Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spectroscopy of YO from first principles†
Alexander N.
Smirnov
 a,
Victor G.
Solomonik
a,
Victor G.
Solomonik
 a,
Sergei N.
Yurchenko
a,
Sergei N.
Yurchenko
 *b and
Jonathan
Tennyson
*b and
Jonathan
Tennyson
 b
b
aDepartment of Physics, Ivanovo State University of Chemistry and Technology, Ivanovo 153000, Russia
bDepartment of Physics & Astronomy, University College London, London WC1E 6BT, UK. E-mail: s.yurchenko@ucl.ac.uk
First published on 2nd October 2019
Abstract
We report an ab initio study on the spectroscopy of the open-shell diatomic molecule yttrium oxide, YO. The study considers the six lowest doublet states, X2Σ+, A′2Δ, A2Π, B2Σ+, C2Π, D2Σ+, and a few higher-lying quartet states using high levels of electronic structure theory and accurate nuclear motion calculations. The coupled cluster singles, doubles, and perturbative triples, CCSD(T), and multireference configuration interaction (MRCI) methods are employed in conjunction with a relativistic pseudopotential on the yttrium atom and a series of correlation-consistent basis sets ranging in size from triple-ζ to quintuple-ζ quality. Core–valence correlation effects are taken into account and complete basis set limit extrapolation is performed for CCSD(T). Spin–orbit coupling is included through the use of both MRCI state-interaction with spin–orbit (SI-SO) approach and four-component relativistic equation-of-motion CCSD calculations. Using the ab initio data for bond lengths ranging from 1.0 to 2.5 Å, we compute 6 potential energy, 12 spin–orbit, 8 electronic angular momentum, 6 electric dipole moment and 12 transition dipole moment (4 parallel and 8 perpendicular) curves which provide a complete description of the spectroscopy of the system of six lowest doublet states. The Duo nuclear motion program is used to solve the coupled nuclear motion Schrödinger equation for these six electronic states. The spectra of 89Y16O simulated for different temperatures are compared with several available high resolution experimental studies; good agreement is found once minor adjustments are made to the electronic excitation energies.
1 Introduction
Oxides of transition metals and lanthanides have rich and complex spectra due to the presence of many low-lying excited electronic states. This complexity poses particular challenges for experimental1 and theoretical2 studies. The yttrium oxide, YO, is an example of a rare-earth oxide whose electronic structure is very difficult to explore. Yttrium is a relatively abundant rare-earth element both on Earth (the 28th most abundant element3) and in space (the second most abundant rare-earth metal4). As a result, the spectrum of YO has been the subject of many astrophysical observations. In particular, YO has been observed in a variety of spectra of cool stars including R-Cygni,5 Pi-Gruis,6 V838 Mon,7,8 and V4332 Sgr.7 The spectrum of YO has been extensively used as a probe to study high temperature materials at the focus of a solar furnace.9–11 The A2Π1/2 electronic state YO has a relatively short life time of 33 ns12 with large diagonal Franck–Condon factors,13 which makes this molecule well suited for cooling experiments with the potential in quantum information applications.14 Yttrium oxide is one of the very few molecules that have been laser cooled and trapped in a magneto-optical trap.15–18A considerable number of experimental studies have been performed probing the A2Π–X2Σ+,10,11,13,19–34 B2Σ+–X2Σ+,19,21,29,35–37 A′2Δ–X2Σ+,16,38,39 and D2Σ+–X2Σ+37 bands of YO, as well as its microwave rotational spectrum40–42 and its hyperfine structure.26,41,43–47 Chemiluminescence spectra of YO have also been investigated.29,48,49 Many of these spectra were recorded using YO samples which were not in thermodynamic equilibrium, thus, at best, only providing information on the relative intensities. For YO, relative intensity measurements were carried out for the A2Π–X2Σ+ system by Bagare and Murthy.24 However, the permanent dipole moments of YO in both the X2Σ+ and A2Π states were measured using the Stark technique.27,45,47
In the absence of direct intensity measurements, measured lifetimes can provide important information on Einstein A coefficients and hence transition dipole moments.50 The lifetimes of some lower lying vibrational states of YO in its A2Π, B2Σ+, and D2Σ+ states were measured by Liu and Parson12 and Zhang et al.37
YO is a strongly bound system. The compilation by Gaydon51 reports its dissociation energy to be 7.0 ± 2 eV, while Ackermann and Rauh52 recommended a D0 value of 7.290(87) eV based on mass spectrometric determinations.
A few theoretical investigations of YO are available in the literature. The most comprehensive one was carried out by Langhoff and Bauschlicher53 who reported the spectroscopic constants for the lowest five doublet, X2Σ+, A′2Δ, A2Π, B2Σ+, C2Π, and fourteen quartet electronic states of YO. The doublets were studied at the multireference single and double excitation configuration interaction (MRCI) level of theory and, in the case of the X2Σ+, A′2Δ, and A2Π states, also using the modified coupled-pair functional (MCPF) method. All the quartet states were considered at the CASSCF level, and that with the lowest energy, reportedly 4Φ, at the MCPF level as well. Zhang et al.37 have recently reported the CASPT2 spectroscopic constants and excitation energies for a set of lowest doublet states of YO including the D2Σ+ state in addition to the doublets studied previously by Langhoff and Bauschlicher.53 In all of the previous theoretical studies, only modest double-ζ53 or triple-ζ37 basis sets were employed. RKR curves and some Franck–Condon factors of YO were computed by Sriramachandran and Shanmugavel.54
The main objective of the present study is to characterise both the electronic ground state and the plethora of low-lying excited states of YO with high-level ab initio methods, and to accurately describe from first principles the spectroscopy of YO via producing the potential energy curves (PECs) and other data needed to calculate the rovibronic energies and transition probabilities comprising a so-called line list for this molecule. The generation of such line lists is a major object of the ExoMol project.55
Thus far, ExoMol studies of open-shell transition metal (TM) diatomics have struggled due to difficulties in providing reliable ab initio starting points.2,56–58 The intrinsic challenge to theory posed by open-shell systems is associated with several types of problems including spin contamination, symmetry breaking in the reference function, strong nondynamical electron correlation effects, avoided crossings between adiabatic potential energy surfaces, etc. (for the discussion, see, e.g., ref. 59 and 60). In the open-shell TM-containing species, these problems are exacerbated by stronger relativistic effects than those in the molecules made up of relatively light main group elements, and greater number of electronic excited states governing the spectroscopic behaviour of a molecule and hence deserving to be taken into account in a study aimed at accurate description of its spectroscopy. Moreover, the low-lying electronic states of TM species are commonly degenerate or near-degenerate, which complicates their theoretical treatment even more. Multireference methods of quantum chemistry best suited for describing closely spaced electronic states might seem to be the natural choice for studying these systems. However, most routine multireference methods, such as MRCI, are incapable of properly handling dynamical electron correlation and therefore do not provide high accuracy description of TM-containing species commonly featuring strong dynamical correlation effects. Such effects are best treated with single reference coupled cluster (CC) theory known for its capability to predict highly accurate properties even for molecules with mild to moderate MR character. Unfortunately, the higher likelihood of severe multireference character in the ground and/or low-lying electronic excited states of open-shell TM-containing species makes their treatment by single reference methods very problematic, if possible at all. Particularly this is true for the studies aimed at a description of the molecular potential energy surfaces over a wide range of geometries. It is therefore not surprising that the high-level coupled cluster studies on the open-shell TM-containing species, where a few excited states are treated on an equal footing with the ground state, are very uncommon and only deal with near-equilibrium regions of these states (see, e.g., ref. 61–64). Such a study on a manifold of electronic excited states of a TM-containing diatomic molecule over a wider bond length range has not been reported so far.
It is thus clear that none of routine methods of modern quantum chemistry are entirely satisfactory in all respects for accurately describing from first principles the spectroscopy of open-shell TM-containing species. Nevertheless, one can try to solve this challenging task via the so-called composite approach by which the desired set of molecular properties is obtained using multiple methods of different nature and sophistication rather than a single method.
In this paper, we have examined efficiency of such an approach taking the example of YO. The PECs for the six lowest doublet electronic states of this molecule, X2Σ+, A′2Δ, A2Π, B2Σ+, C2Π, D2Σ+, were obtained from the extensive high-level coupled cluster calculations addressing core–valence correlation and basis set convergence issues, whereas the spin–orbit curves (SOCs), electronic angular momentum curves (EAMCs), electric dipole moment curves (DMCs), and transition dipole moment curves (TDMCs) were obtained at the MRCI level of theory. These curves, with some simple adjustment of the minimum energies of the PECs, are used to solve the coupled nuclear-motion Schrödinger equation with the program Duo.65 The spectroscopic model and ab initio curves are provided as part of the ESI.† Our open source code Duo can be accessed viahttp://exomol.com/software/.
2 Computational details
2.1 Ab initio calculations
Multireference single and double excitation configuration interaction, MRCI,66–68 and underlying complete active space self-consistent field, CASSCF,69,70 calculations were carried out using a relativistic energy-consistent 28-electron core pseudopotential (PP) accompanied with the aug-cc-pVTZ-PP71 basis set on the Y atom, and the aug-cc-pVTZ72 all-electron basis set on the O atom (this combination of sets is hereafter referred to as aVTZ). To obtain a consistent MRCI data set in the widest possible range of bond lengths, the state-averaged CASSCF procedure was employed with density matrix averaging over 22 doublet (six Σ+, seven Π, five Δ, two Φ, and two Σ−) and 9 quartet (two Σ+, three Π, two Δ, one Φ, and one Σ−) states, with equal weights for each of the roots. The active space included 7 electrons distributed in 13 orbitals (6a1, 3b1, 3b2, 1a2) that had predominantly O 2p and Y 4d, 5s, 5p, and 6s character; all lower energy orbitals were constrained to be doubly occupied. All valence electrons (4d, 5s Y; 2s, 2p O) were included in the MRCI correlation treatment.Potential energy, spin–orbit coupling, and dipole moment curves, as well as electronic angular momentum and transition dipole matrix elements were obtained at the MRCI level for the six lowest doublet states. Moreover, the potential curves were calculated using the extended multi-state complete active space second-order perturbation theory,73 XMS-CASPT2, with the basis sets aug-cc-pwCVTZ-PP71 on Y and aug-cc-pwCVTZ74 on O (henceforth abbreviated as awCVTZ). In the respective SA-CASSCF calculations, the (7e,13o) active space was employed together with averaging over the lowest six doublet states. In order to remedy issues pertaining to intruder states, a level shift of 0.4 and an IPEA (ionisation potential, electron affinity) shift of 0.5 were employed for XMS-CASPT2.
To calculate the molecular Ω states and respective spin–orbit curves, we used the spin–orbit – MRCI state-interacting approach:75 the spin-coupled eigenstates were obtained by diagonalizing Hes + HSO in a basis of MRCI eigenstates of electrostatic Hamiltonian Hes. The matrix elements of HSO were constructed using the one-electron spin–orbit operator accompanying the yttrium pseudopotential.
Spin–orbit effects were also treated more rigorously in relativistic four-component (4c) all-electron calculations employing a Gaussian nuclear model and an accurate approximation to the full Dirac–Coulomb Hamiltonian.76 The respective spin-free results were obtained with the spin-free Hamiltonian of Dyall.77 In these calculations, the relativistic TZ-quality basis sets of Dyall78,79 were used for the Y and O atoms (hereafter referred to as TZD). The basis sets were kept uncontracted to provide sufficient flexibility. Electron correlation was taken into account via the equation-of-motion CCSD (EOM-CCSD) method80 with the Y outer-core (4s and 4p) electrons correlated together with the valence electrons. The EOM-EA scheme (adding 1 electron to the closed shell) was applied with the reference defined by the YO+ cation and the active space comprising 12 spinors (Y 5s and 4d). For the YO electronic states inaccessible via the EOM-EA procedure, we employed the EOM-IP scheme (removing 1 electron from the closed shell) with the YO− (Y 5s2) anion taken as the reference and an active space composed of 8 spinors (Y 5s and O 2p). The virtual orbital space was truncated by deleting all virtual spinors with orbital energies larger than 15 a.u. In the relativistic calculations of dipole moments, a finite-field perturbation scheme was employed by adding the z-dipole moment operator as a small perturbation to the Hamiltonian. Perturbations with electric field strengths of ±0.0005 a.u. were applied.
The atomic spin–orbit corrections, ΔESO, utilised in the calculations of the YO atomization energy were obtained from the experimental J-averaged zero-field splittings of the ground state atomic terms:81 ΔESO = −77.975 cm−1 (O) and −318.216 cm−1 (Y).
The most sophisticated PEC computations were performed at the coupled cluster singles, doubles, and perturbative triples, CCSD(T), level of theory82 with a restricted open-shell Hartree–Fock reference and with an allowance for a small amount of spin contamination in the solution of the CCSD equations, i.e., RHF-UCCSD(T).83,84 Symmetry equivalencing of the ROHF orbitals was performed for the degenerate atomic and molecular electronic states. Both valence (4d, 5s Y; 2s, 2p O) and outer-core (4s, 4p Y; 1s O) electrons were correlated. Scalar relativistic effects were treated with the yttrium pseudopotential described above. Sequences of aug-cc-pwCVnZ-PP71 (n = T, Q, 5) basis sets for Y were used in conjunction with the corresponding all-electron basis sets aug-cc-pwCVnZ74 for the O atom. These combinations of basis sets are denoted below as awCVTZ, awCVQZ, and awCV5Z, respectively.
For each point in a grid of r(Y–O) bond lengths, the CCSD(T) calculated energies were extrapolated to the complete basis set (CBS) limit. Three extrapolation schemes were employed. First, a two-point extrapolation of total energies was performed using the formula:85
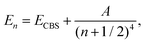 | (1) |
| En = ECBS + A(n + 1)exp(−9.03n1/2); | (2) |
En = ECBS + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−(n − 1)) + B exp(−(n − 1)) + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−(n − 1)2), exp(−(n − 1)2), | (3) |
The spectroscopic constants re, ωe, ωexe, and αe of YO were obtained from a conventional Dunham analysis88 using polynomial fits of total energies for bond lengths in the vicinity of the minimum for a given electronic state.
The CCSD(T) calculations of the equilibrium dipole moments, μe, for a few lowest states were carried out at the corresponding CCSD(T)/CBS1 equilibrium bond lengths. The dipole moments were computed by numerical differentiation of the total energy in the presence of a weak electric field. Finite perturbations with electric field strengths of ±0.0025 a.u. were applied. Since hierarchical sequences of basis sets have been used, the dipole moments were also extrapolated to the CBS limit using the three extrapolation schemes described above.
Most of the ab initio calculations were carried out using the MOLPRO electronic structure package.89 The relativistic 4c-EOM-CCSD calculations were performed using the DIRAC program.90
2.2 Nuclear motion calculations
We use the program Duo65,91 to solve the coupled Schrödinger equation for 6 lowest electronic states of YO. Duo is a variational program capable of solving rovibronic problems for a general (open-shell) diatomic molecule with an arbitrary number of couplings, see, for example, ref. 58 and 92–94. All ab initio couplings between these 6 states are taken into account as described below. The goal of this paper is to provide a qualitative simulation of the electronic spectra of YO based on the ab initio curves. We therefore do not attempt a systematic refinement of the ab initio curves by fitting to the experiment, which will be the subject of future work. In order to facilitate the comparison with the experimental data, we, however, perform some shifts of the Te values and simple scaling of the SOCs (see below).In Duo calculations, the coupled Schrödinger equation was solved on an equidistant grid of 301 bond lengths ri ranging from r = 1.2 to 3 Å using the sinc DVR method. Our ab initio curves are represented by sparser and less extended grids (see below). For the bond length values ri overlapping with the ab initio ranges, the ab initio curves were projected onto the denser Duo grid using the cubic spline interpolation. The PECs outside the ab initio range were reconstructed using the standard Morse potential form
| fPEC(r) = Ve + De(1 − e−a(r−re))2. |
| fshortTDMC(r) = Ar + Br2, |
| fshortother(r) = A + Br, |
| flongEAMC(r) = A + Br, |
| flongother(r) = A/r2 + B/r3, |
The vibrational basis set was taken as eigensolutions of the six uncoupled 1D problems for each PEC. The corresponding basis set constructed from 6 × 301 eigenfunctions was then contracted to include 60 lowest (in terms of the vibrational energy) X functions and 20 from each other state (160 in total). These vibrational basis functions were then combined with the spherical harmonics for the rotational and electronic spin basis set functions. All calculations were performed for 89Y16O using atomic masses.
This study is the first where a Duo calculation has been performed for a system with avoided crossings between curves of the same symmetry. The current version of Duo does not allow for non-adiabatic couplings, and therefore these were ignored in this study. However, despite the expectation that the non-adiabatic coupling effects should be relatively important in the regions near an avoided curve crossing, as we show below, our model neglecting these effects is justified by close agreement with the available experimental spectra.
3 Results and discussion
3.1 Results of ab initio calculations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 above the ground state.
000 cm−1 above the ground state.
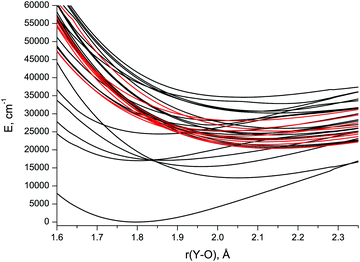 | ||
| Fig. 1 CASSCF/aVTZ spin-free potential energy curves of the low-lying doublet (black) and quartet (red) states in YO. | ||
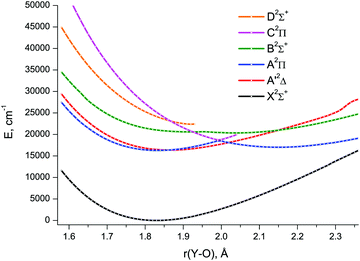
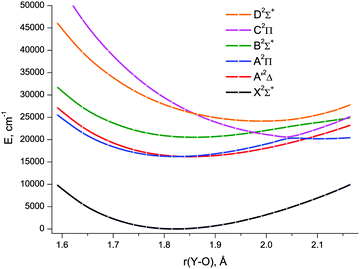
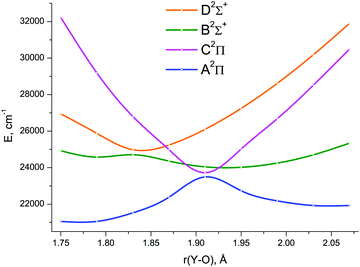
The lowest six doublet MRCI PECs (X2Σ+, A′2Δ, A2Π, B2Σ+, C2Π, D2Σ+) are shown in Fig. 2. For the states X2Σ+, A′2Δ, A2Π, and B2Σ+, the PECs were obtained in the full bond length range amenable to the underlying CASSCF procedure, 1.58 ≤ r ≤ 2.36 Å, while the C2Π and D2Σ+ curves were calculated through r = 2.04 Å and 1.93 Å, respectively. Extending the MRCI curves for the two upper states beyond those distances would require requesting a greater number of states (while exceeding 4 in a single irreducible representation with the chosen active space would make the MRCI computation unfeasible on the hardware used) or selecting the order of the states in the initial internal CI (e.g., 1, 2, 3, 5 rather than 1, 2, 3, 4), leaving some of them out for each MRCI point. This appeared to affect the smoothness of the resulting PECs. Therefore, we refrained from further pursuing the computations with the same number of MRCI roots in the entire bond length range and simply reduced the number of requested states for longer internuclear distances. For all six doublet states, the XMS-CASPT2 PECs could be obtained in the range 1.59 ≤ r ≤ 2.165 Å, Fig. 3; at larger bond lengths the underlying CASSCF procedure failed to converge. As can be seen from Fig. 2 and 3, the A2Π and C2Π curves feature an avoided crossing at bond lengths around 2 Å, as do the B2Σ+ and D2Σ+ curves in approximately the same region, see Fig. 3. The avoided crossings of both pairs of curves are also seen in the EOM-IP-CCSD calculated PECs, Fig. 4, albeit at shorter distances (1.8–1.9 Å).
 | ||
| Fig. 4 The avoided crossing regions of the EOM-IP-CCSD/TZD spin-free potential energy curves for the A2Π, B2Σ+, C2Π, and D2Σ+ electronic states of YO. | ||
In order to provide deeper insight into the electronic structure of YO, we analysed the dominant configurations (configuration state functions) in the MRCI wave functions for the lowest electronic states, Table 1, and the leading atomic orbital contributions in the respective molecular orbitals, Table 2.
| State | Configurationsa | Weights, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10σ | 11σ | 12σ | 13σ | 5π+ | 5π− | 6π+ | 6π− | 2δ+ | 2δ− | 1.79 Å | 2.04 Å | 2.19 Å | |
| a The orbital names π+, π−, δ+, and δ− indicate π(b1), π(b2), δ(a1), and δ(a2) orbitals, respectively. The MO occupancies represented by 2, 0 and + or − denote double, zero, and single occupancies with the total spin raised or lowered by 1/2. | |||||||||||||
| X2Σ+ | 2 | 2 | + | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 79.2 | 71.4 | 63.3 |
| 2 | + | + | − | 2 | 2 | 0 | 0 | 0 | 0 | 2.1 | 3.1 | 3.5 | |
| 2 | + | − | + | 2 | 2 | 0 | 0 | 0 | 0 | 2.3 | 2.8 | ||
| 2 | 2 | + | 0 | 2 | + | 0 | − | 0 | 0 | 3.6 | |||
| 2 | 2 | + | 0 | + | 2 | − | 0 | 0 | 0 | 3.6 | |||
| 2 | 2 | + | 0 | − | 2 | + | 0 | 0 | 0 | 2.2 | |||
| 2 | 2 | + | 0 | 2 | − | 0 | + | 0 | 0 | 2.2 | |||
| A′2Δ (a1) | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | + | 0 | 78.9 | 67.3 | 53.1 |
| 2 | + | − | 0 | 2 | 2 | 0 | 0 | + | 0 | 8.1 | 21.6 | ||
| 2 | + | 0 | − | 2 | 2 | 0 | 0 | + | 0 | 4.8 | 4.2 | 4.1 | |
| A2Π (b1) | 2 | 2 | 0 | 0 | 2 | 2 | + | 0 | 0 | 0 | 79.9 | ||
| 2 | + | 0 | − | 2 | 2 | + | 0 | 0 | 0 | 3.2 | |||
| 2 | 2 | 2 | 0 | + | 2 | 0 | 0 | 0 | 0 | 82.6 | 82.3 | ||
| 2 | 2 | 0 | 0 | + | 2 | 2 | 0 | 0 | 0 | 2.4 | 2.1 | ||
| 2 | 2 | 0 | 0 | + | 2 | 0 | 2 | 0 | 0 | 2.1 | |||
| B2Σ+ | 2 | 2 | 0 | + | 2 | 2 | 0 | 0 | 0 | 0 | 73.5 | ||
| 2 | + | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 4.5 | 80.2 | 78.9 | |
| 2 | + | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2.1 | |||
| 2 | + | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 2.3 | 2.0 | ||
| 2 | + | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 2.3 | 2.0 | ||
| C2Π (b1) | 2 | 2 | 2 | 0 | + | 2 | 0 | 0 | 0 | 0 | 83.2 | ||
| 2 | 2 | 0 | 0 | + | 2 | 2 | 0 | 0 | 0 | 2.8 | |||
| 2 | 2 | 0 | 0 | + | 2 | 0 | 2 | 0 | 0 | 2.3 | |||
| 2 | 2 | 0 | 0 | 2 | 2 | + | 0 | 0 | 0 | 68.7 | |||
| 2 | + | − | 0 | 2 | 2 | + | 0 | 0 | 0 | 4.3 | |||
| 2 | + | 0 | − | 2 | 2 | + | 0 | 0 | 0 | 3.3 | |||
| D2Σ+ | 2 | + | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 79.1 | ||
| 2 | 2 | 0 | + | 2 | 2 | 0 | 0 | 0 | 0 | 4.0 | |||
| 2 | + | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 2.6 | |||
| 2 | + | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 2.6 | |||
| MO | 1.79 Å | 2.04 Å | 2.19 Å |
|---|---|---|---|
| a See footnote to Table 1 for designations. | |||
| 10σ | 52% 2s O + 43% 4pσ Y | 71% 2s O + 27% 4pσ Y | 84% 2s O + 15% 4pσ Y |
| 11σ | 63% 2pσ O + 10% 4dσ Y | 72% 2pσ O | 77% 2pσ O |
| 12σ | 86% 5s Y | 82% 5s Y + 12% 5pσ Y | 81% 5s Y + 13% 5pσ Y |
| 13σ | 50% 5pσ Y + 36% 4dσ Y | 39% 5pσ Y + 53% 4dσ Y | 34% 5pσ Y + 60% 4dσ Y |
| 5π+(−) | 94% 2pπ O | 95% 2pπ O | 96% 2pπ O |
| 6π+(−) | 67% 5pπ Y + 32% 4dπ Y | 46% 5pπ Y + 52% 4dπ Y | 34% 5pπ Y + 63% 4dπ Y |
| 2δ+(−) | 100% 4dδ Y | 100% 4dδ Y | 99% 4dδ Y |
The analysis indicates that the X2Σ+ ground state consists mainly of …10σ211σ25π412σ1 electron configuration. The principal contribution to the singly occupied 12σ MO comes from the yttrium 5s atomic orbital, with an admixture of the 5p AO particularly noticeable at longer internuclear distances. The three lowest active MOs, 10σ, 11σ, and 5π+(−), primarily consist of the oxygen 2s and 2p orbitals whose contributions increase with the bond stretching. Therefore, the bonding in the X2Σ+ YO ground state can be roughly described as ionic, Y2+O2−, however, with significant covalent character mainly owing to an appreciable participation of the yttrium 4pσ AO in the 10σ MO. Upon the Y–O bond stretching, there is a rapid decrease in the 4p contribution (see Table 2) reducing the covalency and resulting in a concomitant increase in the magnitude of the electric dipole moment in the YO ground state (see below).
The first excited state, A′2Δ, mainly consists of …10σ211σ25π42δ1 configuration with the 2δ MO clearly assigned to the Y 4dδ orbital. At shorter bond lengths this state can be reasonably described by the single electron excitation from the ground state, 5s1 → 4dδ1. Upon bond elongation, the weight of the main configuration gradually decreases, approaching ∼50% at bond lengths of about 2.2 Å, whereas at r > ∼2 Å, a few other large-weight configurations emerge, e.g., the three-open-shell …10σ211σα12σβ5π42δα configuration (with a weight of 22% at 2.19 Å) that corresponds to the Y+O− bonding (Y 5s14d1, O 2p5).
At bond lengths up to ∼1.85 Å, the principal configurations of the A2Π and B2Σ+ excited states, …10σ211σ25π46π1 and …10σ211σ25π413σ1, respectively, include the 6π and 13σ singly occupied MOs mainly composed of the yttrium 5pπ and 5pσ atomic orbitals, respectively, yet with a significant admixture of the 4d AOs. Therefore, these states can be viewed as arising from 5s → 5pπ and 5s → 5pσ atomic electron promotions. In the same range of bond length values, the C2Π and D2Σ+ upper lying states consist mainly of …10σ211σ212σ25π3 and …10σ211σ112σ25π4 electron configurations, respectively. The YO bonding in the C2Π and D2Σ+ states is hence well described by the Y+O− scheme consistent with the Y 5s2, O 2p5 electron configuration.
As the Y–O distance approaches the avoided crossing point from below, the principal configurations of the A2Π and B2Σ+ states change to those specified above for the C2Π and D2Σ+ states, respectively, while, vice versa, the principal configurations of the C2Π and D2Σ+ states turn into those being the main ones for the A2Π and B2Σ+ states at shorter bond lengths. This alteration of main configurations describes an oxygen-to-metal charge back-transfer, Y2+O2− → Y+O−, in the A2Π and B2Σ+ states of YO upon the Y–O bond stretching through the avoided crossing region of bond lengths.
Specifically, the avoided crossing point, rac, chosen to be the point of closest approach of two curves, for the A2Π and C2Π states amounts to 2.046 Å (XMS-CASPT2) and 1.994 Å (MRCI), with the energy gap, ΔEac, of 366 cm−1 and 243 cm−1, respectively. For the XMS-CASPT2 B2Σ+ and D2Σ+ curves, rac = 2.064 Å and ΔEac = 1484 cm−1. Notably, the principal configuration interchange between the B2Σ+ and D2Σ+ states occurs at a slightly shorter internuclear distance: ∼1.95 Å (XMS-CASPT2), ∼1.92 Å (MRCI). At the EOM-IP-CCSD level, the avoided crossing characteristics are: rac = 1.911 Å, ΔEac = 231 cm−1 for A2Π and C2Π curves, and rac = 1.832 Å, ΔEac = 272 cm−1 for B2Σ+ and D2Σ+ curves.
The CCSD(T) calculations were carried out for the six lowest doublet states. The reference configurations for each state were as follows:
| X2Σ+ …10σ211σ25π412σ1 |
| A′2Δ …10σ211σ25π42δ1 |
| A2Π …10σ211σ25π46π1 |
| B2Σ+ …10σ211σ25π413σ1 |
| C2Π …10σ211σ212σ25π3 |
| D2Σ+ …10σ211σ112σ25π4 |
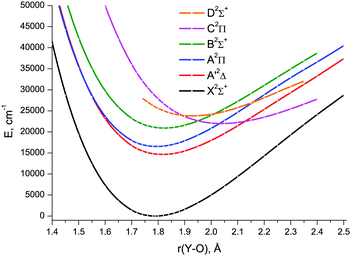
The CCSD(T) energies were obtained in the ranges: 1.0 ≤ r ≤ 2.5 Å for X2Σ+, A′2Δ and A2Π; 1.0 ≤ r ≤ 2.4 Å for B2Σ+; 1.4 ≤ r ≤ 2.4 Å for C2Π; 1.74 ≤ r ≤ 2.35 Å for D2Σ+. At longer distances (as well as shorter ones for C2Π and D2Σ+), the coupled-cluster calculations failed due to severe CCSD convergence problems. For the X2Σ+, A′2Δ, A2Π, and B2Σ+ states, the distances shorter than 1.0 Å were not considered because relative energies of excited states already exceed 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 at this point. The respective CCSD(T)/CBS1 PECs are shown in Fig. 5. Since single reference methods are not suitable for describing avoided crossings, e.g., those between the PECs of the A2Π and C2Π, and B2Σ+ and D2Σ+ states, the CCSD(T) calculated PECs can be viewed as corresponding to the diabatic presentation of these states. It is clearly seen that the CCSD(T) A2Π and C2Π curves cross each other at 2.031 Å, as do the B2Σ+ and D2Σ+ ones at 2.013 Å.
000 cm−1 at this point. The respective CCSD(T)/CBS1 PECs are shown in Fig. 5. Since single reference methods are not suitable for describing avoided crossings, e.g., those between the PECs of the A2Π and C2Π, and B2Σ+ and D2Σ+ states, the CCSD(T) calculated PECs can be viewed as corresponding to the diabatic presentation of these states. It is clearly seen that the CCSD(T) A2Π and C2Π curves cross each other at 2.031 Å, as do the B2Σ+ and D2Σ+ ones at 2.013 Å.
| X2Σ+ | A′2Δ | A2Π | B2Σ+ | C2Π | D2Σ+ | ||
|---|---|---|---|---|---|---|---|
| a EOM-IP for C2Π and D2Σ+, EOM-EA for the remaining states. b Calculated for each electronic state at the respective CCSD(T)/CBS1 equilibrium bond length. c Calculated at a bond length of 1.7932 Å. | |||||||
| T e | XMS-CASPT2/awCVTZ | 0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183 183 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 521 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 139 |
| MRCI/aVTZ | 0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 287 287 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 029 029 |
|||
| EOM-CCSD/TZDa | 0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 096 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 817 817 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 896 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 995 |
|
| MCPF53 | 0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 288 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
||||
| MRCI53 | 0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 853 853 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655 655 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039 039 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
||
| CASPT237 | 0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
|
| r e | XMS-CASPT2/awCVTZ | 1.822 | 1.851 | 1.824 | 1.858 | 2.107 | 1.991 |
| MRCI/aVTZ | 1.830 | 1.864 | 1.833 | 2.149 | |||
| EOM-CCSD/TZDa | 1.787 | 1.811 | 1.789 | 1.818 | 2.049 | 1.934 | |
| MCPF53 | 1.811 | 1.842 | 1.813 | ||||
| MRCI53 | 1.814 | 1.838 | 1.817 | 1.842 | 2.073 | ||
| CASPT237 | 1.79 | 1.82 | 1.77 | 1.84 | 1.97 | 1.91 | |
| ω e | XMS-CASPT2/awCVTZ | 796 | 726 | 763 | 696 | 592 | 696 |
| MRCI/aVTZ | 777 | 693 | 750 | 542 | |||
| EOM-CCSD/TZDa | 881 | 822 | 847 | 804 | 606 | 648 | |
| MCPF53 | 855 | 785 | 832 | ||||
| MRCI53 | 866 | 801 | 834 | 789 | 638 | ||
| μ e | MRCI/aVTZb | 4.410 | 7.871 | 4.343 | 2.971 | 1.329 | |
| EOM-EA-CCSD/TZDc | 4.905 | 7.867 | 4.147 | 2.164 | |||
| MCPF53 | 3.976 | 7.493 | 3.244 | ||||
The results of our EOM-CCSD calculations indicate that the anionic reference is less suitable for describing YO than the cationic one. In Table 3, more accurate cationic-reference EOM-EA-CCSD spectroscopic constants are listed for all states except for the C2Π and D2Σ+ ones which are not accessible via the electron attachment procedure and therefore were described at the EOM-CCSD level only via EOM-IP.
The results given in Table 3 are obviously inferior to those obtained from high-level CCSD(T) calculations including core–valence correlation and extrapolation to the CBS limit. The CCSD(T) results are collected in Table 4 together with the experimental data available to date. The spread in the CCSD(T)/CBS results from different CBS extrapolation schemes serve as a rough estimate of the uncertainty in extrapolation. The good agreement between the CBS estimates and experimentally determined spectroscopic properties of the X2Σ+, A′2Δ, A2Π, and B2Σ+ electronic states demonstrates the high accuracy in the CCSD(T)/CBS PECs of these states for bond lengths in the vicinity of the PECs minima, and is indicative of the mild MR character of the respective electronic wave functions.
| X2Σ+ | A′2Δ | A2Π | B2Σ+ | C2Π | D2Σ+ | a4Π | ||
|---|---|---|---|---|---|---|---|---|
| D 0, Te | awCVTZ | 7.060 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 924 |
|||||
| awCVQZ | 7.207 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809 809 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555 555 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893 893 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 423 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296 |
|
| awCV5Z | 7.260 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712 712 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 898 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 528 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 |
|
| CBS1 | 7.298 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 633 633 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901 901 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 745 745 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
|
| CBS2 | 7.298 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 629 629 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 917 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741 741 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 592 592 |
|
| CBS3 | 7.289 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 564 |
||||||
| Expt | 7.290(87)52 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70138 70138 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53035 53035 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79335 79335 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97237 97237 |
|||
| r e | awCVTZ | 1.7978 | 2.0902 | |||||
| awCVQZ | 1.7927 | 1.8201 | 1.7971 | 1.8268 | 2.0408 | 1.9345 | 2.0841 | |
| awCV5Z | 1.7905 | 1.8177 | 1.7950 | 1.8244 | 2.0384 | 1.9323 | 2.0817 | |
| CBS1 | 1.7887 | 1.8157 | 1.7932 | 1.8225 | 2.0365 | 1.9306 | 2.0797 | |
| CBS2 | 1.7890 | 1.8160 | 1.7935 | 1.8228 | 2.0368 | 1.9308 | 2.0799 | |
| CBS3 | 1.7892 | 2.0802 | ||||||
| Expt | 1.788211 | 1.81739 | 1.793113 | 1.825235 | ||||
| ω e | awCVTZ | 855.2 | 546.0 | |||||
| awCVQZ | 861.4 | 794.0 | 822.5 | 780.6 | 601.8 | 661.2 | 550.6 | |
| awCV5Z | 864.2 | 797.1 | 825.1 | 783.2 | 603.3 | 662.3 | 552.1 | |
| CBS1 | 866.5 | 799.7 | 827.1 | 785.3 | 604.6 | 663.1 | 553.3 | |
| CBS2 | 866.2 | 799.4 | 826.8 | 785.1 | 604.6 | 663.1 | 553.3 | |
| CBS3 | 865.8 | 552.9 | ||||||
| Expt | 861.511 | 79449 | 82035 | 76521 | ||||
| 75935 | ||||||||
| ω e x e | awCVTZ | 2.79 | 2.52 | |||||
| awCVQZ | 2.78 | 3.06 | 3.17 | 2.94 | 2.58 | 2.60 | 2.53 | |
| awCV5Z | 2.79 | 3.05 | 3.18 | 2.98 | 2.57 | 2.60 | 2.57 | |
| CBS1 | 2.79 | 3.04 | 3.19 | 3.01 | 2.57 | 2.61 | 2.60 | |
| CBS2 | 2.79 | 3.03 | 3.18 | 3.01 | 2.57 | 2.61 | 2.60 | |
| CBS3 | 2.79 | 2.59 | ||||||
| Expt | 2.8411 | 3.2338 | 3.1511 | 3.9735 | ||||
| 3.3513 | ||||||||
| α e × 103 | awCVTZ | 1.70 | 1.83 | |||||
| awCVQZ | 1.68 | 1.85 | 1.90 | 1.86 | 1.79 | 1.85 | 1.83 | |
| awCV5Z | 1.68 | 1.85 | 1.91 | 1.87 | 1.80 | 1.86 | 1.83 | |
| CBS1 | 1.68 | 1.85 | 1.91 | 1.87 | 1.80 | 1.87 | 1.83 | |
| CBS2 | 1.68 | 1.84 | 1.91 | 1.87 | 1.80 | 1.87 | 1.83 | |
| CBS3 | 1.68 | 1.83 | ||||||
| Expt | 1.7311 | 1.738 | 2.0113 | 2.4935 | ||||
| μ e | awCVTZ | 4.615 | 7.595 | 3.711 | 1.749 | 2.059 | 1.256 | 3.605 |
| awCVQZ | 4.614 | 7.620 | 3.724 | 1.764 | 2.082 | 1.275 | 3.615 | |
| awCV5Z | 4.611 | 7.626 | 3.728 | 1.770 | 2.090 | 1.281 | 3.618 | |
| CBS1 | 4.609 | 7.630 | 3.730 | 1.775 | 2.097 | 1.287 | 3.621 | |
| CBS2 | 4.609 | 7.630 | 3.731 | 1.777 | 2.097 | 1.287 | 3.621 | |
| CBS3 | 4.609 | 7.629 | 3.729 | 1.774 | 2.095 | 1.285 | 3.620 | |
| Expt | 4.45(7)27 | 3.68(2)27 | ||||||
| 4.524(7)45 | ||||||||
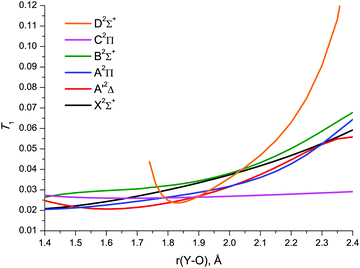
An insight into the reliability of the CCSD(T) PECs over the entire bond length range explored, and for all electronic states considered, including those not yet characterised experimentally, can be provided by using the MR diagnostic criteria commonly employed to identify the suitability of single reference wavefunction-based methods: T1,95 the Frobenius norm of the coupled cluster amplitude vector related to single excitations, and D1,96 the matrix norm of the coupled cluster amplitude vector arising from coupled cluster calculations. The utility of different MR diagnostics has been examined in earlier studies97,98 on various 3d and 4d TM species. The following criteria have been proposed98 as a gauge for the latter to predict the possible need to employ multireference wavefunction-based methods while describing energetic and spectroscopic molecular properties: T1 ≥ 0.045, D1 ≥ 0.120, %TAE[(T)] ≥ 10%. The symbol %TAE[(T)] denotes here the percent total atomization energy corresponding to a relationship between energies determined with CCSD and CCSD(T) calculations.99,100 Obviously, the %TAE[(T)] diagnostic is applicable for judging the SR/MR character of the electronic ground state only. For the YO molecule, the CCSD/awCV5Z calculated %TAE[(T)] of 5.6% is well below the proposed MR threshold. This fact provides further evidence for single reference character of the X2Σ+ wavefunction in the near-equilibrium region of Y–O bond lengths.
The CCSD/awCV5Z T1 plots vs. Y–O bond length are shown in Fig. 6. The similar D1 plots are illustrated in Fig. S1 of the ESI.† At shorter bond lengths, the diagnostics amount to 0.02–0.03 (T1) and 0.05–0.12 (D1), remaining below the MR thresholds down to 1.4 Å for most states. Upon bond stretch, T1 and D1 rapidly increase, typically exceeding the MR threshold at 2.1–2.2 Å. The behaviour of these diagnostics for the C2Π state is a notable exception: the numerical values of both T1 and D1 remain well below the MR threshold throughout the bond length range studied. The D2Σ+ state is also noteworthy: its T1 and D1 diagnostics are indicative of the CCSD D2Σ+ wave function retaining its SR character in much narrower range of bond lengths compared to the other doublet states under study.
The relative importance of SR/MR character of YO can also be guessed with the use of spin contamination appearing in RHF-UCCSD calculations as a result of unrestricted spin at the CCSD level. According to Jiang et al.,97 spin contamination with 〈S2 − Sz2 − Sz〉 greater than 0.1 in an RHF-UCCSD wave function can be viewed as a strong indication of nondynamical correlation in an open-shell system. Plotting spin contamination vs. bond length, Fig. 7, clearly indicates the severe admixture of higher spin states in the CCSD A′2Δ, A2Π, B2Σ+ and D2Σ+ wave functions at bond lengths beyond 2.2–2.3 Å. Greater extent of spin contamination at longer internuclear distances can obviously be associated with larger values of T1 and D1 (Fig. 6 and Fig. S1 of the ESI†) exceeding the established MR thresholds.
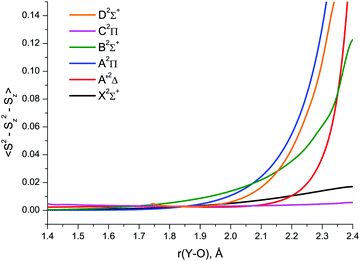 | ||
| Fig. 7 RHF-UCCSD/awCV5Z wave function spin contamination in the low-lying doublet electronic states of YO. | ||
It is instructive to compare the MR diagnostics discussed above with the weights of the principal configurations, C02, in the MRCI wavefunctions of YO (see Table 1). At shorter bond lengths, the C02 values amount to ∼0.73 for the B2Σ+ state and 0.79–0.83 for the remaining doublet electronic states under study. These values are smaller than the threshold, C02 ≥ 0.90, proposed in ref. 97 and 98 to recognise the wave function strongly dominated by a single configuration. It should, however, be noted that this criterion was established by analyzing the CASSCF wavefunctions, whereas the C02 of the entire MRCI wavefunction differs from that of the CASSCF reference function due to the contributions of external configurations, which make C02 a smaller number.
Upon the YO bond stretch, there is a gradual decrease in the weights of the configurations serving as a reference for the coupled cluster treatment of the X2Σ+, A′2Δ, and A2Π states. This indicates greater multireference character of the respective wave functions at longer bond distances, as do the CC-based MR diagnostics. The reference configuration for the C2Π state has approximately the same weight, C02 ≅ 0.83, in the MRCI wavefunctions of YO throughout the bond length range explored, behaving just like the respective CC-based MR diagnostics. These examples of the CCSD-MRCI correlations imply that the CC-based MR diagnostics can be capable of providing qualitative data about the relative accuracy in the single-reference coupled cluster calculation results not only for near-equilibrium regions of electronic ground states, but also for excited states in a wider range of molecular geometries.
In general, the present analysis indicates essentially single reference character of the YO low-lying doublet states over most part of bond length range explored in our work and hence high accuracy in the respective domains of the CCSD(T) PECs. It may also be indicative of accuracy degradation at larger bond lengths, implying the need for additional adjustments of the CCSD(T) PECs. Nevertheless, the bond length range associated with high-energy sections of PECs is expected to have a limited impact on the simulated spectra.
| 4Π | 4Φ | 4Σ+ | 4Δ | 4Σ− | ||
|---|---|---|---|---|---|---|
| a The energies of the 4Φ, 4Σ+, 4Δ and 4Σ− states are given here with respect to the 4Π state which is the lowest-lying quartet state of YO. b In ref. 53, the symmetry of the lowest quartet state of YO was reported to be 4Φ rather than 4Π. c In ref. 53, the adiabatic excitation energy of the lowest quartet state was obtained from the MCPF calculations, and the relative energies of various quartet states were determined at the CASSCF level. | ||||||
| T e | CASSCF | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 460 |
68 | 1062 | 1825 | 2542 |
| CASPT2 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
56 | 1432 | 2158 | 2785 | |
| CASPT3 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772 772 |
53 | 1450 | 2175 | 2788 | |
| MRCI | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 046 |
55 | 1394 | 2128 | 2748 | |
| MRCI + Q | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 274 |
59 | 1357 | 2089 | 2699 | |
| CASSCF53 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975b,c 975b,c |
52 | 1933 | 2664 | 3359 | |
| r e | CASSCF | 2.141 | 2.143 | 2.110 | 2.110 | 2.114 |
| CASPT2 | 2.197 | 2.198 | 2.192 | 2.191 | 2.196 | |
| CASPT3 | 2.210 | 2.211 | 2.207 | 2.208 | 2.214 | |
| MRCI | 2.213 | 2.214 | 2.209 | 2.209 | 2.214 | |
| MRCI + Q | 2.218 | 2.219 | 2.218 | 2.218 | 2.222 | |
| CASSCF53 | 2.121b | 2.122 | 2.108 | 2.109 | 2.114 | |
| MCPF53 | 2.126b | |||||
| ω e | CASSCF | 517 | 516 | 522 | 521 | 519 |
| CASPT2 | 515 | 515 | 517 | 494 | 477 | |
| CASPT3 | 519 | 518 | 524 | 494 | 474 | |
| MRCI | 507 | 507 | 501 | 495 | 492 | |
| MRCI + Q | 506 | 506 | 501 | 496 | 493 | |
| CASSCF53 | 543 | 543 | 524 | 522 | 520 | |
| MCPF53 | 526b | |||||
| State | Configurationsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10σ | 11σ | 12σ | 13σ | 5π+ | 5π− | 6π+ | 6π− | 2δ+ | 2δ− | |
| a See footnote to Table 1 for designations. | ||||||||||
| 4Π(b1) | 2 | 2 | + | 0 | + | 2 | 0 | 0 | + | 0 |
| 2 | 2 | + | 0 | 2 | + | 0 | 0 | 0 | + | |
| 4Φ(b1) | 2 | 2 | + | 0 | + | 2 | 0 | 0 | + | 0 |
| 2 | 2 | + | 0 | 2 | + | 0 | 0 | 0 | + | |
| 4Σ+ | 2 | 2 | + | 0 | + | 2 | + | 0 | 0 | 0 |
| 2 | 2 | + | 0 | 2 | + | 0 | + | 0 | 0 | |
| 4Δ(a1) | 2 | 2 | + | 0 | + | 2 | + | 0 | 0 | 0 |
| 2 | 2 | + | 0 | 2 | + | 0 | + | 0 | 0 | |
| 4Σ− | 2 | 2 | + | 0 | + | 2 | 0 | + | 0 | 0 |
| 2 | 2 | + | 0 | 2 | + | + | 0 | 0 | 0 | |
The results for the YO quartet states obtained in our work agree with those of Langhoff and Bauschlicher,53Table 5, except for the symmetry of the lowest quartet state that was reported53 to be 4Φ rather than 4Π.
The single-reference CCSD(T) method is expected to yield quite accurate results for the a4Π state of YO since its MR diagnostics, C02 = 0.90, T1 = 0.024, and D1 = 0.078, indicate essentially SR character of the a4Π wave function in the vicinity of the a4Π PEC minimum, 2.00–2.25 Å. The very large CCSD(T)/CBS excitation energy of the a4Π state, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1, suggests that the quartet states in YO are too high in energy to significantly affect the spectroscopy of its low-lying doublet states.
600 cm−1, suggests that the quartet states in YO are too high in energy to significantly affect the spectroscopy of its low-lying doublet states.
| r e | ΔSOre | ω e | ΔSOωe | T e | ΔSOTe | |
|---|---|---|---|---|---|---|
| X2Σ+1/2 | 1.7866 | 0.0000 | 880.9 | 0.0 | 0 | 0 |
| A′2Δ3/2 | 1.8109 | +0.0003 | 821.5 | −0.5 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 937 |
−159 |
| A′2Δ5/2 | 1.8102 | −0.0004 | 822.6 | +0.6 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
+157 |
| A2Π1/2 | 1.7892 | +0.0005 | 846.9 | −0.5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591 591 |
−226 |
| A2Π3/2 | 1.7884 | −0.0003 | 847.8 | +0.4 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028 028 |
+212 |
| B2Σ+1/2 | 1.8181 | −0.0003 | 804.5 | +0.6 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
+17 |
| C2Π3/2 | 2.0488 | −0.0004 | 605.7 | +0.1 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 808 |
−88 |
| C2Π1/2 | 2.0495 | +0.0003 | 605.6 | −0.0 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 994 |
+98 |
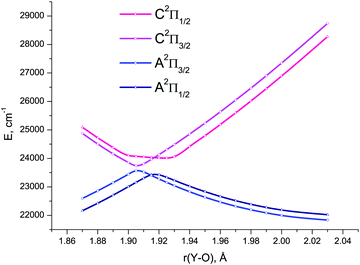 | ||
| Fig. 8 4c-EOM-IP-CCSD/TZD potential energy curves for the spin-coupled components of the A2Π and C2Π electronic states of YO in the avoided crossing region of bond length values. | ||
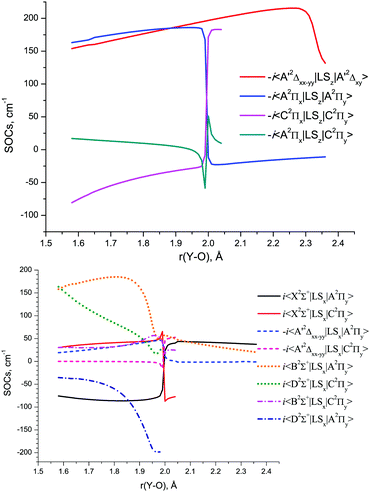
The SOC matrix elements between various doublet states of YO, which also accurately account for the corresponding phases, are shown in Fig. 9 as a function of r(Y–O). The relative phases of the couplings are important when used for solving the nuclear motion problem as part of the coupled Schrödinger equation, see, for example, discussion by Patrascu et al.92 The full details of the ab initio coupling curves including the magnetic quantum numbers are provided as part of the ESI.†
To shed more light on the alleged different order of the dipole moment values for the spin–orbit components of the A2Π state in YO compared to ScO and LaO, we performed additional 4c-EOM-EA-CCSD/TZD computations for the two latter molecules at the experimental equilibrium bond lengths104,105 of 1.6826 Å (ScO) and 1.8400 Å (LaO). These resulted in the following values: μ(A2Π1/2) = 4.543 D, μ(A2Π3/2) = 4.532 D (ScO), μ(A2Π1/2) = 3.011 D, μ(A2Π3/2) = 2.907 D (LaO), i.e., the ab initio predicted difference between the two spin–orbit components monotonically increases on passing in the series ScO → YO → LaO: 0.01 → 0.04 → 0.10 D, respectively. The experimental counterparts101,103 are: μ(A2Π1/2) = 4.43(2) D, μ(A2Π3/2) = 4.06(3) D (ScO), μ(A2Π1/2) = 2.44(2) D, μ(A2Π3/2) = 1.88(6) D (LaO). Since the 4c-EOM-EA-CCSD dipole moments are expected to be overestimated by at least 0.5 D, one can consider the theoretical results to be in reasonable agreement with experiment. In light of our results, the experimental dipole moments27 for the two Ω components of the A2Π state of YO need to be revisited.
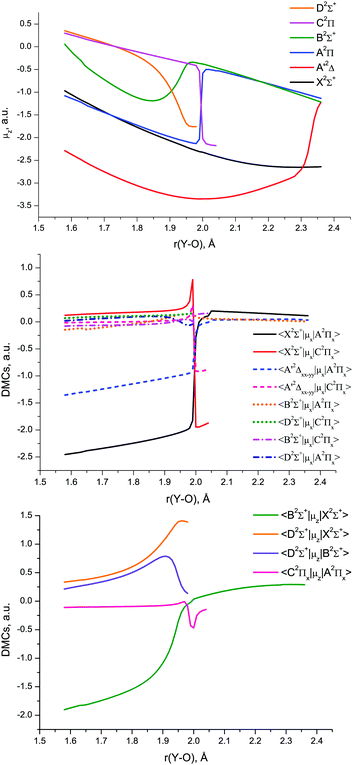
The MCPF dipole moments obtained by Langhoff and Bauschlicher,53 3.976 D (X2Σ+), 7.493 D (A′2Δ) and 3.244 D (A2Π), are systematically smaller than our CCSD(T) (Table 4), MRCI, and EOM-EA-CCSD (Table 3) results.
The MRCI DMCs and TDMCs of YO are shown in Fig. 10. The EAMCs are shown in Fig. S2 of the ESI.† All these curves as well as the SOC ones (Fig. 9) exhibit irregular behaviour at bond lengths around r ∼ 2 Å due to strong changes in the A2Π, B2Σ+, C2Π and D2Σ+ wave functions over the avoided crossing region.
3.2 Results of Duo calculations
For Duo calculations, we selected the following set of curves representing our highest level of theory: the CCSD(T)/CBS PECs shown in Fig. 5 and MRCI SOCs, (T)DMCs, and EAMCs shown in Fig. 9 and 10, as well as Fig. S2 of the ESI.† Due to limitations of single reference CCSD(T), the CCSD(T) curves for the A2Π, C2Π and B2Σ+, D2Σ+ states do not exhibit avoided crossings and hence are not consistent with the MRCI property curves. To alleviate this deficiency, these four PECs were transformed by simply switching the corresponding energy points between A and C (2Π states) as well as those between B and D (2Σ+ states) at r > rac. We have decided to apply this rather simplistic procedure because it has marginal effect on the overall accuracy of our model and is sufficient for the goal of this pure ab initio study, not aiming at spectroscopic accuracy. A proper diabatic representation of the YO electronic states will be, however, important when refining the ab initio curves by fitting to experiment,93 which is a goal of future work. In this study we work directly with the ab initio data in the grid representation without representing ab initio curves analytically. We do not perform any diabatizations here, which is often useful for representing the variation of the data with respect to the bond length in an intuitive and more compact form. One of the artifacts of this choice to use the ab initio curves directly is that the crossing points of the MRCI PECs hence the points of drastic change in the MRCI property curves differ by a few hundredths of Å from the crossing points of the CCSD(T)/CBS PECs. Again, this has small impact on the overall agreement of the current model with the experiment. However, a more accurate study will require a more consistent treatment of the crossing points. Our preferred choice would be to use the CCSD(T)/CBS values of the corresponding crossing points.The Duo rovibronic wavefunctions of YO in conjunction with the ab initio TDMCs were then used to produce Einstein A coefficients for all rovibronic transitions between states considered in this work covering the wavenumber range from 0 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and J ≤ 180.5. These Einstein A coefficients and the corresponding energies from the lower and upper states involved in each transition were organised as a line list using the ExoMol format.106 This format uses a two file structure with the energies included into the States file (.states) and Einstein coefficients appearing in the Transitions file (.trans). This ab initio line list is available from http://www.exomol.com. The ExoMol format has the advantage of being compact and compatible with our intensity simulation program ExoCross107 (see below).
000 cm−1 and J ≤ 180.5. These Einstein A coefficients and the corresponding energies from the lower and upper states involved in each transition were organised as a line list using the ExoMol format.106 This format uses a two file structure with the energies included into the States file (.states) and Einstein coefficients appearing in the Transitions file (.trans). This ab initio line list is available from http://www.exomol.com. The ExoMol format has the advantage of being compact and compatible with our intensity simulation program ExoCross107 (see below).
| υ | J | Ω | Duo | Obs. | ||
|---|---|---|---|---|---|---|
| a Band centres. | ||||||
| ref. 109 | ref. 13 | |||||
| Xa | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 860.879 | 855.2 | 855.7463(52) | ||
| 2 | 0 | 1716.156 | 1704.4 | 1705.8339(90) | ||
| 3 | 0 | 2565.836 | 2547.9 | 2550.2684(65) | ||
| 4 | 0 | 3409.931 | 3385.4 | 3389.0242(90) | ||
| 5 | 0 | 4248.450 | 4217.1 | 4222.085(11) | ||
| 6 | 0 | 5081.364 | 5049.454(13) | |||
| ref. 13 | ||||||
| A | 0 | 0.5 | 0.5 | 16295.492 | 16295.453 | |
| 0 | 1.5 | 1.5 | 16724.499 | 16724.541 | ||
| 1 | 1.5 | 1.5 | 17117.400 | 17109.845 | ||
| 1 | 1.5 | 1.5 | 17545.141 | 17538.459 | ||
| 2 | 1.5 | 1.5 | 17931.536 | 17916.880 | ||
| 2 | 1.5 | 1.5 | 18358.848 | 18345.768 | ||
| 3 | 1.5 | 1.5 | 18740.505 | 18716.674 | ||
| 3 | 1.5 | 1.5 | 19169.245 | 19146.593 | ||
| 4 | 1.5 | 1.5 | 19547.495 | 19510.064 | ||
| 4 | 1.5 | 1.5 | 19964.360 | 19940.488 | ||
| 5 | 1.5 | 1.5 | 20350.928 | 20296.636 | ||
| 5 | 1.5 | 1.5 | 20732.561 | 20727.595 | ||
| ref. 39 | ||||||
| A′ | 0 | 2.5 | 1.5 | 14500.074 | 14502.010 | |
| ref. 21 | ref. 35 | |||||
| B | 0 | 0.5 | 0.5 | 20741.630 | 20741.688 | 20741.6877 |
| 1 | 0.5 | 0.5 | 21516.945 | 21492.4773 | ||
| 2 | 0.5 | 0.5 | 22294.044 | |||
| 3 | 0.5 | 0.5 | 23102.252 | 22941.71 | ||
| 4 | 0.5 | 0.5 | 23850.742 | 23615.3 | ||
| ref. 37 | ||||||
| D | 0 | 0.5 | 0.5 | 23969.916 | 23969.940 | |
| 1 | 0.5 | 0.5 | 24659.745 | 24723.766 | ||
To allow for a direct comparison with the observed spectra of 89Y16O, we generated a line list covering rotational excitations up to J = 190 and the energy/wavenumber range up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, with a lower state energy cutoff of 16
000 cm−1, with a lower state energy cutoff of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.
000 cm−1.
3.3 Partition function
The partition function of 89Y16O computed using our ab initio line list is shown in Fig. 11, which is compared to that recently reported by Barklem and Collet.110 Since 89Y has a nuclear spin degeneracy of two, we have multiplied Barklem and Collet's partition function by a factor of two to compensate for the different conventions used; we follow HITRAN111 and include the full nuclear spin in our partition functions. The partition function of YO was also reported by Vardya,112 which is shown in Fig. 11. All three partition functions are almost identical for their ranges of validity.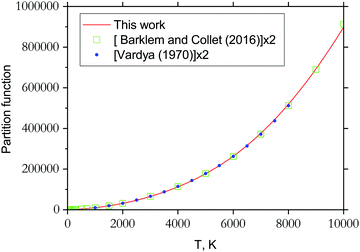 | ||
| Fig. 11 Partition functions of YO: solid line is from this work computed using the energies of the six lowest electronic states; filled circles represent the partition function values by Vardya112 generated using spectroscopic constants of 3 lowest electronic states X, A and B (multiplied by a factor of 2 to account for the nuclear statistics); open squares represent values by Barklem and Collet110 (times the factor 2). | ||
3.4 Spectral comparisons
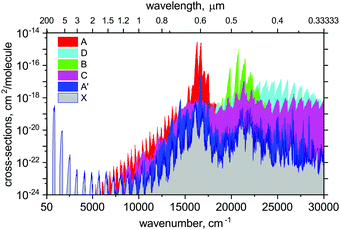
Using the ab initio89Y16O line list, spectral simulations were performed with our code ExoCross.107 ExoCross is an open source Fortran 2003 code with the primary use to produce spectra of molecules at different temperatures and pressures in the form of cross sections using molecular line lists as input. Here we use the YO line list generated with Duo in the ExoMol format, the description of which can be found, e.g., in Yurchenko et al.107 or Tennyson et al.106 ExoCross can be accessed viahttp://exomol.com/software/ or directly at https://github.com/exomol. Amongst other features, ExoCross can generate spectra for non-local thermal equilibrium conditions characterised with different vibrational and rotational temperatures, lifetimes, Landé g-factors, partition and cooling functions.An overview of the YO absorption spectra in the form of cross sections at the temperature T = 2000 K is illustrated in Fig. 12. Here, a Gaussian line profile with a half-width-at-half-maximum (HWHM) of 5 cm−1 was used. This figure shows contributions from each electronic band originating from the ground electronic state. The strongest bands are A2Π–X2Σ+ and B2Σ+–X2Σ+. The visible A–X band is known to be important for the spectroscopy of cool stars. The C state is of the same symmetry as A, however, the corresponding band C–X is much weaker due to the small Franck–Condon effects. The A′2Δ–X2Σ+ band is forbidden and barely seen in Fig. 12, however, it is strong enough to be experimentally known.39
Fig. 13 shows a simulated emission spectrum of the strongest orange system YO (A2Π–X2Σ+, (0,0)), which is compared to the experiment of Badie and Granier31 (from the plume emission close to the liquid Y2O3 surface). It is remarkable that even pure ab initio calculations (after modest adjustment of the corresponding Te value by +9.509 cm−1) provide very close reproduction of experiment. It shows that our line list at the current, ab initio quality should be useful for modelling spectroscopy of exoplanets and cool stars in the visible region.
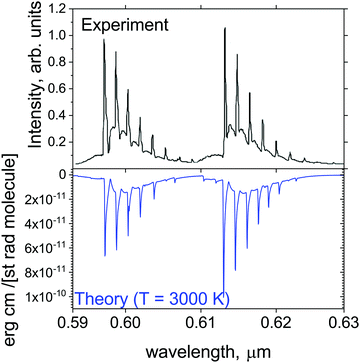 | ||
| Fig. 13 Comparison of the computed A2Π–X2Σ+ orange band with the observations of Badie and Granier.31 Our simulations assume T = 3000 K and Gaussian line profile of HWHM = 1 cm−1. | ||
Fig. 14 illustrates the A′2Δ–X2Σ+ (0,0) forbidden band in emission simulated for T = 77 K compared to the experimental spectrum of Simard et al.39 Here, a shift of +81.096 cm−1 was applied to the Te value of the A′2Δ state. In spectral simulations, this region appears to be contaminated by the dipole-allowed hot A–X transitions, which are not necessarily very accurate in this region. We therefore applied a filter to select the A′2Δ–X2Σ+ transitions only. The difference in shape of the spectra can be attributed either to the non-LTE (Local Thermal Equilibrium) effects present in the experiment or broadening effects, which we have not attempted to model properly. This figure is only to illustrate the generally good agreement of the positions of the rovibronic lines in this band.
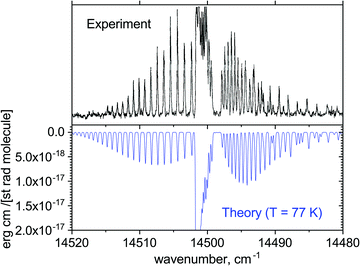 | ||
| Fig. 14 Comparison of the computed emission A′2Δ–X2Σ+ (0,0) band with the measurements of Simard et al.39 at T = 77 K and Gaussian line profile of HWHM = 0.1 cm−1. | ||
Fig. 15 shows a series of absorption bands compared to the measurements of Zhang et al.37 Zhang et al.37 who observed bands in both the B2Σ+–X2Σ+ and D2Σ+–X2Σ+ systems in a heavily non-thermal environment where the vibrations were hot and the rotations cooled to liquid nitrogen temperatures. In this case of multi-band system it was important to include at least some non-LTE effects by treating it using two temperatures, vibrational and rotational, assuming that the corresponding degrees of freedom are in LTE. The rotational temperature Trot = 77 K was set to value specified by Zhang et al.,37 while the vibrational temperature was adjusted to Tvib = 2000 K to better reproduce the experimental spectrum. The spectrum is divided into five spectroscopic windows (I–V) which are also detailed in Table 9. In order to match the positions of the vibronic bands in the experiment, some of the windows were shifted. For example, the D2Σ+–X2Σ+ (1,0) band was shifted by about 76.5 cm−1. This shift is an indication of the inaccuracy with which our model reproduces the vibrationally excited states of D2Σ+. This is not surprising considering the complexity of the quantum-chemistry part of these systems as well as of the nuclear motion part. The avoided crossing with the B2Σ+ state leads to very complex shapes of the D2Σ+ PEC and of the SO and electronic angular momentum coupling curves with the A and C states. The corresponding SOCs of the B and D states with the nearby state C are also relatively large, ∼30 cm−1 and 80 cm−1, respectively (see Fig. 9), and therefore important. Besides, the D PEC is rather shallow with the equilibrium in the vicinity of the avoided crossing point, which also complicates the solution. An accurate description of the B and D curves would require diabatic representations before attempting any empirical refinement by fitting to the experiment. In all cases our simulations, while not perfect, show striking agreement with the observed spectra.
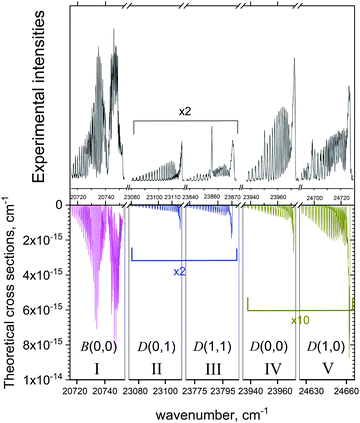 | ||
| Fig. 15 Comparison of our computed emission spectra to the measurements of Zhang et al.37 Our simulations assumed a cold rotational temperature of Trot = 77 K and a hot vibrational temperature of Tvib = 2000 K. The Gaussian line profile of HWHM = 0.1 cm−1 was used. | ||
| Experiment | Theory | Band | |
|---|---|---|---|
| I | 20714.5–20753.5 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715–20 715–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 754 |
B(0,0) |
| II | 23078.5–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073–23 073–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 112 |
D(0,1) |
| III | 23837.5–23874.5 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 769–23 769–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 806 |
D(1,1) |
| IV | 23934.5–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 973 |
23934.5–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 973 |
D(0,0) |
| V | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 689–24 689–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
24625.5–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 666 |
D(1,0) |
3.5 Lifetimes
The lifetimes of 89Y16O in the A2Π and B2Σ+ states (v ≤ 2) were measured by Liu and Parson12 using laser fluorescence detection of nascent product state distributions in the reactions of Y with O2, NO, and SO2. Some lifetimes were also measured by Zhang et al.37 and computed ab initio by Langhoff and Bauschlicher.53Table 10 presents a comparison of these results with our calculations with Duo,50 showing value of the states with corresponding lowest J and the positive parity. It can be seen that our A2Π state lifetimes appear to be shorter than the observed ones. This suggests that the A2Π–X2Σ+ transition dipoles may be slightly too large. Good agreement is obtained for the lifetimes of the B2Σ+ states, while the D2Σ+ state lifetimes are underestimated by a factor of 2 indicating that the corresponding transition dipole moments D–B and D–X, or at least one of them might be too large.4 Conclusion
In this work, a composite approach to accurate first-principles description of the spectroscopy of open-shell TM-containing diatomics is proposed and its high efficiency is demonstrated taking the example of the yttrium oxide molecule. The approach is based on the combined use of single reference coupled cluster and multireference methods of electronic structure theory, accompanied with a thorough joint analysis of the SR/MR character of the molecular wave function. A full set of potential energy, (transition) dipole moment, spin–orbit, and electronic angular momenta curves for the lowest 6 electronic states of YO was produced ab initio using a combination of the CCSD(T)/CBS and MRCI methods. These curves were then used to solve the fully coupled Schrödinger equation for the nuclear motion using the Duo program. Given the complexity of the system under study, the results show remarkably good agreement with the experiment. Our ultimate goal is to produce an accurate, empirical line list for 89Y16O for applications in modelling the spectroscopy of atmospheres of exoplanets and cool stars. This will require a refinement of the ab initio curves by fitting to the experimental data in the diabatic representation as well as inclusion of the non-adiabatic coupling effects and will be addressed in future work. The A2Π band of YO has strong absorption in the visible region, i.e. where the stellar radiation usually peaks. Such systems are known to cause the temperature inversion in atmospheres of exoplanets, similar to the inversion caused by TiO and VO in giant exoplanets.113 Opacities of such species are crucial in modelling the degree of temperature inversion in giant exoplanets. YO is yet to be detected in exoplanetary atmospheres and this work is meant to provide the necessary spectroscopic data.YO is one of the few molecules with the strong potential for laser-cooling techniques,18 which have widely ranging applications, from quantum information and chemistry to searches for new fundamental physics. The results of this work will help to model the cooling properties of YO and thus will be important for designing and implementing laser-cooling experiments.
The ab initio curves of YO obtained in this study are provided as part of the supplementary material to this paper along with our spectroscopic model in a form of a Duo input file. The computed line list can be obtained from http://www.exomol.com. This is given in the ExoMol format106 which also includes state-dependent lifetimes. The line list can be directly used with the ExoCross program to simulate the spectral properties of YO.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by STFC Projects No. ST/M001334/1 and ST/R000476/1. The authors acknowledge the use of the UCL Legion High Performance Computing Facility (Legion@UCL), and associated support services, in the completion of this work, along with the Cambridge COSMOS SMP system, part of the STFC DiRAC HPC Facility supported by BIS National E-infrastructure capital grant ST/P002307/1 and ST/R002452/1 and STFC operations grant ST/R00689X/1. DiRAC is part of the National e-Infrastructure. A. N. S. and V. G. S. acknowledge support from the Ministry of Science and Higher Education of the Russian Federation (Project No. 4.3232.2017/4.6).References
- P. F. Bernath, Int. Rev. Phys. Chem., 2009, 28, 681–709 Search PubMed.
- L. K. McKemmish, S. N. Yurchenko and J. Tennyson, Mol. Phys., 2016, 114, 3232–3248 CrossRef CAS.
- S. A. Cotton, Scandium, Yttrium & the Lanthanides: Inorganic & Coordination Chemistry, John Wiley & Sons, Ltd., 2006 Search PubMed.
- E. Anders and N. Grevesse, Geochim. Cosmochim. Acta, 1989, 53, 197–214 CrossRef CAS.
- P. S. Murty, Astrophys. Lett. Commun., 1982, 23, 7–9 CAS.
- P. S. Murty, Astrophys. Space Sci., 1983, 94, 295–305 CrossRef CAS.
- V. P. Goranskii and E. A. Barsukova, Astron. Rep., 2007, 51, 126–142 CrossRef CAS.
- T. Kaminski, M. Schmidt, R. Tylenda, M. Konacki and M. Gromadzki, Astrophys. J., Suppl. Ser., 2009, 182, 33–50 CrossRef CAS.
- J. M. Badie, L. Cassan and B. Granier, Eur. Phys. J.: Appl. Phys., 2005, 29, 111–114 CrossRef CAS.
- J. M. Badie, L. Cassan and B. Granier, Eur. Phys. J.: Appl. Phys., 2005, 32, 61–64 CrossRef CAS.
- J. M. Badie, L. Cassan and B. Granier, Eur. Phys. J.: Appl. Phys., 2007, 38, 177–181 CrossRef CAS.
- K. Liu and J. M. Parson, J. Chem. Phys., 1977, 67, 1814–1828 CrossRef CAS.
- A. Bernard and R. Gravina, Astrophys. J., Suppl. Ser., 1983, 52, 443–450 CrossRef CAS.
- M. T. Hummon, M. Yeo, B. K. Stuhl, A. L. Collopy, Y. Xia and J. Ye, Phys. Rev. Lett., 2013, 110, 143001 CrossRef PubMed.
- M. Yeo, M. T. Hummon, A. L. Collopy, B. Yan, B. Hemmerling, E. Chae, J. M. Doyle and J. Ye, Phys. Rev. Lett., 2015, 114, 223003 CrossRef PubMed.
- A. L. Collopy, M. T. Hummon, M. Yeo, B. Yan and J. Ye, New J. Phys., 2015, 17, 055008 CrossRef.
- G. Quéméner and J. L. Bohn, Phys. Rev. A: At., Mol., Opt. Phys., 2016, 93, 012704 CrossRef.
- A. L. Collopy, S. Ding, Y. Wu, I. A. Finneran, L. Anderegg, B. L. Augenbraun, J. M. Doyle and J. Ye, Phys. Rev. Lett., 2018, 121, 213201 CrossRef CAS PubMed.
- J. B. Shin and R. W. Nicholls, Spectrosc. Lett., 1977, 10, 923–935 CrossRef CAS.
- C. Linton, J. Mol. Spectrosc., 1978, 69, 351–364 CrossRef CAS.
- A. Bernard, R. Bacis and P. Luc, Astrophys. J., 1979, 227, 338–348 CrossRef CAS.
- K. Liu and J. M. Parson, J. Phys. Chem., 1979, 83, 970–973 CrossRef CAS.
- T. Wijchers, H. A. Dijkerman, P. J. T. Zeegers and C. T. J. Alkemade, Spectrochim. Acta, Part B, 1980, 35, 271–279 CrossRef.
- S. P. Bagare and N. S. Murthy, Pramana, 1982, 19, 497–499 CrossRef CAS.
- T. Wijchers, H. A. Dijkerman, P. J. T. Zeegers and C. T. J. Alkemade, Chem. Phys., 1984, 91, 141–159 CrossRef CAS.
- W. J. Childs, O. Poulsen and T. C. Steimle, J. Chem. Phys., 1988, 88, 598–606 CrossRef CAS.
- T. C. Steimle and J. E. Shirley, J. Chem. Phys., 1990, 92, 3292–3296 CrossRef CAS.
- R. Dye, R. Muenchausen and N. Nogar, Chem. Phys. Lett., 1991, 181, 531–536 CrossRef CAS.
- D. Fried, T. Kushida, G. P. Reck and E. W. Rothe, J. Appl. Phys., 1993, 73, 7810–7817 CrossRef CAS.
- C. E. Otis and P. M. Goodwin, J. Appl. Phys., 1993, 73, 1957–1964 CrossRef CAS.
- J. M. Badie and B. Granier, Chem. Phys. Lett., 2002, 364, 550–555 CrossRef CAS.
- J. M. Badie and B. Granier, Eur. Phys. J.: Appl. Phys., 2003, 21, 239–242 CrossRef CAS.
- T. Kobayashi and T. Sekine, Chem. Phys. Lett., 2006, 424, 54–57 CrossRef CAS.
- J. M. Badie, L. Cassan, B. Granier, S. A. Florez and F. C. Janna, J. Sol. Energy Eng., 2007, 129, 412–415 CrossRef CAS.
- A. Bernard and R. Gravina, Astrophys. J., Suppl. Ser., 1980, 44, 223–239 CrossRef CAS.
- J. W. H. Leung, T. M. Ma and A. S. C. Cheung, J. Mol. Spectrosc., 2005, 229, 108–114 CrossRef CAS.
- D. Zhang, Q. Zhang, B. Zhu, J. Gu, B. Suo, Y. Chen and D. Zhao, J. Chem. Phys., 2017, 146, 114303 CrossRef PubMed.
- C. L. Chalek and J. L. Gole, J. Chem. Phys., 1976, 65, 2845–2859 CrossRef CAS.
- B. Simard, A. M. James, P. A. Hackett and W. J. Balfour, J. Mol. Spectrosc., 1992, 154, 455–457 CrossRef CAS.
- U. Uhler and L. Akerlind, Ark. Fys., 1961, 19, 1–16 CAS.
- T. C. Steimle and Y. Alramadin, Chem. Phys. Lett., 1986, 130, 76–78 CrossRef CAS.
- J. Hoeft and T. Torring, Chem. Phys. Lett., 1993, 215, 367–370 CrossRef CAS.
- P. H. Kasai and W. Weltner, Jr., J. Chem. Phys., 1965, 43, 2553 CrossRef CAS.
- T. C. Steimle and Y. Alramadin, J. Mol. Spectrosc., 1987, 122, 103–112 CrossRef CAS.
- R. D. Suenram, F. J. Lovas, G. T. Fraser and K. Matsumura, J. Chem. Phys., 1990, 92, 4724–4733 CrossRef CAS.
- L. B. Knight, J. G. Kaup, B. Petzoldt, R. Ayyad, T. K. Ghanty and E. R. Davidson, J. Chem. Phys., 1999, 110, 5658–5669 CrossRef CAS.
- T. C. Steimle and W. Virgo, J. Mol. Spectrosc., 2003, 221, 57–66 CrossRef CAS.
- D. M. Manos and J. M. Parson, J. Chem. Phys., 1975, 63, 3575–3585 CrossRef CAS.
- C. L. Chalek and J. L. Gole, Chem. Phys., 1977, 19, 59–90 CrossRef CAS.
- J. Tennyson, K. Hulme, O. K. Naim and S. N. Yurchenko, J. Phys. B: At., Mol. Opt. Phys., 2016, 49, 044002 CrossRef.
- A. G. Gaydon, Dissociation Energies and Spectra of Diatomic Molecules, Chapman and Hall, Ltd., London, 3rd edn, 1968 Search PubMed.
- R. J. Ackermann and E. G. Rauh, J. Chem. Phys., 1974, 60, 2266–2271 CrossRef CAS.
- S. R. Langhoff and C. W. Bauschlicher, J. Chem. Phys., 1988, 89, 2160–2169 CrossRef CAS.
- P. Sriramachandran and R. Shanmugavel, New Astron., 2011, 16, 110–113 CrossRef CAS.
- J. Tennyson and S. N. Yurchenko, Mon. Not. R. Astron. Soc., 2012, 425, 21–33 CrossRef CAS.
- L. Lodi, S. N. Yurchenko and J. Tennyson, Mol. Phys., 2015, 113, 1559–1575 CrossRef.
- L. K. McKemmish, S. N. Yurchenko and J. Tennyson, Mon. Not. R. Astron. Soc., 2016, 463, 771–793 CrossRef CAS.
- L. K. McKemmish, T. Masseron, J. Hoeijmakers, V. V. Pérez-Mesa, S. L. Grimm, S. N. Yurchenko and J. Tennyson, Mon. Not. R. Astron. Soc., 2019, 488, 2836–2854 CrossRef.
- J. F. Stanton and J. Gauss, Adv. Chem. Phys., 2003, 125, 101–146 CrossRef CAS.
- J. Tennyson, L. Lodi, L. K. McKemmish and S. N. Yurchenko, J. Phys. B: At., Mol. Opt. Phys., 2016, 49, 102001 CrossRef.
- Ľ. Horný, A. Paul, Y. Yamaguchi and H. F. Schaefer III, J. Chem. Phys., 2004, 121, 1412–1418 CrossRef PubMed.
- C. Puzzarini and K. A. Peterson, J. Chem. Phys., 2005, 122, 084323 CrossRef PubMed.
- A. Paul, Y. Yamaguchi, H. F. Schaefer III and K. A. Peterson, J. Chem. Phys., 2006, 124, 034310 CrossRef PubMed.
- A. Paul, Y. Yamaguchi and H. F. Schaefer III, J. Chem. Phys., 2007, 127, 154324 CrossRef PubMed.
- S. N. Yurchenko, L. Lodi, J. Tennyson and A. V. Stolyarov, Comput. Phys. Commun., 2016, 202, 262–275 CrossRef CAS.
- H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1988, 89, 5803–5814 CrossRef CAS.
- P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1988, 145, 514–522 CrossRef CAS.
- P. J. Knowles and H.-J. Werner, Theor. Chem. Acc., 1992, 84, 95–103 Search PubMed.
- H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1985, 82, 5053–5063 CrossRef CAS.
- P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1985, 115, 259–267 CrossRef CAS.
- K. A. Peterson, D. Figgen, M. Dolg and H. Stoll, J. Chem. Phys., 2007, 126, 124101 CrossRef PubMed.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- T. Shiozaki, W. Györffy, P. Celani and H.-J. Werner, J. Chem. Phys., 2011, 135, 081106 CrossRef PubMed.
- K. A. Peterson and T. H. Dunning, J. Chem. Phys., 2002, 117, 10548 CrossRef CAS.
- A. Berning, M. Schweizer, H.-J. Werner, P. J. Knowles and P. Palmieri, Mol. Phys., 2000, 98, 1823–1833 CrossRef CAS.
- L. Visscher, Theor. Chem. Acc., 1997, 98, 68–70 Search PubMed.
- K. G. Dyall, J. Chem. Phys., 1994, 100, 2118–2127 CrossRef CAS.
- K. G. Dyall, Theor. Chem. Acc., 2007, 117, 483–489 Search PubMed.
- K. G. Dyall, Theor. Chem. Acc., 2016, 135, 128 Search PubMed.
- A. Shee, T. Saue, L. Visscher and A. S. P. Gomes, J. Chem. Phys., 2018, 149, 174113 CrossRef PubMed.
- A. Kramida, Yu. Ralchenko, J. Reader and NIST ASD Team, NIST Atomic Spectra Database (ver. 5.6.1), [Online]. Available: https://physics.nist.gov/asd [2019, April 13]. National Institute of Standards and Technology, Gaithersburg, MD., 2019.
- C. Hampel, K. A. Peterson and H.-J. Werner, Chem. Phys. Lett., 1992, 190, 1–12 CrossRef CAS.
- J. D. Watts, J. Gauss and R. J. Bartlett, J. Chem. Phys., 1993, 98, 8718–8733 CrossRef CAS.
- P. J. Knowles, C. Hampel and H. J. Werner, J. Chem. Phys., 1993, 99, 5219–5227 CrossRef CAS.
- J. M. L. Martin, Chem. Phys. Lett., 1996, 259, 669–678 CrossRef CAS.
- A. Karton and J. M. L. Martin, Theor. Chem. Acc., 2006, 115, 330–333 Search PubMed.
- K. A. Peterson, D. Woon and T. H. Dunning, J. Chem. Phys., 1994, 100, 7410–7415 CrossRef CAS.
- J. L. Dunham, Phys. Rev., 1932, 41, 721–731 CrossRef CAS.
- H. J. Werner, P. J. Knowles, G. Knizia, F. R. Manby, M. Schütz, P. Celani, W. Györffy, D. Kats, T. Korona, R. Lindh, A. Mitrushenkov, G. Rauhut, K. R. Shamasundar, T. B. Adler, R. D. Amos, A. Bernhardsson, A. Berning, D. L. Cooper, M. J. O. Deegan, A. J. Dobbyn, F. Eckert, E. Goll, C. Hampel, A. Hesselmann, G. Hetzer, T. Hrenar, G. Jansen, C. Köppl, Y. Liu, A. W. Lloyd, R. A. Mata, A. J. May, S. J. McNicholas, W. Meyer, M. E. Mura, A. Nicklass, D. P. O'Neill, P. Palmieri, D. Peng, K. Pflüger, R. Pitzer, M. Reiher, T. Shiozaki, H. Stoll, A. J. Stone, R. Tarroni, T. Thorsteinsson and M. Wang, MOLPRO, version 2015.1, a package of ab initio programs, http://www.molpro.net, 2015 Search PubMed.
- DIRAC, a relativistic ab initio electronic structure program, Release DIRAC18, written by T. Saue, L. Visscher, H. J. Aa. Jensen, and R. Bast, with contributions from V. Bakken, K. G. Dyall, S. Dubillard, U. Ekström, E. Eliav, T. Enevoldsen, E. Faßhauer, T. Fleig, O. Fossgaard, A. S. P. Gomes, E. D. Hedegård, T. Helgaker, J. Henriksson, M. Iliaš, Ch. R. Jacob, S. Knecht, S. Komorovský, O. Kullie, J. K. Lærdahl, C. V. Larsen, Y. S. Lee, H. S. Nataraj, M. K. Nayak, P. Norman, G. Olejniczak, J. Olsen, J. M. H. Olsen, Y. C. Park, J. K. Pedersen, M. Pernpointner, R. di Remigio, K. Ruud, P. Sałek, B. Schimmelpfennig, A. Shee, J. Sikkema, A. J. Thorvaldsen, J. Thyssen, J. van Stralen, S. Villaume, O. Visser, T. Winther, and S. Yamamoto, 2018, available at https://doi.org/10.5281/zenodo.2253986, see also http://www.diracprogram.org.
- J. Tennyson and S. N. Yurchenko, Int. J. Quantum Chem., 2017, 117, 92–103 CrossRef CAS.
- A. T. Patrascu, C. Hill, J. Tennyson and S. N. Yurchenko, J. Chem. Phys., 2014, 141, 144312 CrossRef PubMed.
- S. N. Yurchenko, I. Szabo, E. Pyatenko and J. Tennyson, Mon. Not. R. Astron. Soc., 2018, 480, 3397–3411 CrossRef CAS.
- H.-Y. Li, J. Tennyson and S. N. Yurchenko, Mon. Not. R. Astron. Soc., 2019, 486, 2351–2365 CrossRef.
- T. J. Lee and P. R. Taylor, Int. J. Quantum Chem., 1989, 36, 199–207 CrossRef.
- C. L. Janssen and I. M. B. Nielsen, Chem. Phys. Lett., 1998, 290, 423–430 CrossRef CAS.
- W. Jiang, N. J. DeYonker and A. K. Wilson, J. Chem. Theory Comput., 2012, 8, 460–468 CrossRef CAS PubMed.
- J. Wang, S. Manivasagam and A. K. Wilson, J. Chem. Theory Comput., 2015, 11, 5865–5872 CrossRef CAS PubMed.
- A. Karton, E. Rabinovich, J. M. L. Martin and B. Ruscic, J. Chem. Phys., 2006, 125, 144108 CrossRef PubMed.
- A. Karton, S. Daon and J. M. L. Martin, Chem. Phys. Lett., 2011, 510, 165–178 CrossRef CAS.
- J. Shirley, C. Scurlock and T. Steimle, J. Chem. Phys., 1990, 93, 1568–1575 CrossRef CAS.
- C. W. Bauschlicher and S. R. Langhoff, J. Chem. Phys., 1986, 85, 5936–5942 CrossRef CAS.
- T. C. Steimle and W. Virgo, J. Chem. Phys., 2002, 116, 6012–6020 CrossRef CAS.
- R. Stringat, C. Athénour and J. L. Féménias, Can. J. Phys., 1972, 50, 395–403 CrossRef CAS.
- A. Bernard and J. Vergés, J. Mol. Spectrosc., 2000, 201, 172–174 CrossRef CAS PubMed.
- J. Tennyson, S. N. Yurchenko, A. F. Al-Refaie, E. J. Barton, K. L. Chubb, P. A. Coles, S. Diamantopoulou, M. N. Gorman, C. Hill, A. Z. Lam, L. Lodi, L. K. McKemmish, Y. Na, A. Owens, O. L. Polyansky, T. Rivlin, C. Sousa-Silva, D. S. Underwood, A. Yachmenev and E. Zak, J. Mol. Spectrosc., 2016, 327, 73–94 CrossRef CAS.
- S. N. Yurchenko, A. F. Al-Refaie and J. Tennyson, Astron. Astrophys., 2018, 614, A131 CrossRef.
- C. M. Western, J. Quant. Spectrosc. Radiat. Transfer, 2017, 186, 221–242 CrossRef CAS.
- R. R. Reddy, Y. Nazeer Ahammed, K. Rama Gopal, P. Abdul Azeem and S. Anjaneyulu, Astrophys. Space Sci., 1998, 262, 223–240 CrossRef CAS.
- P. S. Barklem and R. Collet, Astron. Astrophys., 2016, 588, A96 CrossRef.
- R. R. Gamache, C. Roller, E. Lopes, I. E. Gordon, L. S. Rothman, O. L. Polyansky, N. F. Zobov, A. A. Kyuberis, J. Tennyson, S. N. Yurchenko, A. G. Császár, T. Furtenbacher, X. Huang, D. W. Schwenke, T. J. Lee, B. J. Drouin, S. A. Tashkun, V. I. Perevalov and R. V. Kochanov, J. Quant. Spectrosc. Radiat. Transfer, 2017, 203, 70–87 CrossRef CAS.
- M. S. Vardya, Astron. Astrophys., 1970, 5, 162–164 CAS.
- N. Madhusudhan and S. Gandhi, Mon. Not. R. Astron. Soc., 2017, 472, 2334–2355 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cp03208h |
| This journal is © the Owner Societies 2019 |