Efficient electrospinning fabrication and the underlying formation mechanism of one-dimensional monoclinic Li2FeSiO4 nanofibers†
Abstract
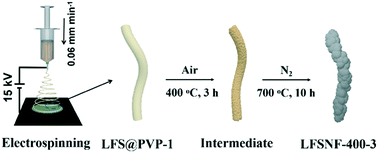
The purposeful exploration of efficient methodologies towards synthesizing phase-pure multi-compositional nanostructures is an everlasting topic for material scientists. In this work, we first devise a smart strategy to fabricate high-purity Li2FeSiO4 nanofibers (LFSNFs) with a P21/n structure via controllable electrospinning and subsequent calcination in air and N2 atmospheres. Systematic physicochemical characterization confirms the significant roles of the regulation of polyvinyl pyrrolidone (PVP) content via pretreatment in air, and concentrations of inorganic salts in the formation of one-dimensional LFSNFs. Specifically, the excess PVP-derived carbon content will result in the carbon-thermal reduction of high-valence Fe species, and high concentrations of precursors will increase the diameter of the nanofibers, and even induce the conversion from nanofibers to nanobelts. Finally, the optimum synthetic parameters are convincingly proposed. More significantly, we strongly believe that the unusual formation mechanism of the LFSNFs will have enormous potential in enriching synthetic methods of polyoxyanion nano-architectures.


 Please wait while we load your content...
Please wait while we load your content...