Self-assembly of Pt nanocrystals into three-dimensional superlattices results in enhanced electrocatalytic performance for methanol oxidation†
Abstract
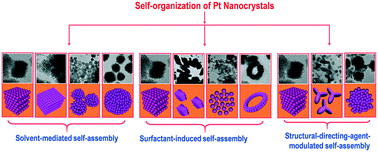
Because of the collective effects emerging from integrated nanostructures, more and more research endeavors have been dedicated to realizing the self-organization of nanostructures into complex integrated architectures with multiple functionalities. Thus, the self-organization of nanostructures into higher-order superstructures is becoming an attractive theme in materials research of nanoscience. Herein, we develop a simple and low-temperature solution approach without the need of any preformed Pt seeds to directly realize a series of three-dimensional (3D) Pt nanocrystal superlattices (NSLs) composed of well-defined interior Pt nanocrystals assembled into 3D face-centered cubic (fcc) superlattice structures. The effects of experimental parameters including solvents, surfactants and structure-directing agents on the assembly behavior, structural configuration, and morphological arrangement of 3D Pt NSLs are systematically studied. Through modulating the experimental parameters, the self-organization of 3D Pt NSLs can be elegantly controlled, and an in-depth mechanism depicting the pathway for Pt nanocrystals self-organized into various 3D Pt NSLs is proposed. The optimal 3D Pt NSLs used as an efficient electrocatalyst exhibit excellent catalytic performance with CO-tolerant catalytic ability and long-term stability for the methanol oxidation reaction (MOR) with a mass activity of 403 mA mgPt−1, which is 2 times higher than that of a commercial 20% Pt/C electrocatalyst (200 mA mgPt−1). This work provides a pathway to realize robust higher-order 3D Pt NSLs with enhanced electrocatalytic performance for the MOR. The long-term stability and the CO-tolerant catalytic ability of 3D Pt NSLs are anticipated to lead to an ideal system with relevance to applications in the renewable energy field.


 Please wait while we load your content...
Please wait while we load your content...