (b) by solution methods, to compare the hysteresis effect. Reproduced from ref. 45 with permission from the RSC, copyright 2015. | ||
In another account, de Miguel et al. reported the formation of hybrid perovskites by ball milling methylammonium (MA), formamidinium (FA) and guanidinium (Gua) iodide with PbI2 in stoichiometric ratios.46 In this manner, 1D, 2D, and 3D hybrid perovskites (GuaPbI3, Gua2PbI4 and MAPbI3 and FAPbI3, respectively) were obtained and characterised by ex situ PXRD analysis. Their procedure offers a simple, efficient and highly reproducible route to polycrystalline materials on a multigram scale for use in optoelectronic applications. These two examples highlight the ability of mechanochemistry to enable precise stoichiometric control over the composition and formation of perovskite materials for manufacturing of devices.
Combining ligand capping nanoparticle (NP) strategies with bulk perovskite synthesis, Wang et al. recently reported the synthesis of main group CsPbX3 perovskite quantum dots (QDs).47 These QDs exhibited high fluorescence quantum yield and high photostability, with tuneable spectral range, representing good candidates for use in optoelectronic devices (i.e., such as photodetectors, solar cells etc.). NP-Sized CsPbBr3 was obtained by ball milling CsBr and PbBr2, with oleylamine as a surface capping agent. In addition, by altering the stoichiometry of the starting materials, a series of mixed halide perovskites CsPbX3 (X = Cl, Br or I) with varying optical properties were obtained (Fig. 20). This stoichiometric control afforded by mechanochemistry enables the straightforward fine-tuning of optical properties (Fig. 20), opening new avenues towards novel inorganic perovskite nanomaterials.
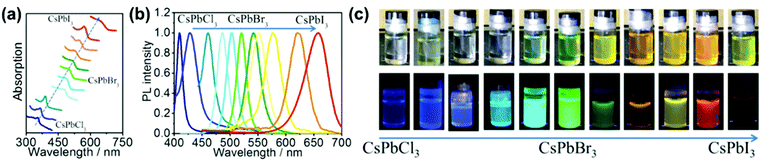 | ||
| Fig. 20 (a) Stacked UV-vis absorption spectra and (b) normalized fluorescence spectra of the mechanosynthesised CsPbX3. (c) QDs with varying halide ratios. Reproduced from ref. 47 with permission from the ACS, copyright 2017. | ||
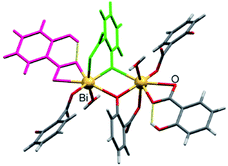 | ||
| Fig. 21 Fragment of a bismuth disalicylate sheet highlighting different bonding modes of doubly deprotonated sal (green) and singly deprotonated Hsal (purple) anions. Reproduced from ref. 48 with permission from Willey, copyright 2011. | ||
Generally, mechanochemical reactions between solids/solids phases/powders are thought to occur through one of two mechanisms, either (i) gradual reactions induced by non-thermal deformation and mixing, or (ii) reactions promoted by exothermic processes that result in an autocatalytic self-propagation of the reaction.
Humphry-Baker et al. described a third type of mechanochemical reaction mechanism that shares several characteristics of both (i) and (ii) in which the heat released by mechanical work leads to localised melting triggering local chemical reactions. In this case, the impact force exerted by a stainless-steel ball in a ball drop reactor was used to produce bismuth telluride (Bi2Te3) from a stoichiometric powdered mixture of elemental Bi and Te (Fig. 22).49 Similarly to mechanism (i), there is an initial deformation produced by the impact however, in this case, localised pinpoint heating and melting occurs, triggering the reaction. Although there is liquefaction, it is restricted to individual particles and does not classify as a mechanically-induced self-propagating reaction as in (ii). Furthermore, a combination of SEM and in situ temperature monitoring determined that the process is not gradual as it would have been in a type (i) mechanism.
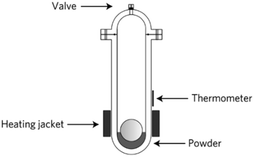 | ||
| Fig. 22 Diagram depicting the ball drop reactor for the impact force induced formation of Bi2Te3. Reproduced from ref. 49 with permission from the NPG, copyright 2016. | ||
 | ||
| Fig. 23 Pictures of the eccentric vibratory mill with attached closed satellite and open satellite filled with balls. Reproduced from ref. 51 with permission from the ACS, copyright 2018. | ||
Ex situ PXRD analysis revealed full conversion of the chalcopyrite after only 120 minutes of milling. In addition, their protocol was used to synthesise kesterite, Cu2ZnSnS4, from its elemental solids. This report emphasizes how mechanochemistry can be employed for the low-cost scaling and manufacturing of technologically relevant material from raw components.
Although the use of mechanochemistry to synthesis transition metal-based NPs and composite materials has already been covered in previous reviews,4,7,9,10 we will highlight a few examples of mechanochemically synthesised nano-sized materials comprising s- and p-block main group elements.
4. Synthesis of nanoparticles containing main group elements
4.1 Top-down approach to nanoparticles
Boron carbide (B4C) is characterised by its low density, high melting point and hardness, properties that are desirable in a wide range of engineering applications. Generally, B4C is synthesised by reduction of cheap and readily accessible boron oxide, B2O3. In contrast to the traditional reduction of B2O3 by carbon (which is highly endothermic and energy intensive), ball milling B2O3 in the presence of magnesium and carbon under inert atmosphere conditions affords B4C in good yield.52In situ temperature and ex situ PXRD monitoring revealed an initial size reduction of the reaction mixture without product formation after 60 hrs (confirmed by SEM, Fig. 24). Upon milling for 80 hours, an exothermic reduction reaction occurred in a self-propagating combustion process, type (i) mechanism, to form B4C and MgO by-products, which are readily removed by washing with mild acids.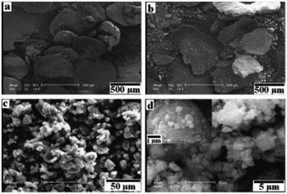 | ||
| Fig. 24 SEM images of the powders after (a) 2 hours, (b) 20 hours (c) 60 hours and (d) 80 hours of milling times, for the reaction between Mg, C and B2O3 to form B4C. Reproduced from ref. 52 with permission from Elsevier, copyright 2011. | ||
Main group metal oxides, such as stannates, have gained significant interest due to their potential applications in photoelectrical devices, anode materials in Li-ion batteries, etc. Šepalák et al. reported the synthesis of nanocrystalline Ca2SnO4via high-energy milling of CaO with SnO4 at low temperatures in a single step,53 representing both a novel and sustainable approach to their production. Noteworthy, is the combination of 119Sn-MAS-NMR, 119Sn Mössbauer spectroscopy, PXRD and TEM to study the mechanically-induced formation of Ca2SnO4 Their comprehensive structural studies showed a fully ordered orthorhombic core with disordered near-surface layers where the tin(IV) atoms displayed a distorted octahedral geometry.
As previously discussed, the addition of inert milling auxiliaries to grinding mixtures helps convert viscous mixtures into “powder-like” solids. These milling auxiliaries comprise organic polymers, salts (e.g., NaCl) and inert oxides (e.g., silica). However, despite their “chemically innocent” nature, the addition of milling auxiliaries can be regarded as “diluting” the mechanochemical reaction. Their addition can influence the kinetics of milling reactions, act as an abrasive to induce surface defects or promote amorphization and reduction of particle size.
Blair et al. reported the size-controlled synthesis of zirconium silicides (i.e., ZrSi2) by ball milling of ZiCl4 and CaSi, using CaCl2 as a milling auxiliary.54 By varying the loadings of the CaCl2 auxiliary acting as a diluent they were able to fine-tune the size of the ZrSi2 crystallite. The “effective” concentration of this reaction can be defined as moles of reagent per litre of free volume of the vessel (volume of the vessel excluding the volume of the milling media and reactants). PXRD analysis revealed a linear correlation between ZrCl4 concentration and the ZrSi2 crystallite size (Fig. 25).
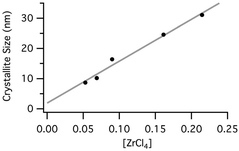 | ||
| Fig. 25 Correlation between the particle size and the effective concentration of ZrCl4, as expressed in terms of moles per litre of free volume 53. | ||
4.2 Bottom-up approach to nanoparticles
The bottom-up approach to synthesising NPs can often occur under mild milling conditions. In such cases, ball milling the reagents for a few minutes provides sufficient “mechanical activation” to enable reactions to progress to completion without further input of energy to the system.Bismuth sulfide, Bi2S3, NPs are promising nanomaterials for biomedical purposes as they can be used as contrast agents for computed tomography (CT) imaging. Recently, Malca et al. reported the synthesis of ultrafine Bi2S3 NPs by a short mechanical activation followed by aging, whereby a solid mixture is allowed to sit (or “age”) under controlled ambient conditions without agitation or external input of energy.55
The Bi2S3 NPs were obtained by ball milling commercially available Bi(NO3)3·5H2O with L-cysteine, sources of bismuth and sulfur respectively, in the presence of capping agents (i.e., oleylamine or 6-aminohexanoate). TEM analysis revealed the formation of a highly disperse and narrow Bi2S3 NP size distribution (2–3 nm), which is the smallest reported to date (Fig. 26). Interestingly, the same ultrafine NP distribution can also be obtained by manual grinding for a few minutes followed by ageing overnight. Subsequently, the authors demonstrated that multi-gram scale synthesis of the Bi2S3 NPs can be performed by using a planetary ball mill and ageing in air for 48 hours, highlighting the straightforward scalability of their solvent-free methodology.
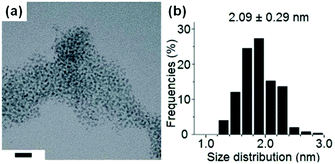 | ||
| Fig. 26 (a) TEM images and (b) size distribution histograms of the oleylamine-capped Bi2S3 NPs synthesised using a combination of mechanical milling and activation, and ageing. Scale bars in the TEM image represent 20 nm. Reproduced from ref. 55 with permission from the ACS, copyright 2017. | ||
5. Conclusions and outlook
In summary, this tutorial review serves as an introduction to the fast-emerging field of main group mechanochemistry. Although many examples were beyond the scope of this review, we have showcased a variety of seminal work and unique examples in the field, along with the various techniques involved in their analysis.Whilst we do not suggest that mechanochemistry will ever fully replace solution-based methods, it is clearly a powerful and environmentally-benign synthetic tool that should be better exploited. Its rapid renaissance has been realised by a group of pioneering researchers from a variety of fields who have transformed mechanochemistry from a mere curiosity to a well-established research area within the chemical and materials sciences.
However, and in spite of its many benefits, the application of mechanochemistry in the main group arena still represents an underutilised alternative to solution-based methods, and its adoption by the main group community remains a significant challenge.
Many main group compounds are air- and moisture-sensitive and hence, require special handling, manipulation and storage. Solution-based techniques (i.e., Schlenk techniques) have been developed for their handling, however, despite accumulating examples of the same species being produced mechanochemically, the latter trails behind, lacking standardised protocols, especially for large-scale continuous flow processes (i.e., TSE).13
Advancement of current mechanistic understanding of mechanochemistry is vital to enabling its incorporation as a mainstream methodology for synthetic and materials chemists alike. Recently, significant efforts have been placed towards understanding the fundamental mechanisms underlying mechanochemical reactions.4,5,7 However, further enhancement of current monitoring techniques and development of new approaches for in situ measurements are required to fully comprehend mechanochemical transformations and allow for better reaction control.
Both the air-sensitive apparatus and the development of more sophisticated in situ measurement will require interdisciplinary research efforts between chemists, materials scientists, physicists and engineers sharing a common vision of “engineering mechanochemical technologies” and we foresee many more collaborative advancements in these areas in the next few years.
On a longer term, we anticipate that the implementation of disruptive technologies such as automation, robotics, machine learning and artificial intelligence to mechanochemistry will transform the way it is executed in a similar way it has revolutionised the way new molecules are discovered in solution. This, in our humble opinion, is the next frontier of mechanochemistry.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank A*STAR AME IRG (A1783c0003), NTU start-up grant (M4080552), and MOE AcRF Tier 1 grants (M4011441 and M4011709) for financial support. We would like to thank X. Y. Shi, Y. Sim, H. C. Ong and K. A. Martin for constructive discussion.Notes and references
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC , and references therein.
- J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed , and references therein.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed , and references therein.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC , and references therein.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC , and references therein.
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC , and references therein.
- E. Boldyreva, Chem. Rev., 2013, 42, 7719–7738 CAS , and references therein.
- D. Braga, S. L. Giaffreda, F. Grepioni, A. Pettersen, L. Maini, M. Curzi and M. Polito, Dalton Trans., 2006, 1249 RSC , and references therein.
- M. J. Muñoz-Bautista, D. Rodriguezpadron, A. R. Puente-Santiago and R. Luque, ACS Sustainable Chem. Eng., 2018, 9, 9530–9544 CrossRef , and references therein.
- C. Xu, S. De, A. M. Balu, M. Ojeda and R. Luque, Chem. Commun., 2015, 51, 6698–6713 RSC , and references therein.
- A. A. Geciauskaite and F. García, Beilstein J. Org. Chem., 2017, 13, 2068–2077 CrossRef CAS PubMed , and references therein.
- L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC , and references therein.
- D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC , and references therein.
- J. M. Anderson and J. Mack, Chem. Sci., 2017, 8, 5447–5453 RSC , and references therein.
- G. A. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC , and references therein.
- D. Tan, L. Loots and T. Friščić, Chem. Commun., 2016, 52, 7760 RSC , and references therein.
- K. Užarević, I. Halasz and T. Friščić, J. Phys. Chem. Lett., 2015, 6, 4129 CrossRef PubMed , and references therein.
- J. Mack, D. Fulmer, S. Stofel and N. Santos, Green Chem., 2007, 9, 1041–1043 RSC , and references therein.
- V. P. Balema, V. K. Pecharsky and K. W. Dennis, J. Alloys Compd., 2000, 313, 69–74 CrossRef CAS.
- I. Z. Hlova, S. Gupta, J. F. Goldston, T. Kobayashi, M. Pruski and V. K. Pecharsky, Faraday Discuss., 2014, 170, 137–153 RSC.
- N. C. Boyde, N. R. Rightmire, T. P. Hanusa and W. W. Brennessel, Inorganics, 2017, 5, 36–47 CrossRef.
- D. W. Peters and R. G. Blair, Faraday Discuss., 2014, 170, 83–91 RSC.
- D. B. Ravnsbæk, L. H. Sørensen, Y. Filinchuk, F. Besenbacher and T. R. Jensen, Angew. Chem., Int. Ed., 2012, 51, 3582–3586 CrossRef PubMed.
- Z. Li, J. Zhang, S. Wang, L. Jiang, M. Latroche, J. Dub and F. Cuevas, Phys. Chem. Chem. Phys., 2015, 17, 21927–21934 RSC.
- S. Soiron, A. Rougier, L. Aymard and J.-M. Tarascon, J. Power Sources, 2001, 402–405 CrossRef CAS.
- X. Yang, Z. Wen, X. Xu, B. Lin and S. Huang, J. Power Sources, 2007, 164, 880–884 CrossRef CAS.
- A. Düvel, S. Wegner, K. Efimov, A. Feldhoff, P. Heitjans and M. Wilkening, J. Mater. Chem., 2011, 21, 6238–6250 RSC.
- G. Scholz, S. Breitfeld, T. Krahl, A. Düvel, P. Heitjans and E. Kemnitz, Solid State Sci., 2015, 50, 32–41 CrossRef CAS.
- N. R. Rightmire, D. L. Bruns, T. P. Hanusa and W. W. Brennessel, Organometallics, 2016, 35, 1698–1706 CrossRef CAS.
- J. Wang, R. Ganguly, L. Yongxin, J. Díaz, H. S. Soo and F. García, Dalton Trans., 2016, 45, 7941–7946 RSC.
- M. Glavinović, M. Krause, L. Yang, J. A. McLeod, L. Liu, K. M. Baines, T. Friščić and J.-P. Lumb, Sci. Adv., 2017, 3, e1700149 CrossRef PubMed.
- V. Chandrasekhar, V. Baskar, R. Boomishankar, K. Gopal, S. Zacchini, J. F. Bickley and A. Steiner, Organometallics, 2003, 22, 3710–3716 CrossRef CAS.
- R. F. Koby, T. P. Hanusa and N. D. Schley, J. Am. Chem. Soc., 2018, 140, 15934–15942 CrossRef CAS PubMed.
- V. P. Balema, J. W. Wiench, M. Pruski and V. K. Pecharsky, J. Am. Chem. Soc., 2002, 124, 6244–6245 CrossRef CAS PubMed.
- V. P. Balema, J. W. Wiench, M. Pruski and V. K. Pecharsky, Chem. Commun., 2002, 724–725 RSC.
- R. Kumar, S. Kumar, M. K. Pandey, V. S. Kashid, L. Radhakrishna and M. S. Balakrishna, Eur. J. Inorg. Chem., 2018, 1028–1037 CrossRef CAS.
- Y. Sim, Y. X. Shi, R. Ganguly, Y. Li and F. García, Chem. – Eur. J., 2017, 23, 11279–11285 CrossRef CAS PubMed.
- Y. Sim, D. Tan, R. Ganguly, Y. Li and F. García, Chem. Commun., 2018, 54, 6800–6803 RSC.
- X. Shi, K. Xu, J. K. Clegg, R. Ganguly, H. Hirao, T. Friščić and F. García, Angew. Chem., Int. Ed., 2016, 55, 12736–12740 CrossRef PubMed.
- J. G. Hernández, N. A. J. Macdonald, C. Mottillo, I. S. Butler and T. Friščić, Green Chem., 2014, 16, 1087–1092 RSC.
- J. G. Hernández, I. S. Butler and T. Friščić, Chem. Sci., 2014, 5, 3576–3582 RSC.
- Z. Xie, M. Graule, N. Orlovskaya, E. A. Payzant, D. A. Cullen and R. G. Blair, J. Solid State Chem., 2014, 215, 16–21 CrossRef CAS.
- N. Biliškov, A. Borgschulte, K. Užarević, I. Halasz, S. Lukin, S. Milošević, I. Milanović and J. G. Novakovic, Chem. – Eur. J., 2017, 23, 16274–16282 CrossRef PubMed.
- J. Kano, E. Kobayashi, W. Tongamp and F. Saito, J. Alloys Compd., 2008, 464, 337–339 CrossRef CAS.
- D. Prochowicz, M. Franckevičius, A. M. Cieślak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC.
- A. D. Jodlowski, A. Yépez, R. Luque, L. Camacho and G. de Miguel, Angew. Chem., Int. Ed., 2016, 55, 14972–14977 CrossRef CAS PubMed.
- Z.-Y. Zhu, Q.-Q. Yang, L.-F. Gao, L. Zhang, A.-Y. Shi, C.-L. Sun, Q. Wang and H.-L. Zhang, J. Phys. Chem. Lett., 2017, 8, 1610–1614 CrossRef CAS PubMed.
- V. André, A. Hardeman, I. Halasz, R. S. Stein, G. J. Jackson, D. G. Reid, M. J. Duer, C. Curfs, M. T. Duarte and T. Friščić, Angew. Chem., Int. Ed., 2011, 50, 7858–7861 CrossRef PubMed.
- S. A. Humphry-Baker, S. Garroni, F. Delogu and C. A. Schuh, Nat. Mater., 2016, 15, 1280–1286 CrossRef CAS PubMed.
- P. Baláž, M. Baláž, M. Achimovičová, Z. Bujňaková and E. Dutková, J. Mater. Sci., 2017, 52, 11851–11890 CrossRef.
- P. Baláž, M. Hegedüs, M. Achimovičová, M. Baláž, M. Tešinský, E. Dutková, M. Kaňuchová and J. Briančin, ACS Sustainable Chem. Eng., 2018, 6, 2132–2141 CrossRef , and references therein.
- E. M. Sharifi, F. Karimzadeh and M. H. Enayati, Adv. Powder Technol., 2011, 22, 354–358 CrossRef CAS.
- V. Šepalák, K. D. Becker, I. Bergmann, S. Suzuki, S. Indris, A. Feldhoff, P. Heitjans and C. P. Grey, Chem. Mater., 2009, 21, 2518–2524 CrossRef.
- D. Restrepo, S. M. Hick, C. Griebel, J. Alarcón, K. Giesler, Y. Chen, N. Orlovskaya and R. G. Blair, Chem. Commun., 2013, 49, 707–709 RSC.
- M. Y. Malca, H. Bao, T. Bastaille, N. K. Saadé, J. M. Kinsella, T. Friščić and A. Moores, Chem. Mater., 2017, 29, 7766–7773 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cs00813a |
| This journal is © The Royal Society of Chemistry 2019 |