Microstructure of ionic liquid (EAN)-rich and oil-rich microemulsions studied by SANS†
Jan C.
Thater
a,
Cosima
Stubenrauch
 a,
Otto
Glatter
b,
Helge
Klemmer
a,
Otto
Glatter
b,
Helge
Klemmer
 c and
Thomas
Sottmann
c and
Thomas
Sottmann
 *a
*a
aUniversität Stuttgart, Institut für Physikalische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany. E-mail: thomas.sottmann@ipc.uni-stuttgart.de
bInstitut für Anorganische Chemie, TU Graz, Stremayrgasse 9, 8010 Graz, Austria
cUniversität zu Köln, Department Chemie, Physikalische Chemie, Luxemburger Straße 116, 50939 Cologne, Germany
First published on 26th November 2018
Abstract
In a previous study we investigated the phase behavior of microemulsions consisting of the ionic liquid ethylammonium nitrate (EAN), an n-alkane and a nonionic alkyl polyglycolether (CiEj). We found the same general trends as for the aqueous counterparts, i.e. a transition from an oil-in-EAN microemulsion via a bicontinuous microemulsion to an EAN-in-oil microemulsion with increasing temperature. However, unlike what happens in the corresponding aqueous systems, in EAN-in-oil microemulsions only a very small amount of EAN was detected by NMR-measurements. This is why we investigated the phase behavior and microstructure of EAN-rich n-dodecane-in-EAN microemulsions and oil-rich EAN-in-n-octane microemulsions. We found that the ionic liquid emulsification failure boundary has an extraordinarily small slope, which suggests that the amphiphilic film loses its ability to solubilize EAN with an increase in temperature by only a few degrees. The analysis of the small angle neutron scattering (SANS) curves unambiguously shows that this behavior is due to the fact that the EAN molecules form a substructure with a characteristic length scale of Λ ≈ 8 Å inside the EAN-in-oil droplets. In more detail, the analysis of the SANS data with the GIFT method revealed a transition from spherical to cylindrical structures approaching the respective critical endpoint temperatures. By using the respective form factors and combining them with a Gaussian spatial intensity distribution to account for the EAN sub-structure we were able to describe the scattering curves nearly quantitatively.
1 Introduction
The correlations between phase behavior, interfacial tension, microstructure and composition of microemulsions composed of water, hydrocarbons and nonionic alkyl polyglycolether surfactants (CiEj) are well understood.1–6 The influence of molecular properties such as the size of the head group or the length of the hydrocarbon chain3,5 as well as the addition of additives like electrolytes,3 alcohols7,8 and block co-polymers9,10 on the phase behavior have been studied extensively, too.In the past ten years several groups have used ionic liquids (ILs) to formulate microemulsions. Since ionic liquids exhibit unique solvent properties,11–13 these systems are interesting, for example, as reaction media.12,14 Generally speaking, the solvophobicity15 of an IL strongly depends on its molecular structure. Several examples of microemulsions with an IL as either the polar16–20 or the nonpolar21–23 component have been reported. Ethylammonium nitrate (EAN), which was in fact the first ionic liquid to be reported in the literature,24 was used as a replacement for water in microemulsions stabilized by both ionic16,17 and nonionic19 surfactants. Due to their high boiling temperature, microemulsions containing EAN (or other polar ionic liquids) can be utilized in high temperature applications.16 The microstructure of EAN-in-oil microemulsions stabilized by an ionic surfactant was studied with small angle neutron scattering (SANS) over a broad temperature range. It was found that spherical droplets are formed with radii in the range of 2 nm to 3 nm, depending on the volume of solubilized EAN while no pronounced temperature dependency was observed.17 Atkin and Warr observed a correlation peak in a small angle X-ray scattering (SAXS) curve for a microemulsion composed of equal mass fractions of EAN and n-dodecane, stabilized by the surfactant triethylene glycolmonododecylether (C12E3) and fitted this curve with the Teubner–Strey model.19 For microemulsions and lamellar phases with propylammonium nitrate (PAN) instead of EAN as well as for micelles of CiEj surfactants in EAN, SANS spectra have also been recorded.25,26
We published a systematic study on the interfacial tension, microstructure and composition of a microemulsion consisting of EAN, an n-alkane and the nonionic surfactant C12E327 to complement the data published by Atkin and Warr on the very same system.19 We were able to show that with increasing temperature the system undergoes a phase inversion with (a) the characteristic minimum of the interfacial tension at the phase inversion temperature and (b) the characteristic change of the self-diffusion coefficients of the solvents which is known from water–n-alkane–CiEj microemulsions.5,28,29 Unlike what happens in the corresponding aqueous systems, in EAN-in-oil microemulsions only a very small amount of EAN could be detected by NMR-measurements. We speculated that this behavior can be connected to the fact that EAN itself forms a substructure with a domain size of about 1 nm.30
In order to understand the different behavior of EAN-in-oil microemulsions compared to water-in-oil microemulsions and in order to clarify whether the EAN nanostructure is responsible for the difference, we studied the phase behavior and microstructure of both EAN-rich and oil-rich microemulsions. To study the properties of EAN-rich microemulsions we chose the system EAN–n-dodecane–C12E3, while the system EAN–n-octane–C12E3 was chosen to study oil-rich microemulsions. We have chosen two different oils in order to work at similar temperatures, namely between 30–37 °C. If we worked with one system only, we would have to deal with temperature differences of about 20 °C. In this case, a quantitative comparison of the data would have been difficult, since temperature has an enormous effect on the monomeric solubility of C12E3 in both n-alkanes and EAN. We measured the phase behavior of both EAN-rich and n-octane-rich microemulsions and studied the evolution of the microstructure along the respective emulsification failure boundary31,32 with SANS.
2 Experimental
2.1 Materials
Ethylammonium nitrate (EAN, purity > 97%) was purchased from Iolitec (Heilbronn, Germany) and used without further purification. The nonionic surfactant triethylene glycolmonododecylether (C12E3, purity > 95%) was purchased from TCI (Zwijndrecht, Belgium). Deuterated n-octane (D18, 98%) and n-dodecane (D26, 98%) were provided by Cambridge Isotope Laboratories (Andover, USA). The oils n-dodecane (H26, purity ≥ 99%) and n-octane (H18, purity ≥ 99%) were purchased from Sigma-Aldrich (Steinheim, Germany).2.2 Phase behavior
Samples of ∼1 ml total volume were prepared in sealed glass tubes. The masses of EAN (mA), oil (mB), and surfactant (mC) were determined with an accuracy of ±0.001 g. For the determination of the phase diagram, a magnetic stirring bar was added and the glass tubes were placed in a thermostated water bath. For a given composition, the number and type of phases were determined visually as a function of temperature with an accuracy of ±0.05 K.The phase behavior of IL-rich and oil-rich microemulsions is characterized performing vertical sections (so called funnel cuts) through the phase prism.31,32 For EAN-rich microemulsions the phase boundaries were determined as a function of temperature T and mass fraction
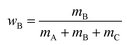 | (1) |
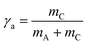 | (2) |
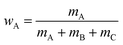 | (3) |
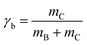 | (4) |
2.3 SANS-measurements
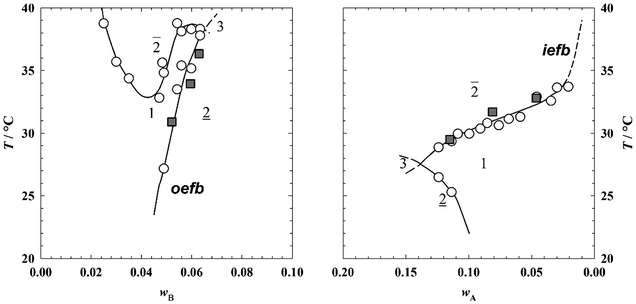
The scattering curves were recorded with the D11 spectrometer of the Institut Laue Langevin (ILL), Grenoble, France. Sample to detector distances of 1.75 m and 10.00 m with a collimation length of 8.0 m and 10.5 m, respectively, were chosen for the experiments. The wavelength of the neutrons was set to λ = 6 Å so that the scattering vector q = 4π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ/2)/λ ranged from 0.015 to 0.350 Å−1 (θ is the scattering angle). The wavelength distribution of the spectrometer is Δλ/λ = 0.09 as specified by the ILL. All samples were prepared following the procedure for the investigation of the phase behavior. With a view to adjusting film contrast conditions (equal scattering length density of EAN and oil) the protonated oils were partially replaced by deuterated oils. The mass mB of deuterated and protonated oil were chosen such that the total volume fraction of oil was equal to the volume fraction of the non-deuterated sample. Prior to the SANS experiments the phase-transition temperatures, i.e. the oil (oefb) and the IL emulsification failure boundary (iefb), were measured, which revealed that the partial deuteration of the oil causes only a slight shift of the phase boundaries (see Fig. 1). After that the samples were filled into a Hellma-quartz QS glass cell (1 mm path length) which is then transferred to the homebuilt cell holder ensuring that the sample stays homogeneous. The SANS measurements were then performed at temperatures slightly above the oefb (EAN–n-dodecane–C12E3) and slightly below the iefb (EAN–n-octane–C12E3), respectively, with an accuracy of ±0.05 K. The data were recorded using a two dimensional 3He-gas detector with 128 × 128 detector elements of 1 cm2. The intensity was radially averaged in order to obtain a one-dimensional scattering curve and the incoherent scattering of H2O was used as secondary calibration standard in order to normalize the scattering intensity. All measured scattering intensities were corrected for the background and the dead time of the detector was taken into account.
sin(θ/2)/λ ranged from 0.015 to 0.350 Å−1 (θ is the scattering angle). The wavelength distribution of the spectrometer is Δλ/λ = 0.09 as specified by the ILL. All samples were prepared following the procedure for the investigation of the phase behavior. With a view to adjusting film contrast conditions (equal scattering length density of EAN and oil) the protonated oils were partially replaced by deuterated oils. The mass mB of deuterated and protonated oil were chosen such that the total volume fraction of oil was equal to the volume fraction of the non-deuterated sample. Prior to the SANS experiments the phase-transition temperatures, i.e. the oil (oefb) and the IL emulsification failure boundary (iefb), were measured, which revealed that the partial deuteration of the oil causes only a slight shift of the phase boundaries (see Fig. 1). After that the samples were filled into a Hellma-quartz QS glass cell (1 mm path length) which is then transferred to the homebuilt cell holder ensuring that the sample stays homogeneous. The SANS measurements were then performed at temperatures slightly above the oefb (EAN–n-dodecane–C12E3) and slightly below the iefb (EAN–n-octane–C12E3), respectively, with an accuracy of ±0.05 K. The data were recorded using a two dimensional 3He-gas detector with 128 × 128 detector elements of 1 cm2. The intensity was radially averaged in order to obtain a one-dimensional scattering curve and the incoherent scattering of H2O was used as secondary calibration standard in order to normalize the scattering intensity. All measured scattering intensities were corrected for the background and the dead time of the detector was taken into account.
2.4 Scattering theory
Scattering curves contain valuable information about the microstructure of a sample. An approach aimed at extracting this information from experimental data is to calculate theoretical scattering curves using a structural model that describes the scattering of a sample of a given microstructure. By fitting the theoretical curve to the experimental data one can then obtain parameters such as the size, and the polydispersity of the structure.For a sample consisting of an ensemble of scattering particles, the scattering intensity I(q) can be modeled using the decoupling approximation.33 Following this approach, the total scattering intensity can be factorized into inter- and intra-particle scattering contributions according to
| I(q) = nP(q)S(q). | (5) |
With increasing concentration of the microemulsion droplets, inter-particle interactions contribute to the scattering intensity, which can be accounted for by using an appropriate structure factor. For spherical microemulsion droplets it was found that the averaged Percus Yevick structure factor SPY(q) for polydisperse hard spheres37,38 can be used to describe the influence of the inter-droplet interactions. However, in the system under discussion these inter-droplet interactions seem to be significantly weaker than the hard-sphere interaction considered by the Percus Yevick structure factor (Fig. 2, inlet).
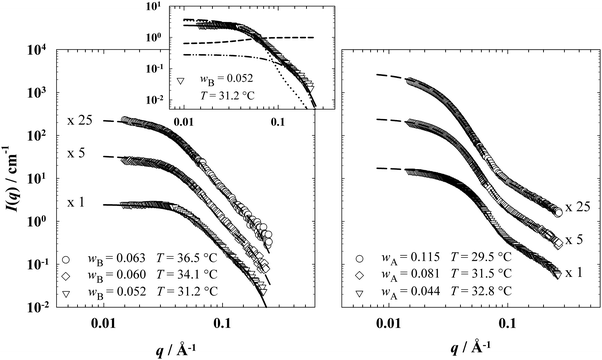 | ||
| Fig. 2 SANS curves of (left) EAN-rich microemulsions (EAN–n-dodecane–C12E3) at γa = 0.08 and different n-dodecane mass fractions wB and of (right) oil-rich microemulsions (EAN–n-octane–C12E3) at γb = 0.16 and different EAN mass fractions wA. The samples were prepared almost in film contrast using a mixture of protonated and deuterated oils. All measurements were performed at temperatures near the oefb and iefb, respectively. The inlet shows the individual scattering contributions by means of the sample with the lowest n-dodecane mass fraction wB = 0.052: form factor for polydisperse spherical droplets Psphere using a diffuse scattering length density distribution suggested by Foster36 (dotted line), averaged Percus Yevick structure factor SPY for polydisperse hard spheres38 (using a much smaller droplet volume fraction, short dashed line) and Gaussian spatial intensity distribution Igauss47,48 describing the contribution of the EAN substructure (dashed-dotted line). Note, that neglecting the inter-particle interactions the scattering data are almost quantitative described apart from small but systematic deviations at low and intermediate q. | ||
Another approach aimed at gaining structural information from the experimental scattering data is to make use of the Generalized Inverse Fourier Transformation (GIFT). Here, the scattering data are transformed into real space, yielding the pair distance distribution function (PDDF) p(r).39,40 This method does not depend on a priori information about the shape of the investigated particle. However, an appropriate structure factor has to be applied in the decoupling approximation (see eqn (5)) and hence is not model-free. In order to extract the PDDF from the experimental scattering curves the GIFT software developed by Bergmann, Fritz and Glatter41,42 was used.
3 Results and discussion
3.1 Phase behavior
Recently, we published phase diagrams of the systems EAN–n-octane–C12E3 and EAN–n-dodecane–C12E3 containing equal mass fractions of EAN and n-alkane. The phase diagrams were measured as a function of the temperature and the surfactant mass fraction.27 We found that the phase boundaries of both systems have the same fish-like shape as those known from microemulsions composed of water–n-alkane–CiEj.2 Furthermore, the shift of the phase inversion temperature (PIT) to lower temperatures when n-dodecane (![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) = 40 °C) is replaced with n-octane (
= 40 °C) is replaced with n-octane (![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) = 23 °C) is similar in both aqueous and EAN-containing microemulsion systems.2 However, we found by NMR-measurements that only a very small amount of EAN is solubilized in EAN-in-oil microemulsions, whereas a reasonable amount of oil could be detected in oil-in-EAN microemulsions.
= 23 °C) is similar in both aqueous and EAN-containing microemulsion systems.2 However, we found by NMR-measurements that only a very small amount of EAN is solubilized in EAN-in-oil microemulsions, whereas a reasonable amount of oil could be detected in oil-in-EAN microemulsions.
In order to clarify whether this behavior is related to the fact that EAN itself forms a nanostructure with a domain size of about 1 nm,30 we investigated the phase behavior and the microstructure of both EAN-rich and oil-rich microemulsions. The system EAN–n-dodecane–C12E3 was chosen to study EAN-rich microemulsions, while the system EAN–n-octane–C12E3 was chosen to study oil-rich microemulsions. In the former case, the phase behavior was studied as a function of the temperature and the n-dodecane mass fraction (wB), keeping the fraction of C12E3 in the EAN/C12E3 mixture constant at γa = 0.08. In the second case, the phase behavior was studied as a function of the temperature and the EAN mass fraction (wA), keeping the fraction of C12E3 in the n-octane/C12E3-mixture constant at γb = 0.16. As already mentioned, we decided to work with two different oils in order to work at very similar temperatures, namely between 30–37 °C, which, in turn, allows us to neglect the temperature dependent monomeric solubility of C12E3 in both EAN and oil.
On the left hand side of Fig. 1 the T–wB phase diagram of the system EAN–n-dodecane–C12E3 is shown. As can be seen, the phase behavior of the EAN-rich microemulsion is analogous to that of oil-in-water (o/w)-microemulsions.3 The funnel shaped one-phase region is limited by the (lower, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) → 1) oil emulsification failure boundary (oefb) and the (upper, 1 →
→ 1) oil emulsification failure boundary (oefb) and the (upper, 1 → ![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) near critical boundary (ncb). The slope of the oefb is rather steep, i.e. the amount of solubilized oil and thus the size of the microemulsion droplets increases moderately with increasing temperature similar to what is known for oil-in-water microemulsions.43 Starting from the cloud temperature of the binary EAN/C12E3-system at γa = 0.08 and T = 88.2 °C the ncb decreases with increasing wB and eventually runs through a narrow minimum. The existence of such a narrow minimum44 is an indication that C12E3 is neither a weak nor a strong surfactant on the EAN-rich side, which is in agreement with its rather low efficiency.27 The intersection of oefb and ncb determines the maximum amount of n-dodecane that can be solubilized in the EAN–C12E3 mixture. At higher values of wB the three-phase region exists. The phase diagram of the oil-rich microemulsion system EAN–n-octane–C12E3 is shown on the right hand side of Fig. 1. While the general trend of the phase boundaries is analogous to the aqueous counterparts stabilized by rather weak surfactants, the slope of the (upper, 1 →
) near critical boundary (ncb). The slope of the oefb is rather steep, i.e. the amount of solubilized oil and thus the size of the microemulsion droplets increases moderately with increasing temperature similar to what is known for oil-in-water microemulsions.43 Starting from the cloud temperature of the binary EAN/C12E3-system at γa = 0.08 and T = 88.2 °C the ncb decreases with increasing wB and eventually runs through a narrow minimum. The existence of such a narrow minimum44 is an indication that C12E3 is neither a weak nor a strong surfactant on the EAN-rich side, which is in agreement with its rather low efficiency.27 The intersection of oefb and ncb determines the maximum amount of n-dodecane that can be solubilized in the EAN–C12E3 mixture. At higher values of wB the three-phase region exists. The phase diagram of the oil-rich microemulsion system EAN–n-octane–C12E3 is shown on the right hand side of Fig. 1. While the general trend of the phase boundaries is analogous to the aqueous counterparts stabilized by rather weak surfactants, the slope of the (upper, 1 → ![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) IL emulsification failure boundary (iefb) is extraordinarily small. Thus, the surfactant C12E3 loses its ability to solubilize EAN within a few degrees. This unusual feature might be caused by the EAN nanostructure (correlation length of 1 nm30) which might be altered under the confinement, i.e. within the EAN-in-oil droplets. Moreover, the slope of the (lower,
) IL emulsification failure boundary (iefb) is extraordinarily small. Thus, the surfactant C12E3 loses its ability to solubilize EAN within a few degrees. This unusual feature might be caused by the EAN nanostructure (correlation length of 1 nm30) which might be altered under the confinement, i.e. within the EAN-in-oil droplets. Moreover, the slope of the (lower, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) → 1) ncb is rather steep, which is an indication that C12E3 is a rather weak surfactant44 on the oil-rich side of EAN-in-oil microemulsions.
→ 1) ncb is rather steep, which is an indication that C12E3 is a rather weak surfactant44 on the oil-rich side of EAN-in-oil microemulsions.
3.2 SANS
In order to elucidate the significantly different behavior of EAN-in-oil microemulsions compared to the well-known water-in-oil microemulsions as well as to clarify whether the EAN nanostructure is responsible for this behavior, we studied the microstructure of both EAN- and oil-rich microemulsions with SANS. In order to measure in film contrast, the scattering length density of the respective oils were adjusted to match the scattering length density of EAN (ρ(EAN) = 3.6 × 10−6 Å−2) by adding deuterated oils. Accordingly, the oils were prepared with volume fractions of ϕd-C8 = 0.58 and ϕd-C12 = 0.56 of the corresponding deuterated oils in the mixture of protonated and deuterated oil. Note that the monomeric solubility of the surfactant in the solvents (EAN and oil) was not taken into account. As can be seen in Fig. 1, the partial deuteration of the oils causes only a slight shift of the phase boundaries, which is in agreement with SANS studies on water–n-decane–CiEj microemulsions.45 In these systems a shift of the phase boundaries by +0.3 K was found if one replaces protonated by deuterated n-alkanes. The composition of the samples as well as the measuring temperatures and the fit parameters used to describe the scattering curves are compiled in Table 1. The scattering curves of EAN-rich (EAN–n-dodecane–C12E3, γa = 0.08) and oil-rich (EAN–n-octane–C12E3, γb = 0.16) microemulsions recorded at different weight fractions of n-dodecane (wB) and EAN (wA) close to the respective emulsification failure boundary are shown in Fig. 2, left and right. The incoherent scattering contribution Iincoh was subtracted from the scattering curves.| EAN–n-dodecane–C12E3 | EAN–n-octane–C12E3 | ||||||
|---|---|---|---|---|---|---|---|
| w B | 0.052 | 0.060 | 0.063 | w A | 0.044 | 0.081 | 0.115 |
| γ a | 0.08 | 0.08 | 0.08 | γ b | 0.16 | 0.16 | 0.16 |
| T/°C | 31.2 | 34.1 | 36.5 | T/°C | 32.8 | 31.5 | 29.5 |
| R 0/Å | 25 | 29 | 23 | R 0/Å | 25 | 21 | 28.5 |
| σ R | 0.4R0 | 0.4R0 | 0.4R0 | σ R | 0.4R0 | 0.4R0 | 0.4R0 |
| d/Å | 4 | 4 | 4 | d/Å | 8 | 9 | 8 |
| L 0/Å | — | — | 90 | L 0/Å | — | 100 | 135 |
| σ L | — | — | 0.4L0 | σ L | — | 0.4L0 | 0.4L0 |
| χ/Å | 2 | 2 | 2 | χ/Å | 6 | 6 | 6 |
| ϕ C,i | 0.080 | 0.078 | 0.078 | ϕ C,i | 0.070 | 0.068 | 0.062 |
| F | 1.0 | 0.9 | 1.1 | F | 0.8 | 1.2 | 1.1 |
| Δρcore/10−6 Å2 | 0.93 | 1.10 | 1.43 | Δρcore/10−6 Å2 | 1.19 | 1.61 | 1.80 |
| Δρfilm/10−6 Å2 | 1.79 | 1.79 | 1.79 | Δρfilm/10−6 Å2 | 3.58 | 3.58 | 3.58 |
| Λ/Å | 14 | 13 | 13 | Λ/Å | 8.0 | 8.0 | 8.0 |
| I 0,gauss/cm−1 | 0.28 | 0.20 | 0.20 | I 0,gauss/cm−1 | 0.28 | 0.28 | 0.28 |
The shape of the scattering curves of both the EAN-rich (left) and the oil-rich (right) microemulsions is somehow unexpected. Under film contrast conditions a more or less pronounced minimum of the scattering intensity at q ≈ π/R0 is expected to be observed. The lack of this minimum is an indication for a comparatively large polydispersity as well as for the fact that the scattering intensity contains contributions from both bulk and film contrast. Furthermore, additional scattering contributions, which might be caused by the EAN substructure known to be of the size of about 1 nm,30 can be observed at large values of the scattering vector q. Note that this contribution is particularly pronounced for the oil-rich microemulsions. However, the trends observed at low q values correspond to those known from aqueous microemulsions.34,46 Thus, approaching the respective critical endpoint temperatures one observes an increasing forward scattering intensity which points towards an increasing elongation of the structures.49
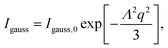 | (6) |
Thus, the total scattering intensity I of this sample (solid line) was modeled by
| I = SPY(FnPsphere + Igauss), | (7) |
As both the model analysis of the EAN-rich microemulsion with the smallest weight fraction of n-dodecane (Fig. 2, inlet) and the GIFT-analysis (see below) show only a small influence of the structure factor, we neglected the inter-particle interactions and used only the combination of form factor and Gaussian spatial intensity distribution to describe the scattering data (long dashed line). To be more precise, the scattering data of the samples with wB = 0.060 and wB = 0.063 were fitted using the form factors for polydisperse spherical and cylindrical droplets, respectively. Note that for the latter a Gaussian distribution of the cross section radius and the length of the cylindrical droplets was assumed.
In the same way the scattering data of the EAN-in-n-octane microemulsions were analyzed using the form factor for polydisperse spherical droplets (eqn (S6), ESI†) for the sample with an EAN mass fraction of wA = 0.044, while the form factor for cylindrical droplets (eqn (S8), ESI†) was used for the samples with wA = 0.081 and wA = 0.115. Taking into account the scattering contribution of the EAN substructure and neglecting contributions of the inter-particle structure factor also the scattering data of the n-octane-rich microemulsions are described almost quantitatively. Note, that the systematic deviations around q ≈ 0.09 Å−1 are again most probably caused by the fact that we neglected the cross-term of the scattering amplitudes from microemulsion droplets and the EAN substructure.
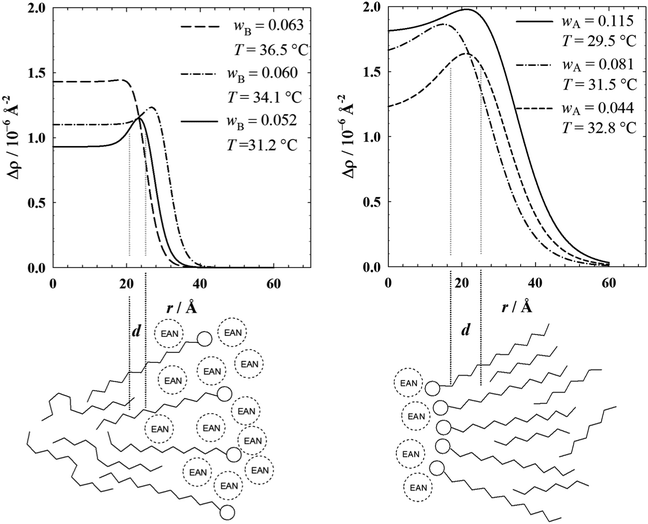
The fit parameters used to describe the scattering curves are compiled in Table 1. Interestingly, the values obtained for the characteristic length scale of the EAN substructure Λ = 13.0 Å nm (n-dodecane-in-EAN microemulsions) and Λ = 8 Å nm (EAN-in-n-octane microemulsions) are of the same order of magnitude as the domain size of 10.1 Å found by Hayes et al. in pure EAN.30 The observation, that the length scale of the EAN substructure is smaller in EAN-in-n-octane microemulsions, might be caused by two effects, namely a confinement of the EAN in the core of the EAN-in-n-octane droplets and an interaction of the EAN molecules with the surfactant head groups.
Fig. 3 illustrates the scattering length density profiles (top) calculated from the radial distribution function (eqn (S5), ESI†) with the parameters obtained from the fitting of the experimental scattering data and the schematic representations of the interfacial layer (bottom).
The scattering length density profiles of the n-dodecane-in-EAN microemulsions (left, top) clearly show that the profile of all samples is quite diffuse. It possesses a relative large core contrast Δρcore = ρbulk − ρcore although we aimed for Δρcore = 0. Furthermore, the maximum of the scattering length density profile is by more than a factor of two smaller than the value Δρfilm = 3.6 × 10−6 A2 obtained for full film contrast. Both results, namely the large core contrast and the comparably small film contrast, are a consequence of the strong penetration of the oil molecules in the alkyl chains of the surfactant and of the solvation of the surfactant head groups by EAN molecules (depicted in Fig. 3 left, bottom). The latter effect was also reported by Araos and Warr,26 who studied micelles of C12E5 in EAN. Interestingly, as a consequence of the smaller cross sectional radius the scattering length densities of core and film are almost identical in the case of the cylindrical droplets (wB = 0.063) which leads to an almost pure bulk contrast.
The scattering length density profiles of the inverse EAN-in-n-octane microemulsions (right, top) are even more diffuse. Here, too, the core contrast Δρcore differs clearly from Δρcore = 0, which suggests that the comparably small droplets have no “pure” EAN core, but rather an extended EAN solvation shell around the surfactant head groups. Due to the larger compactness of the EAN solvation shell, which might come from the inverse structure of the microemulsion droplets, the maximum of the scattering length density profile is located at a somewhat larger value of Δρ. Compared to the n-dodecane-in-EAN microemulsions, the decrease of the scattering length density is also less steep, indicating that the penetration of the shorter n-octane molecules in the alkyl chains of the surfactant is more pronounced for the inverse droplet microemulsions.
As for the model fits we first used an averaged Percus Yevick structure factor for polydisperse hard spheres.37,38 However, for all samples the influence of the structure factor on the scattering is surprisingly low, as found in the model fits. The resulting normalized p(r) functions are shown in Fig. 4. The PDDFs of the EAN-rich samples with wB = 0.052 and wB = 0.060 (left) as well as the PDDF of the oil-rich sample with wA = 0.044 (right) have a nearly symmetrical shape, which suggests that the microemulsion droplets are almost spherical.50,52 The PDDFs of the other samples are asymmetric, indicating the existence of elongated droplets.51
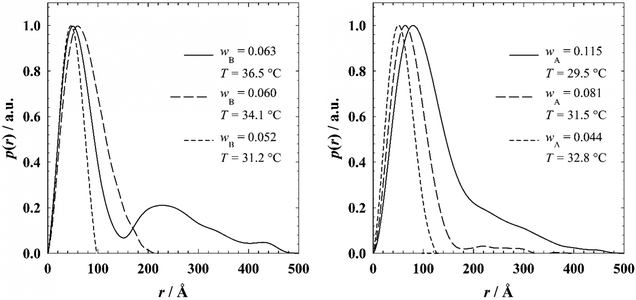 | ||
| Fig. 4 Pair distance distribution functions p(r) of (left) EAN-rich EAN–n-dodecane–C12E3 microemulsions for γb = 0.08 at different n-dodecane mass fractions wB and (right) oil-rich EAN–n-octane–C12E3 microemulsions for γa = 0.16 at different EAN mass fractions wA obtained by the GIFT analysis.41,42 | ||
For the n-dodecane-in-EAN microemulsions (Fig. 4, left), the maximum of the PDDF shifts from 46 Å at wB = 0.052 (T = 31.2 °C) to 62 Å at wB = 0.060 (T = 34.1 °C) and 50 Å at wB = 0.063 (T = 36.5 °C). The shift of the maximum to smaller values of r from 62 Å to 50 Å, also found in other microemulsion systems,53 is connected to the elongation of the initially spherical droplets. The maximum length scale of the PDDF, which corresponds either to the diameter of spherical droplets or to the length of cylindrical droplets, grows with increasing mass fraction of n-dodecane and with increasing temperature, respectively. The PDDF of the sample at wB = 0.063 (36.5 °C) shows an additional maximum at larger distances, which may be caused by the interaction of cylinders.54 Note that near the intersection of oefb and ncb, i.e. the lower critical end point temperature Tl, the formation of networks might start as it is known to occur in the corresponding water-rich microemulsions.55 For the oil-rich EAN-in-n-octane microemulsions the maximum of the PDDF shifts from a value of 52 Å at wA = 0.044 (T = 32.8 °C) to 64 Å at wA = 0.081 (T = 31.5 °C) and 80 Å at wA = 0.115 (T = 29.5 °C). Approaching the upper critical end point temperature (temperature Tu where iefb and ncb meet) by decreasing the temperature, one observes an extension of the PDDFs towards larger distances which unambiguously indicates the elongation of the droplets.
Comparing the length of the cylinders obtained from the model analysis with the maximum length scale observed in the PDDFs, it becomes obvious that the latter value is much larger. This result is not surprising as the length L0 obtained from the form factor analysis corresponds to the mean length of the polydisperse cylindrical droplets, while the maximum length scale observed in the PDDF corresponds to the length of the longest cylinders present in the sample. Additionally, we assumed a Gaussian distribution for the length of the cylindrical droplets, while according to some studies56 an exponential distribution of the cylinder length is more probable in microemulsion systems.
 | (8) |
For details see the ESI.† Furthermore, due to the diffuse nature of the scattering length profile the radius R0 obtained from the form factor analysis underestimates the radius of the droplets. In order to account for this phenomenon, we defined the radius RFFA as the distance r where the scattering length density differs by 5% from the scattering length density ρbulk of the bulk, i.e.
| Δρ(RFFA) = 0.95ρbulk. | (9) |
Another way to determine the radius of spherical microemulsion droplets is based on purely geometrical considerations. Accordingly, the radius Rcomp is given by the composition of the sample as
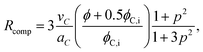 | (10) |
![[small gamma, Greek, tilde]](https://www.rsc.org/images/entities/char_e0dd.gif) which are the surfactant mass fractions at the “fish-head” and “fish-tail” in the T—γ diagram, respectively.5 According to Kahlweit et al. the monomeric solubility of nonionic surfactants in oil increases with temperature.43 Thus, a monomeric solubility γmon,b ≈ 0.095 ± 0.01 of C12E3 in n-octane was used for the EAN-in-n-octane microemulsions.
which are the surfactant mass fractions at the “fish-head” and “fish-tail” in the T—γ diagram, respectively.5 According to Kahlweit et al. the monomeric solubility of nonionic surfactants in oil increases with temperature.43 Thus, a monomeric solubility γmon,b ≈ 0.095 ± 0.01 of C12E3 in n-octane was used for the EAN-in-n-octane microemulsions.
Having determined the radius of the microemulsion droplets for different samples along both the oefb and the iefb, we will discuss the evolution of the characteristic domain size ξ (note that ξ = R for droplet microemulsions along the respective efb) with the temperature in the following. For microemulsions of the type water–n-alkane–CiEj it has been shown that the domain size runs through a distinct maximum at the phase inversion temperature ![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) , where the microstructure is known to be bicontinuous.4 In order to prove whether or not this trend is also found for microemulsions containing EAN, the radii obtained by the analysis of the SANS curves and from the composition are plotted as a function of the temperature T–
, where the microstructure is known to be bicontinuous.4 In order to prove whether or not this trend is also found for microemulsions containing EAN, the radii obtained by the analysis of the SANS curves and from the composition are plotted as a function of the temperature T–![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) in Fig. 5 and compiled in Table 2. The relative temperature T–
in Fig. 5 and compiled in Table 2. The relative temperature T–![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) is used to compare the temperature dependence of the domain size of different systems. Since the system EAN–n-octane–C12E3 exhibits a very similar behavior as the system water–n-octane–C8E3 with respect to efficiency, and interfacial tension,27,35 the temperature dependence of the domain size of the latter system (stars) is included in Fig. 5.
is used to compare the temperature dependence of the domain size of different systems. Since the system EAN–n-octane–C12E3 exhibits a very similar behavior as the system water–n-octane–C8E3 with respect to efficiency, and interfacial tension,27,35 the temperature dependence of the domain size of the latter system (stars) is included in Fig. 5.
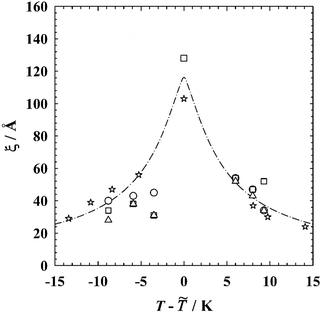 | ||
Fig. 5 Characteristic domain size ξ plotted as a function of the relative temperature T–![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) for the systems EAN–n-dodecane–C12E3 and EAN–n-octane–C12E3. Shown are the radii obtained from GIFT (RGIFT, triangles), form factor and Teubner–Strey34 analysis (RFFA, squares), and composition (Rcomp, circles). The temperature variation of the domain size in the system water–n-octane–C8E3 is shown for comparison (stars).32 The drawn line describes the temperature variation of the principal curvatures of the amphiphilic film.3,32 for the systems EAN–n-dodecane–C12E3 and EAN–n-octane–C12E3. Shown are the radii obtained from GIFT (RGIFT, triangles), form factor and Teubner–Strey34 analysis (RFFA, squares), and composition (Rcomp, circles). The temperature variation of the domain size in the system water–n-octane–C8E3 is shown for comparison (stars).32 The drawn line describes the temperature variation of the principal curvatures of the amphiphilic film.3,32 | ||
| EAN–n-dodecane–C12E3 | EAN–n-octane–C12E3 | ||||||
|---|---|---|---|---|---|---|---|
| w B | 0.052 | 0.060 | 0.063 | w A | 0.044 | 0.081 | 0.115 |
| T/°C | 31.2 | 34.1 | 36.5 | T/°C | 32.8 | 31.5 | 29.5 |
| R FFA/Å | 34 | 38 | 31 | R FFA/Å | 52 | 47 | 54 |
| R GIFT/Å | 28 | 38 | 31 | R GIFT/Å | 34 | 43 | 52 |
| R comp/Å | 40 | 43 | 45 | R comp/Å | 34 | 47 | 54 |
As can be seen, both systems, the EAN and the aqueous microemulsion, show the same general trend. The maximum domain size ξmax = 128 Å found for the bicontinuous EAN microemulsion35 is slightly larger than the maximum domain size of the ternary system water–n-octane–C8E3 where ξmax = 103 Å.32 This result is in line with the finding that, although the two surfactants have a similar efficiency, the monomeric solubility of C12E3 in EAN and n-octane is much larger than the monomeric solubility of C8E3 in water and n-octane, which results in a smaller internal interface and thus in a larger domain size. All in all, the droplet radii RGIFT, RFFA and Rcomp obtained from the GIFT and form factor analysis of the SANS data as well as from the composition are in almost quantitative agreement (within ±6 Å, except the samples at wB = 0.063 and wA = 0.044) despite the approximations made.
Examining the data more thoroughly, one finds that in the case of aqueous microemulsions the droplets on the water-rich side are slighly larger than on the oil-rich side. This small but systematic difference indicates that in aqueous microemulsions nonionic surfactants act less strongly on the oil-rich side compared to the water-rich side, which can partly be ascribed to the higher temperature. For the EAN microemulsions the droplets on the EAN-rich side seem to be smaller than on the oil-rich side. This apparently different behavior of aqueous and EAN microemulsions is, however, caused by the fact that different EAN-microemulsion systems are studied on the EAN-rich and oil-rich side as explained above. As C12E3 solubilizes n-octane more efficiently than n-dodecane,27 the n-dodecane-in-EAN droplets are smaller than the EAN-in-n-octane droplets.
Finally, the evolution of the domain size with increasing temperature is discussed in more detail. We recall that the microstructure of the samples studied close to the respective critical endpoint temperatures corresponds to cylindrical structures. Thus, the domain size corresponds to the cross sectional radius of cylinders and not to the radius of droplets. Consequently, a slight jump in the ξ(T–![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) )-curve is found in the temperature regimes where the microstructure changes its geometry. A comparison of the slope of the data points on the EAN- and oil-rich side reveals that the increase of the droplet radius observed with increasing temperature for n-dodecane-in-EAN microemulsions is much less pronounced than the decrease found for EAN-in-n-octane microemulsions. This result is in perfect agreement with the different slopes of the oefb and the iefb in the phase diagrams (Fig. 1). While the oefb is rather steep, i.e. the amount of solubilized oil increases moderately with increasing temperature, the iefb has an extraordinarily small slope, which suggests that the amphiphilic film loses its ability to solubilize EAN with an increase in temperature by only a few degrees. Using SANS we were able to prove that this quite extraordinary behavior is a consequence of the fact that the EAN molecules form a substructure with a characteristic length scale of Λ = 8 Å inside the EAN-in-oil droplets. Furthermore, these results also explain NMR-data published in previous studies27,34 where hardly or even no EAN could be detected on the oil-rich side at temperatures above the phase inversion temperature.
)-curve is found in the temperature regimes where the microstructure changes its geometry. A comparison of the slope of the data points on the EAN- and oil-rich side reveals that the increase of the droplet radius observed with increasing temperature for n-dodecane-in-EAN microemulsions is much less pronounced than the decrease found for EAN-in-n-octane microemulsions. This result is in perfect agreement with the different slopes of the oefb and the iefb in the phase diagrams (Fig. 1). While the oefb is rather steep, i.e. the amount of solubilized oil increases moderately with increasing temperature, the iefb has an extraordinarily small slope, which suggests that the amphiphilic film loses its ability to solubilize EAN with an increase in temperature by only a few degrees. Using SANS we were able to prove that this quite extraordinary behavior is a consequence of the fact that the EAN molecules form a substructure with a characteristic length scale of Λ = 8 Å inside the EAN-in-oil droplets. Furthermore, these results also explain NMR-data published in previous studies27,34 where hardly or even no EAN could be detected on the oil-rich side at temperatures above the phase inversion temperature.
4 Conclusions
In aqueous microemulsion systems the spontaneous curvature depends nearly linearly on temperature and changes its sign at the phase inversion temperature (PIT).4,29,32 As a consequence, the temperature dependence of the phase behavior, interfacial tension and microstructure is symmetrical with respect to the PIT.4,29,32 Although the same general features are found in ternary EAN–n-alkane–surfactant systems,15,23 only a very small amount of EAN is solubilized in oil-rich microemulsions. In order to elucidate whether the asymmetric behavior of EAN microemulsions with respect to the PIT can be ascribed to the presence of an EAN substructure in the EAN droplets, we studied the properties of both oil-in-EAN and EAN-in-oil microemulsions.Studying the phase behavior, we found that the oil emulsification failure boundary (oefb) is rather steep, while the slope of the IL emulsification failure boundary (iefb) is extraordinarily small. The different slopes suggest that the surfactant C12E3 is able to continuously solubilize more and more oil when the PIT is approached, while it loses its ability to solubilize EAN within a few degrees once the PIT is exceeded.
In order to study whether the variation of the spontaneous curvature with temperature in EAN microemulsions is linear or not, we investigated the evolution of the nanostructure along the respective efbs by SANS. The pair distance distribution functions (PDDF) obtained from the GIFT analysis show a transition from spherical to cylindrical structures approaching the respective critical endpoint temperatures. Thereby, the influence of the structure factor on the scattering is surprisingly low, indicating that inter-droplet interactions are much less pronounced than hard sphere interactions. Applying the respective form factors for polydisperse spherical and cylindrical droplets, a Gaussian spatial intensity distribution had to be used to describe the additional scattering contribution at large q. Assigning this contribution to the EAN substructure, we found, that its length scale Λ is comparable to the domain size of 10.1 Å found by Hayes et al. in pure EAN.30 The fact, that the length scale is smaller in EAN-in-oil microemulsions (Λ = 8 Å) than in oil-in-EAN microemulsions (Λ = 13.0 Å) is attributed to the confinement of the EAN molecules in the core of the EAN-in-oil droplets and their interaction with the surfactant head groups.
Finally, the evolution of the domain size with increasing temperature prove our hypothesis of a non-linear variation of the spontaneous curvature in EAN microemulsions. Related to the urge of the EAN molecules to form their own substructure, the radius of the EAN-swollen droplets decreases strongly within a narrow temperature range of only 3 K, while the radius of oil-swollen droplets varies in a less pronounced way over a wider temperature range if the phase inversion is approached.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the German Research Foundation (DFG) for funding (project STU287/3-1). We also thank Diana Zauser, Stefan Lülsdorf, Dr Yvonne Pütz and Dr Lena Grassberger for valuable help with the SANS measurements. Furthermore, we would like to acknowledge the Institut Laue Langevin (ILL) in Grenoble (France) in providing the facilities for the SANS measurements and the valuable support of the local contacts Dr Ralf Schweins and Dr Peter Lindner of the D11 spectrometer.References
- J. H. Schulman, W. Stoeckenius and L. M. Prince, J. Phys. Chem., 1959, 63, 1677–1680 CrossRef CAS.
- M. Kahlweit and R. Strey, Angew. Chem., Int. Ed. Engl., 1985, 24, 654–668 CrossRef.
- M. Kahlweit, R. Strey, P. Firman, D. Haase, J. Jen and R. Schomäcker, Langmuir, 1988, 4, 499–511 CrossRef CAS.
- R. Strey, Colloid Polym. Sci., 1994, 272, 1005–1019 CrossRef CAS.
- S. Burauer, T. Sachert, T. Sottmann and R. Strey, Phys. Chem. Chem. Phys., 1999, 1, 4299 RSC.
- T. Sottmann and R. Strey, J. Chem. Phys., 1997, 106, 8606 CrossRef CAS.
- M. Kahlweit, R. Strey and G. Busse, J. Phys. Chem., 1991, 95, 5344 CrossRef CAS.
- M. Penders and R. Strey, J. Phys. Chem., 1995, 99, 10313 CrossRef CAS.
- B. Jakobs, T. Sottmann, R. Strey, J. Allgaier and D. Richter, Langmuir, 1999, 15, 6707 CrossRef CAS.
- T. Sottmann, Curr. Top. Colloid Interface Sci., 2002, 7, 57 CrossRef CAS.
- O. Zech and W. Kunz, Soft Matter, 2011, 7, 5507 RSC.
- Z. Qiu and J. Texter, Curr. Top. Colloid Interface Sci., 2008, 13, 252 CrossRef CAS.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709 RSC.
- M. Moniruzzaman, N. Kamiya and M. Goto, Langmuir, 2009, 25, 977 CrossRef CAS.
- A. Ray, Nature, 1971, 231, 313 CrossRef CAS.
- O. Zech, S. Thomaier, A. Kolodziejski, D. Touraud, I. Grillo and W. Kunz, Chem. – Eur. J., 2010, 16, 783 CrossRef CAS.
- O. Zech, S. Thomaier, A. Kolodziejski, D. Touraud, I. Grillo and W. Kunz, J. Colloid Interface Sci., 2010, 347, 227 CrossRef CAS.
- O. Zech, P. Bauduin, P. Palatzky, D. Touraud and W. Kunz, Energy Environ. Sci., 2010, 3, 846 RSC.
- R. Atkin and G. G. Warr, J. Phys. Chem. B, 2007, 111, 9309 CrossRef CAS.
- Y. Gao, N. Li, L. Zheng, X. Zhao, J. Zhang, Q. Cao, M. Zhao, Z. Li and G. Zhang, Chemistry, 2007, 13, 2661 CrossRef CAS.
- Y. Gao, S. Han, B. Han, G. Li, D. Shen, Z. Li, J. Du, W. Hou and G. Zhang, Langmuir, 2005, 21, 5681 CrossRef CAS PubMed.
- N. Anjum, M.-A. Guedeau-Boudeville, C. Stubenrauch and A. Mourchid, J. Phys. Chem. B, 2009, 113, 239 CrossRef CAS.
- J. H. Porada, M. Mansueto, S. Laschat and C. Stubenrauch, Soft Matter, 2011, 7, 6850 RSC.
- P. Walden, Bull. Acad. Imp. Sci. Petrograd, 1914, 8, 405 Search PubMed.
- R. Atkin, S. M. C. Bobillier and G. G. Warr, J. Phys. Chem. B, 2010, 114, 1350 CrossRef CAS.
- M. U. Araos and G. G. Warr, Langmuir, 2008, 24, 9354 CrossRef CAS.
- J. C. Thater, V. Gérard and C. Stubenrauch, Langmuir, 2014, 30, 8283 CrossRef CAS PubMed.
- M. Kahlweit, R. Strey, D. Haase, H. Kunieda, T. Schmeling, B. Faulhaber, M. Borkovec, H.-F. Eicke, G. Busse, F. Eggers, T. Funck, H. Richmann, L. Magid, O. Söderman, P. Stilbs, J. Winkler, A. Dittrich and W. Jahn, J. Colloid Interface Sci., 1987, 118, 436 CrossRef CAS.
- B. Lindman and U. Olsson, Bunsen-Ges. Phys. Chem., Ber., 1996, 100, 344–363 CrossRef CAS.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Phys. Chem. Chem. Phys., 2011, 13, 3237 RSC.
- R. Strey, Bunsen-Ges. Phys. Chem., Ber., 1996, 100, 182–189 CrossRef CAS.
- T. Sottmann and R. Strey, in Fundamentals of Interface and Colloid Science, ed. J. Lyklema, Elsevier Academic Press, Heidelberg, 2005, p. 1 Search PubMed.
- E. W. Kaler, NATO ASI Ser., Ser. C, 1995, 451, 329–353 CAS.
- T. Foster, T. Sottmann, R. Schweins and R. Strey, J. Chem. Phys., 2008, 128, 064902 CrossRef PubMed.
- J. C. Thater, T. Sottmann and C. Stubenrauch, Colloids Surf., A, 2016, 494, 139–146 CrossRef CAS.
- T. Foster, J. Phys. Chem. B, 2011, 115, 10207 CrossRef CAS PubMed.
- J. K. Percus and G. J. Yevick, Phys. Rev., 1958, 110, 1–13 CrossRef CAS.
- B. Weyerich, J. Brunner-Popela and O. Glatter, J. Appl. Crystallogr., 1999, 32, 197–209 CrossRef CAS.
- O. Glatter, J. Appl. Crystallogr., 1979, 12, 166–175 CrossRef CAS.
- O. Glatter, J. Appl. Crystallogr., 1980, 13, 7 CrossRef CAS.
- A. Bergmann, G. Fritz and O. Glatter, J. Appl. Crystallogr., 2000, 33, 1212–1216 CrossRef CAS.
- G. Fritz, A. Bergmann and O. Glatter, J. Chem. Phys., 2000, 113, 9733–9740 CrossRef CAS.
- M. Kahlweit and G. Busse, J. Phys. Chem., 1990, 94, 3881–3894 CrossRef CAS.
- M. Kahlweit, R. Strey and G. Busse, Phys. Rev., 1993, 47, 4197 CAS.
- M. Gotter, T. Sottmann, M. Baciu, U. Olsson, H. Wennerström and R. Strey, Eur. Phys. J. E: Soft Matter Biol. Phys., 2007, 24, 277 CrossRef CAS.
- T. Foster, T. Sottmann, R. Schweins and R. Strey, J. Chem. Phys., 2008, 128, 54502 CrossRef.
- M. Shibayama, T. Tanaka and C. C. Han, J. Chem. Phys., 1992, 97, 6829 CrossRef CAS.
- M. Shibayama, T. Tanaka and C. C. Han, J. Chem. Phys., 1992, 97, 6842 CrossRef CAS.
- O. Glatter, R. Strey, K.-V. Schubert and E. W. Kaler, Ber. Bunseng. Phys. Chem., 1996, 100, 323 CrossRef CAS.
- J. Brunner-Popela and O. Glatter, J. Appl. Crystallogr., 1997, 30, 431 CrossRef CAS.
- J. Brunner-Popela, R. Mittelbach, R. Strey, K.-V. Schubert, E. W. Kaler and O. Glatter, J. Chem. Phys., 1999, 110, 10623 CrossRef CAS.
- R. Strey, O. Glatter, K.-V. Schubert and E. W. Kaler, J. Chem. Phys., 1996, 105, 1175 CrossRef CAS.
- A. Müller, Y. Pütz, R. Oberhoffer, N. Becker, R. Strey, A. Wiedenmann and T. Sottmann, Phys. Chem. Chem. Phys., 2014, 16, 18092 RSC.
- O. Glatter, G. Fritz, H. Lindner, J. Brunner-Popela, R. Mittelbach, R. Strey and S. U. Egelhaaf, Langmuir, 2000, 16, 8692–8701 CrossRef CAS.
- T. Tlusty, S. A. Safran and R. Strey, Phys. Rev. Lett., 2000, 84, 1244 CrossRef CAS.
- M. E. Cates and S. J. Candau, J. Phys.: Condens. Matter, 1990, 2, 6869 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cp06228e |
| This journal is © the Owner Societies 2019 |