Open Access Article
Open Access ArticleTriplet state promoted reaction of SO2 with H2O by competition between proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) processes†
Josep M.
Anglada
 *a,
Marilia T. C.
Martins-Costa
b,
Joseph S.
Francisco
*a,
Marilia T. C.
Martins-Costa
b,
Joseph S.
Francisco
 c and
Manuel F.
Ruiz-López
c and
Manuel F.
Ruiz-López
 *b
*b
aDepartament de Química Biològica (IQAC – CSIC), c/Jordi Girona 18, E-08034 Barcelona, Spain. E-mail: anglada@iqac.csic.es
bLaboratoire de Physique et Chimie Théoriques, UMR CNRS 7019, University of Lorraine, CNRS, BP 70239, 54506 Vandoeuvre-lès-Nancy, France. E-mail: manuel.ruiz@univ-lorraine.fr
cDepartment of Earth and Environmental Science and Department of Chemistry, University of Pennsylvania, Philadelphia, PA 19104-6316, USA
First published on 23rd April 2019
Abstract
The SO2 + H2O reaction is proposed to be the starting process for the oxidation of sulfur dioxide to sulfate in liquid water, although the thermal reaction displays a high activation barrier. Recent studies have suggested that the reaction can be promoted by light absorption in the near UV. We report ab initio calculations showing that the SO2 excited triplet state is unstable in water, as it immediately reacts with H2O through a water-assisted proton coupled electron transfer mechanism forming OH and HOSO radicals. The work provides new insights for a general class of excited-state promoted reactions of related YXY compounds with water, where Y is a chalcogen atom and X is either an atom or a functional group, which opens up interesting chemical perspectives in technological applications of photoinduced H-transfer.
Introduction
Hydrogen atom transfer originated by radicals is one of the most fundamental processes for several areas of chemistry, from biological processes, to chemical synthesis, materials science, hydrocarbon combustion or atmospheric chemistry.1–12 In the context of the chemistry of the troposphere, the oxidation processes involving abstraction of hydrogen atoms by radicals are among the most important reactions and many of these processes are well known.7,13 Very recently, it has been shown that the triplet excited state of SO2 can react with water producing hydroxyl radicals and is the most oxidizing species in the Earth's atmosphere,14 and it has also been predicted that this reaction is enhanced by several orders of magnitude at the air–water (air–clouds) interface.15 Because the SO2 molecule is an air pollutant formed as a byproduct of fossil fuel combustion, there is broad interest in the chemistry of SO2 ranging from its industrial clean-up to understanding the chemistry associated with its oxidation to sulfuric acid, a major constituent of acid rain.7,16,17 On a fundamental level, the oxidation of SO2 is thought to proceed by the following steps in the gas-phase:| SO2(g) + OH(g) → HOSO2(g) | (1) |
| HOSO2(g) + O2(g) → SO3(g) + HO2(g) | (2) |
| SO3(g) + H2O(g) → H2SO4(g) | (3) |
| SO2(g) + H2O(l) → H2SO3(aq) | (4) |
| H2SO3(aq) + H2O2(aq) → H2O(l) + H2SO4(aq) | (5) |
Studies in the literature have focused on reactions (1)–(3),18,19 sometimes assuming a water dimer (ref. 17), but those that have addressed the reaction of SO2 with H2O have been few. Recently, Kroll et al.14 proposed that SO2 driven by sunlight could initiate the reaction producing HOSO and OH radicals. However, details on the electronic features triggering the reaction mechanism were not analyzed. In the present work we show that the reaction of the triplet exited state of sulfur dioxide with water yielding HOSO and OH radicals belongs to a subcategory of proton coupled electron transfer (pcet) processes that involve photoexcitation of SO2 followed by intersystem crossing to a triplet state. The effect of further water molecules on these reactions has been also investigated because it has interest in modeling how these processes behave in different environments, such as for instance at the air–water interface, where we have predicted an enhancement of the reaction rate by several orders of magnitude,15 or in the bulk.
Results and discussion
Our investigation has been carried out by doing calculations with B3LYP20 density functional theory and coupled cluster with single, double and extrapolation to triple excitations (CCSD(T)),21 along with the 6-311+G(2df,2p), aug-cc-pVTZ, aug-cc-pVQZ and complete CBS basis sets.22–26 The bonding interactions have been analyzed within the framework of atoms in molecules (AIM),27 and for some elementary reactions we have calculated the corresponding rate constants according to transition state theory. Full details of the theoretical methods employed are discussed in the ESI.†The reaction of the SO2 (X1A1) ground state with H2O
The interaction between SO2 and H2O in the gas phase first forms a complex. Computational studies have determined conformational structures for the SO2–H2O complex.28,29 It is established that the global-minimum has Cs symmetry with H2O lying above the plane of SO2. Such a structure stems mainly from the interaction between the S atom of SO2 and the O atom in H2O. Both theoretical and experimental gas phase microwave spectroscopy and frozen matrix infrared spectroscopy studies30–32 have confirmed and validated this picture. The binding energy of SO2 to H2O was estimated to be between 3.5 and 5.5 kcal mol−1.29,32The transition state for addition of H2O to SO2 to form sulfurous acid, H2SO3, involves a four-centered transition state in which the O–H bond in water is broken while forming a new O–H bond on the SO2, and the OH from water forms an S–OH bond with the SO2. The activation barrier for the addition of H2O to SO2 to form H2SO3 is calculated to be 30–33.9 kcal mol−1.29,33,34 All theoretical computations show a high reaction barrier and suggest that SO2 does not react with H2O in the gas phase and no experimental studies have observed the H2SO3 product in the gas phase; nor has it been isolated in a matrix.
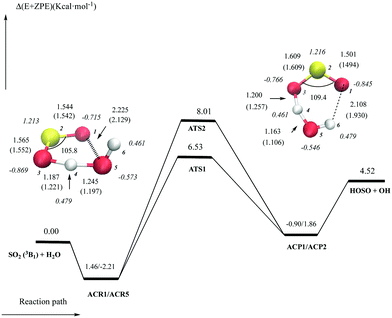
The reaction of the SO2 (a3B1) excited state with H2O
The lowest triplet state of SO2 (a3B1) may be accessed by near-UV solar excitation (absorption band extending from 240 to 330 nm) to its excited 1B1 state followed by rapid intersystem crossing.35–39 The lifetime of the lowest triplet state of SO2 has been estimated to be τ = (7.9 ± 1.7) × 10−4 s.40 Because of the long lifetime, the triplet state has been suggested to react with water to form H2SO3 in the gas phase41 or OH + HOSO.14The potential energy surface for the reaction of SO2 (a3B1) with water is shown in Fig. 1. Only the most stable structures involving the (a3B1) excited state are discussed here but other structures are reported as ESI† (Tables S1, S2 and Fig. S1, S2). We have found that there are two different kinds of processes leading to product formation of HOSO + OH. Fig. 1 shows that the transition state structure having the lowest energy barrier (ATS1) lies 6.53 kcal mol−1 above the energy of the separate reactants and compares quite well with the data reported very recently by Kroll and coworkers.14 In addition, Fig. 1 also shows the existence of a second reaction path (viaATS2) whose energy barrier is 1.48 kcal mol−1 higher. Both elementary processes have different electronic features and we have plotted in Fig. 2 an orbital diagram to illustrate them.
 | ||
| Fig. 2 Orbital diagram for the pcet and hat mechanisms for the SO2 (a3B1) + H2O reaction, along with a picture of the natural orbitals involved in these processes. | ||
In the process going through ATS1, the reaction between SO2 (a3B1) and water (X1A1) involves the interaction of the 8a1 singly occupied orbital of the triplet state of SO2 with a lone pair of water, so that the doubly occupied 20a and the singly occupied 22a orbitals are formed in ATS1. The structure and electronic features of this transition state allow the electronic density of the lone pair of water to interact with the electronic density of the unpaired electron of SO2 (orbital 8a1) localized over the terminal oxygen atom of sulfur dioxide opposite the oxygen atom of the water moiety. Fig. 2 shows that in the doubly occupied orbital 20a of ATS1, the electron density is shared between the oxygen atom of the water moiety and the terminal oxygen atom of SO2, whereas in the singly occupied 22a orbital the anti-bonding combination is formed. This situation corresponds to a two-center three-electron structure in which an electron is transferred from the lone pair of water to the terminal oxygen atom of SO2. This originates a simultaneous jump of a proton from water to the other oxygen atom of SO2 so that the whole process can be described as a proton coupled electron transfer (pcet) mechanism. The 3b1 orbital of SO2 converts into the 21a orbital of ATS1; it has no interaction with water and consequently acts as a spectator in the reaction. Its sole role is to maintain the triplet multiplicity. It is worth pointing out that the electronic features of this pcet mechanism are the same as those described for the oxidation of organic and inorganic species by radicals.9,42–51
The process viaATS2 occurs in a different way. Fig. 2 shows that it takes place by interaction of the 8a1 orbital of SO2 and the (O–H) σ bond of water forming the doubly occupied 20a orbital with bonding character and the singly occupied 22a orbital with anti-bonding character. This situation corresponds to a three-center three-electron system where the electronic density lies over the O–H–O moiety and describes the well-known hydrogen atom transfer mechanism (hat), in which simultaneous breaking and forming of the covalent bonds (OS)O–H–O(H) occurs. It is also interesting to point out that only small differences are found in the natural atomic charges of both transition states, which can be attributed to a minor role played by the unpaired electron in these processes. One should note however that the two oxygen atoms of the SO2 moiety bear different net charge in the two transition structures. Thus, in the case of ATS1, the largest negative charge corresponds to the H+-acceptor O atom. Conversely, in the case of ATS2, the H-acceptor O atom bears the lowest negative charge, and the remaining charge is accumulated on the opposite O atom because it is involved in a H-bond with water.
The electronic features of these two reaction mechanisms are also supported by the bonding analysis according to the AIM theory, which points out the existence of a bond critical point between O1 and O5 in the case of ATS1 and a hydrogen bond interaction between O1 and H6 in the case of ATS2, as discussed in previous work on related systems9 (see also the ESI†). It is also worth mentioning that pcet and hat processes involved in hydrogen transfer reactions have been identified by the electronic features of the orbitals involved and according the vibronic coupling in the self-exchange reactions between these processes.52–54
Fig. 1 shows that the elementary reaction going via the pcet mechanism (ATS1) is more favorable than the process taking place through the hat mechanism (ATS2), despite the last one being additionally stabilized by a hydrogen bond between the terminal oxygen atom of SO2 and one hydrogen of the water moiety. Indeed, elementary reactions occurring through a pcet mechanism in the oxidation of organic and inorganic species by radicals have been found to be more favorable than the oxidation processes taking place via a hat mechanism,9,42–51 which can be easily rationalized looking at the pictorial canonical forms in the transition states. For the hat process, Zavitsas and co-workers55–58 pointed out that the three-center three-electron structure in the transition state can be described by the four canonical forms (a = X↑↓H⋯Y↑; b = ↑X⋯H↓↑Y; c = X↑⋯H↓⋯↑Y; and d = [X⋯H⋯Y]), where X and Y are the two atoms between which the hydrogen atom is being transferred, namely two oxygen atoms in the present case. a and b describe the bonding of the H to both X and Y; c corresponds to the triplet repulsion (antibonding) between X and Y and d describes the resonance of one electron delocalization between the three atoms. Thus, the corresponding energy barrier is related to the triplet repulsion energy of the X/Y pair (canonical form c) in the transition state structure. In the case of the pcet mechanism, the electrons are transferred from the Z to the Y atoms (both oxygen atoms in this case) in a two center three electron mechanism so that we could write two canonical forms for this process, namely e = Z↑↓⋯↑Y; and f = Z↑+⋯↓↑Y−. Thus, it turns out that the pcet mechanism avoids the triplet repulsion occurring in hat, which, in general, results in a lower energy barrier for the proton coupled electron transfer reactions compared to the conventional hydrogen atom transfer processes.
From an energetic point of view, Fig. 1 and Table S2 (ESI†) show that our calculations predict the SO2 (a3B1) + H2O → HOSO + OH reaction to be endothermic by 4.52 kcal mol−1 or endoergic by 3.81 kcal mol−1 in terms of free energy at 298 K, whereas ATS1 and ATS2 are predicted to lie 8.53 and 8.01 kcal mol−1, respectively, above the energy of the separate reactants, or 15.39 and 16.44 kcal mol−1 in terms of free energy at 298 K, which makes the reaction feasible in the atmosphere despite the endothermicity of the whole reaction. For this reaction, Kroll et al.14 report an estimated rate constant of (5–16) × 10−15 cm3 molecule−1 s−1 following photochemical experiments and in the range between 10−14 and 10−16 cm3 molecule−1 s−1 according to theoretical calculations employing transition state theory. Employing the same level of kinetic calculations and our theoretical results, we obtain a rate constant of 4.7 × 10−17 cm3 molecule−1 s−1 for the elementary reaction through ATS1 and 2.6 × 10−20 cm3 molecule−1 s−1 for the elementary reaction through ATS2, indicating that the whole reaction takes place via the pcet mechanism.
Finally, for the sake of completeness, we have also optimized the reactants SO2 (a3B1) and H2O and the ATS1 and ATS2 transition states at the CCSD(T) level of theory and their geometrical parameters compare quite well with those obtained at the DFT level (see Fig. 1). Moreover, the corresponding relative energies obtained with both the CCSD(T)/CBS//B3LYP/aug-cc-pVTZ and CCSD(T)/CBS//CCSD(T)/6-311+G(2df,2p) levels of theory differ by less than 0.2 kcal mol−1 (see Table S2 of the ESI†).
Impact of multiple H2O on the excited state reaction
We now look at the effect of additional water molecules interacting with the SO2·H2O system by taking into account the interaction of SO2 (a3B1) with a water dimer, trimer and tetramer.Fig. 3 displays schematically the zero-point energy corrected potential energy surfaces for the reaction SO2 (a3B1) + (H2O)2, as well as the structures corresponding to the hat and pcet transition states. As shown, hydrogen-bonding with water produces a huge stabilization of the pcet and hat transition structures (respectively BTS1 and BTS2), which lie now only 0.62 and 1.60 kcal mol−1 above the energy of the separated reactants.
The geometry obtained for the transition state structures shows that the additional water molecules essentially play a solvation role, and are not directly involved in the elementary H˙, H+ and e− transfer processes. To rationalize the strong stabilizing solvation effect in this case, it is interesting to look at the geometrical parameters and charge distributions reported in Fig. 1 and 3.
The transition state structure involved in the hat mechanism (BTS2) is a single ring-type structure whose stabilization stems from a cooperative hydrogen-bond network. Remarkably, the hydrogen-bond distance between the two water molecules is very short (1.62 Å) compared to the same bond length in the isolated water dimer (around 1.9 Å). Apart from the hydrogen-bond contribution, the stability of BTS2 can be explained by the relaxation of the strain energy in ATS2 providing greater geometrical flexibility for the atoms involved in the H-transfer.
In the case of the pcet transition state structure (BTS1), instead, the solvating water molecule forms a second ring and two cooperative hydrogen-bonds with the reacting system. The main effect of this solvating water molecule is a significant enhancement of the electron charge transfer between the oxygen atoms (compare net charges on linked O-atoms in BTS1 and ATS1, Fig. 1 and 3). Thus, electron transfer in the transition state structure is sustained by water solvation, and accordingly, the activation barrier decreases. Overall, the solvating water molecule bears a non-negligible positive charge (0.0311 a.u.), which suggests that its role as a proton acceptor prevails over its role as a proton donor, which is also reflected in the H-bond distances. Our results displayed in Fig. 3 and Table S4 (ESI†) show that the formation of HOSO + H2O⋯OH is endothermic by 3.72 kcal mol−1. In terms of free energy at 298 K, the formation of these products is also endoergic by 2.94 kcal mol−1, but the production of HOSO + H2O + OH is endoergic by about 1 kcal mol−1. Our calculations also predict the transition states via pcet and hat to lie between 0.6 and 1.8 kcal mol−1, or between 12.1 and 13.0 kcal mol−1 in terms of free energy at 298 K, and the corresponding rate constants are computed to be 5.8 × 10−15 cm3 molecule−1 s−1via the pcet reaction mechanism, and 2.1 × 10−15 cm3 molecule−1 s−1via the hat reaction mechanism, with a total value of 7.9 × 10−15 cm3 molecule−1 s−1. This means that 74% of the reaction goes through a pcet mechanism and 26% goes via a hat mechanism. The calculated rate constant for the reaction with a water dimer is two orders of magnitude greater than the value computed for the reaction with a single water molecule. However, its importance in the chemistry of the atmosphere is negligible, given the smaller concentration of water dimers compared to the concentration of single water molecules. Only in very hot and humid conditions, where [(H2O)2] is about 2.5 × 102 times smaller than [(H2O)],59 could the reaction with a water dimer play a minor role.
Regarding the reaction with a water trimer, our calculations reveal that the pcet mechanism is even more stabilized than in the case of a water dimer. The corresponding transition state structure (CTS1) is more stable than the separated reactants (−2.75 kcal mol−1, Table S5 and Fig. S5, ESI†). As in the case of BTS1, the two additional water molecules act as a solvent forming a second ring in the transition state structure, which allows the geometry of the transition state to be less strained and results in a more effective interaction of the reactant moiety with the solvent. In contrast, adding a further water molecule does not lead to additional stabilization of the transition state structure of the hat mechanism (CTS2), which lies 2.05 kcal mol−1 above the separate reactants, and similar results are obtained when considering four water molecules (see also Table S5 and Fig. S5 of the ESI†).
It is worth pointing out that the gas phase reactivity of SO2 (a3B1) with a water trimer and tetramer has less interest for gas phase atmospheric purposes, because of the low atmospheric concentration of these water clusters.59 However, these results may be useful for predicting the reactivity of SO2 in the condensed phase. Indeed, in previous work, we have shown that SO2 displays a significant affinity for the air–water interface and that the photoinduced reaction with water at the surface of cloud water droplets has large atmospheric significance.15 The clusters with three or four water molecules studied here represent a simple microsolvation model for these condensed phase reactions, in which relative free energies with respect to the initial SO2–water complexes are the relevant quantities. In Fig. 4, the free energy profiles of the pcet process viaCTS1 or DTS1 are shown (see also Table S5 and S6 of the ESI†). As shown, the free energy barriers are very small, 0.79 and 0.70 kcal mol−1 at 298 K for CTS1 and DTS1, respectively, the processes being almost isoergonic. These results indicates therefore that a very fast reaction of triplet SO2 with water should take place in the condensed phase.
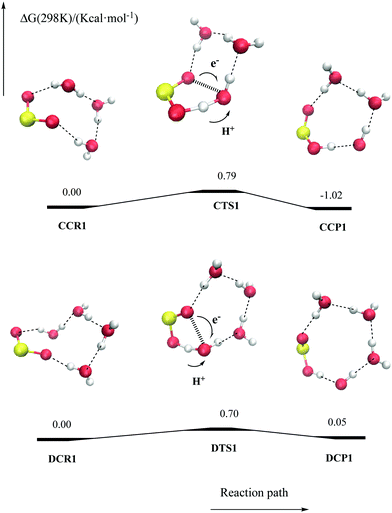 | ||
| Fig. 4 Schematic free energy surface at 298 K (CCSDT/aug-cc-pVTS//B3LYP/aug-cc-pVTZ, values in kcal mol−1) for the reaction of SO2 (a3B1) with three and four water molecule clusters. | ||
Implications
The reported results provide useful information to discuss the actual outcome of the process, which may differ in different environments (gas vs. condensed phase). Overall, they emphasize the fact that in the reaction of triplet SO2 with water, environmental water molecules may act as a catalyst of the pcet mechanism by expediting charge transfer.The pcet reactions cover a wide range of chemical processes that have attracted increasing interest over the last decades because they play a key role in biological processes, photocatalysis and solar energy conversion.3,5,10,12 The mechanism discussed in this work for the reaction of sulfur dioxide with water to yield HOSO and OH radicals belongs to a subcategory of bimolecular pcet processes that involve prior photoexcitation followed by intersystem crossing to a triplet state. We have shown that in this reaction pathway a very low activation barrier needs to be overcome, in contrast to the high stability of SO2 in its ground electronic state. Since, in addition, the reaction is favored through hydrogen-bonding interactions with additional surrounding water molecules, the photoinduced pcet mechanism is expected to be particularly relevant for SO2 exposed to sunlight at the air–water interface and in aqueous environments, which presumably should have significant implications for the atmospheric chemistry of sulfur dioxide adsorbed on cloud droplets or other aqueous aerosols.15
Nevertheless, the implications of the studied process are much wider. Indeed, our results suggest that pcet mechanisms would be involved in the conversion of other YXY systems to the corresponding HYXY products, where X is an atom or possibly a complex functional group, Y is oxygen or another chalcogen atom, and the YXY system must display an open-shell structure. Interestingly, the reaction of water with NO2 in its doublet ground state has been previously studied49 and proceeds through a pcet mechanism that significantly lowers the activation barrier with respect to the traditional H-atom transfer. The data reported here provide chemical insights into such a new general class of reactions by which the electronic excited-state promotes the process. This route opens up interesting chemical perspectives in technological applications of photoinduced H-transfer reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the French CNRS and the Spanish CSIC organizations for funding a collaborative PICS project (PIC2015FR1). MTCMC and MFRL are grateful to the French CINES (project lct2550) for providing computational resources. JMA thanks the Generalitat de Catalunya (Grant 2017SGR348) for financial support, and the Consorci de Serveis Universitaris de Catalunya (CSUC) for providing computational resources.References
- J. L. Dempsey, J. R. Winkler and H. B. Gray, Chem. Rev., 2010, 110, 7024–7039 CrossRef CAS PubMed.
- S. Hammes-Schiffer, ChemPhysChem, 2002, 3, 33–42 CrossRef CAS PubMed.
- S. Hammes-Schiffer and A. A. Stuchebrukhov, Chem. Rev., 2010, 110, 6939–6960 CrossRef CAS PubMed.
- J. M. Mayer, Acc. Chem. Res., 2011, 44, 36–46 CrossRef CAS PubMed.
- J. W. Darcy, B. Koronkiewicz, G. A. Parada and J. M. Mayer, Acc. Chem. Res., 2018, 51, 2391–2399 CrossRef CAS PubMed.
- D. J. Jacob, in Handbook of Weather, Climate and Water: Atmospheric Chemistry, Hydrology and Societal Impacts, ed. T. D. Potter and B. R. Colman, Wiley-Interscience, 1st edn, 2002, pp. 29–46 Search PubMed.
- B. J. Finlayson-Pitts and J. N. Pitts Jr., Atmospheric Chemistry: Fundamental and Experimentals Techniques, John Wiley and Sons, New York, 1986 Search PubMed.
- J. C. Lennox, D. A. Kurtz, T. Huang and J. L. Dempsey, ACS Energy Lett., 2017, 2, 1246–1256 CrossRef.
- S. Olivella, J. M. Anglada, A. Sole and J. M. Bofill, Chem. – Eur. J., 2004, 10, 3404–3410 CrossRef CAS PubMed.
- L. Capaldo and D. Ravelli, Eur. J. Org. Chem., 2017, 2056–2071 CrossRef CAS PubMed.
- Y. J. Sun, Y. Liu and C. Turro, J. Am. Chem. Soc., 2010, 132, 5594–5595 CrossRef CAS PubMed.
- T. J. Whittemore, A. Millet, H. J. Sayre, C. Xue, B. S. Dolinar, E. G. White, K. R. Dunbar and C. Turro, J. Am. Chem. Soc., 2018, 140, 5161–5170 CrossRef CAS PubMed.
- J. M. Anglada, M. Martins-Costa, J. S. Francisco and M. F. Ruiz-Lopez, Acc. Chem. Res., 2015, 48, 575–583 CrossRef CAS PubMed.
- J. A. Kroll, B. N. Frandsen, H. G. Kjaergaard and V. Vaida, J. Phys. Chem. A, 2018, 122, 4465–4469 CrossRef CAS PubMed.
- M. T. C. Martins-Costa, J. M. Anglada, J. S. Francisco and M. F. Ruiz-López, J. Am. Chem. Soc., 2018, 140, 12341–12344 CrossRef CAS PubMed.
- J. P. D. Abbatt, Chem. Rev., 2003, 103, 4783–4800 CrossRef CAS PubMed.
- K. Morokuma and C. Muguruma, J. Am. Chem. Soc., 1994, 116, 10316–10317 CrossRef CAS.
- W. R. Stockwell and J. G. Calvert, Atmos. Environ., 1983, 17, 2231–2235 CrossRef CAS.
- J. T. Jayne, U. Poschl, Y. M. Chen, D. Dai, L. T. Molina, D. R. Worsnop, C. E. Kolb and M. J. Molina, J. Phys. Chem. A, 1997, 101, 10000–10011 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- J. A. Pople, M. Head-Gordon and K. Raghavachari, J. Chem. Phys., 1987, 87, 5968–5975 CrossRef CAS.
- T. H. J. Dunning, J. Chem. Phys., 1989, 90, 1007 CrossRef CAS.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- K. L. Bak, J. Gauss, P. Jorgensen, J. Olsen, T. Helgaker and J. F. Stanton, J. Chem. Phys., 2001, 114, 6548–6556 CrossRef CAS.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS.
- W. J. Hehre, L. Radom, P. v. R. Schleyer and J. A. Pople, Ab Initio Molecular Orbital Theory, Wiley, New York, 1986 Search PubMed.
- R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Clarendon Press, Oxford, UK, 1990 Search PubMed.
- N. C. Baird and K. F. Taylor, J. Comput. Chem., 1981, 2, 225–230 CrossRef CAS.
- W.-K. Li and M. L. McKee, J. Phys. Chem. A, 1997, 101, 9778–9782 CrossRef CAS.
- K. Matsumura, F. J. Lovas and R. D. Suenram, J. Chem. Phys., 1989, 91, 5887–5894 CrossRef CAS.
- A. Schriver, L. Schriver and J. P. Perchard, J. Mol. Spectrosc., 1988, 127, 125–142 CrossRef CAS.
- H. Tachikawa, J. Phys. Chem. A, 2011, 115, 9091–9096 CrossRef CAS PubMed.
- J. J. Liu, S. Fang, W. Liu, M. Y. Wang, F. M. Tao and J. Y. Liu, J. Phys. Chem. A, 2015, 119, 102–111 CrossRef CAS PubMed.
- E. Bishenden and D. J. Donaldson, J. Phys. Chem. A, 1998, 102, 4638–4642 CrossRef CAS.
- L. Anyang, S. Bing, W. Zhenyi and W. Yubin, Sci. China, Ser. B: Chem., 2006, 49, 289–295 CrossRef.
- S. Mai, P. Marquetand and L. González, J. Chem. Phys., 2014, 140, 204302 CrossRef PubMed.
- I. Wilkinson, A. E. Boguslavskiy, J. Mikosch, J. B. Bertrand, H. J. Wörner, D. M. Villeneuve, M. Spanner, S. Patchkovskii and A. Stolow, J. Chem. Phys., 2014, 140, 204301 CrossRef PubMed.
- C. Lévêque, R. Taïeb and H. Köppel, J. Chem. Phys., 2014, 140, 091101 CrossRef PubMed.
- C. Xie, X. Hu, L. Zhou, D. Xie and H. Guo, J. Chem. Phys., 2013, 139, 014305 CrossRef PubMed.
- S. S. Collier, A. Morikawa, D. H. Slater, J. G. Calvert, G. Reinhard and E. Damon, J. Am. Chem. Soc., 1970, 92, 217–218 CrossRef CAS.
- D. J. Donaldson, J. A. Kroll and V. Vaida, Sci. Rep., 2016, 6, 30000 CrossRef CAS PubMed.
- J. M. Anglada, J. Am. Chem. Soc., 2004, 126, 9809–9820 CrossRef CAS PubMed.
- J. M. Anglada, S. Olivella and A. Sole, J. Phys. Chem. A, 2006, 110, 1982–1990 CrossRef CAS PubMed.
- J. Gonzalez and J. M. Anglada, J. Phys. Chem. A, 2010, 114, 9151–9162 CrossRef CAS PubMed.
- J. M. Anglada and J. Gonzalez, ChemPhysChem, 2009, 10, 3034–3045 CrossRef CAS PubMed.
- S. Jorgensen, C. Jensen, H. G. Kjaergaard and J. M. Anglada, Phys. Chem. Chem. Phys., 2013, 15, 5140–5150 RSC.
- J. M. Anglada, S. Olivella and A. Solé, J. Am. Chem. Soc., 2014, 136, 6834–6837 CrossRef CAS PubMed.
- J. M. Anglada, S. Olivella and A. Sole, Phys. Chem. Chem. Phys., 2014, 16, 19437–19445 RSC.
- J. M. Anglada and A. Solé, J. Phys. Chem. A, 2017, 121, 9698–9707 CrossRef CAS PubMed.
- J. M. Anglada, R. Crehuet and A. Solé, Mol. Phys., 2019, 1–12 CrossRef.
- J. M. Anglada, R. Crehuet, S. Adhikari, J. S. Francisco and Y. Xia, Phys. Chem. Chem. Phys., 2018, 20, 4793–4804 RSC.
- J. M. Mayer, D. A. Hrovat, J. L. Thomas and W. T. Borden, J. Am. Chem. Soc., 2002, 124, 11142–11147 CrossRef CAS PubMed.
- J. H. Skone, A. V. Soudackov and S. Hammes-Schiffer, J. Am. Chem. Soc., 2006, 128, 16655–16663 CrossRef CAS PubMed.
- A. Sirjoosingh and S. Hammes-Schiffer, J. Phys. Chem. A, 2011, 115, 2367–2377 CrossRef CAS PubMed.
- A. A. Zavitsas and C. Chatgilialoglu, J. Am. Chem. Soc., 1995, 117, 10645–10654 CrossRef CAS.
- B. P. Roberts, J. Chem. Soc., Perkin Trans. 2, 1996, 2719–2725 RSC.
- A. A. Zavitsas, J. Chem. Soc., Perkin Trans. 2, 1996, 391–393 RSC.
- A. A. Zavitsas, J. Am. Chem. Soc., 1998, 120, 6578–6586 CrossRef CAS.
- J. M. Anglada, G. J. Hoffman, L. V. Slipchenko, M. M. Costa, M. F. Ruiz-López and J. S. Francisco, J. Phys. Chem. A, 2013, 117, 10381–10396 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Contains a description of the theoretical methods employed, of additional elementary reactions, relative energies, and geometrical structures and Cartesian coordinates of all stationary points investigated. See DOI: 10.1039/c9cp01105f |
| This journal is © the Owner Societies 2019 |