MnCo2O4+x nanowire arrays grown on carbon sponge: improved electrochemical and catalytic performances
Abstract
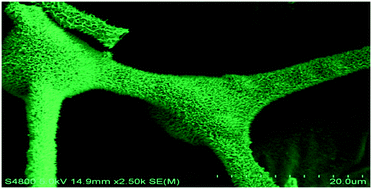
MnCo2O4+x nanowire arrays have been successfully grown on carbon sponge (CS) via a simple hydrothermal route with sequential thermal treatment, employing CoSO4·7H2O, MnSO4·H2O and urea as the initial reactants. The as-prepared product, labeled MnCo2O4+x/CS, was characterized by X-ray powder diffraction (XRD), field emission scanning electron microscopy (FESEM), (high resolution) transmission electron microscopy (TEM/HRTEM), nitrogen adsorption–desorption isotherms, Raman spectrometry and X-ray photoelectron spectroscopy (XPS). The experiments showed that the presence of CS could affect the phase and morphology of the final manganese cobaltite. Without the presence of CS, a MnCo2O4.5 phase with spherical microstructures was produced; and with the presence of CS, a mixed phase of MnCo2O4.5 and MnCo2O4 with wire-like nanostructures was obtained. It was found that the final MnCo2O4+x/CS markedly improved the electrochemical and catalytic properties against MnCo2O4.5. At a current density of 1.0 A g−1, the as-prepared product exhibited a specific capacitance of 405 F g−1, which was far higher than 158.8 F g−1 of pure MnCo2O4.5. Under the same experimental conditions, simultaneously, the MnCo2O4+x/CS catalyst only took ∼6 min for catalyzing 92% of 4-nitrophenol (4-NP) to 4-nitroaniline (4-AP), which was shorter than ∼10 min of the pure MnCo2O4.5 catalyst.


 Please wait while we load your content...
Please wait while we load your content...