Highlights from Faraday Discussion 301: Nanolithography of Biointerfaces, London, UK, July 3–5 2019
Clare S.
Mahon
 *a and
Mia L.
Huang
*a and
Mia L.
Huang
 *b
*b
aDepartment of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: clare.mahon@durham.ac.uk
bDepartment of Molecular Medicine, Scripps Research, 130 Scripps Way, Jupiter, FL, USA. E-mail: miahuang@scripps.edu
First published on 4th November 2019
Abstract
The 2019 Faraday Discussion on the Nanolithography of Biointerfaces brought together a diverse set of interdisciplinary scientists involved in the seemingly disparate fields of materials science, nanolithography and glycoscience. The setting and format of this meeting renders the experience unique, and anyone in the audience is instantly engaged in the debate. This Faraday Discussion attracted about sixty delegates, ranging from graduate students and early career researchers to full professors. The meeting was a reflection on how far lithography techniques, tissue engineering and glycoscience have come, with the aid of scientists working at the realm of the nanoscale. True to its name, this gathering was also a discussion on what the outstanding questions in glycobiology are and how nanolithography can be appropriately applied to answer them. In this report, we will give an overview of the topics and discussions covered during the meeting and highlight the content of each session.
Introduction
On July 3rd, 2019, around 60 delegates from multiple continents arrived in London to attend the 301st Faraday Discussion meeting, dedicated to the Nanolithography of Biointerfaces (Fig. 1). In an unusual occurrence, London welcomed us with sunny skies and warm temperatures, which pervaded throughout the three days of the meeting. The conference took place in the library of the headquarters of the Royal Society of Chemistry (RSC), within the beautiful and historic Palladian-style architecture of Burlington House in Piccadilly. The Faraday Discussions are celebrating more than a century since their inception in 1907, and they have gained a reputation as a platform for presenting and discussing state-of-the art technologies and for spawning new fields.1The theme for our Faraday Discussion explored questions of fundamental biological importance, with scientists from different disciplines presenting diverse, yet often complementary approaches to further our understanding of biological interfaces. Increasingly, biological interfaces including cellular surfaces are recognized as key arenas for many processes of heath and disease. The living cell exists as a pre-disposed intricate network of biomolecules at the microscale. Within the cell or on the cell, and amongst proteins, nucleic acids and lipids, are glycan molecules that are arranged in particular densities and three-dimensional coordinates at the nanoscale. These glycan molecules represent an important frontier that we as a community have yet to conquer, although research interest in this area is expanding rapidly. Whereas proteins and nucleic acids are directly templated from the genetic code, glycans are installed by biosynthetic enzymes as post-translational modifications on proteins and lipids. Although these protein enzymes are encoded by the genome, the frequency and site of the glycan modifications they impart can be unpredictable. Yet, we know that defects in the glycan biosynthetic machinery occur with deleterious consequences towards human health. We have long marvelled at this arrangement of glycans and have sought to dismantle its complexity. Studies to probe, mimic and recapitulate these arrangements have been intensely pursued in the past decade. The maturation of nanolithography, or the process of printing matter at the 1–100 nm scale, has been highly critical for the precise immobilization of molecules onto artificial surfaces, allowing us to generate synthetic interfaces with levels of complexity approaching those seen in nature. Thus, this Faraday Discussion is immediately relevant and the ensuing debate is timely.
This Faraday Discussion is loosely grouped into four sessions: (1) printing technologies, (2) glycan arrays, (3) glycan interactions and (4) surface functionalization. A total of 14 papers were submitted and published in this volume to discuss the latest advances in these areas. Perhaps what we most appreciate about the Faraday Discussion is not just the presentation of new techniques, but its forum to confront what is lacking in the field and how it can be further advanced to interrogate important questions. After all, one of the primary reasons we create new technologies is to create model systems that understand the complexity found within complex biological systems and allow their systematic, rigorous study.
Opening lecture
After an introduction to the Faraday Discussions format (Fig. 2) by Adam Braunschweig (City University of New York), the introductory lecture was provided by Prof. Peter Seeberger (Max Planck Institute of Colloids and Interfaces) who provided an overview of his laboratory's work, starting from the development of organic reactions and towards the study of carbohydrate–carbohydrate interactions that are being used to create new materials. Peter starts with a quote from Frederich Wohler, “Organic chemistry just now is enough to drive one mad. It gives me the impression of a primeval forest full of the most remarkable things, a monstrous and boundless thicket, with no way of escape, into which one may well dread to enter2” to depict how the synthesis of biomolecules have stumped many organic chemists in the past. While the automated chemical synthesis of nucleic acids9 and peptides10 have been long established and are now often viewed as routine, such a feat for carbohydrates has only been accomplished by the Seeberger group in recent years.11 The automated preparation of peptides and oligonucleotides not only revolutionized the field of biochemistry, but provided new inspiration to those working in areas as diverse as materials chemistry and nanoscience, by providing easy access to high purity materials to allow their study, or use as building blocks for subsequent assembly of more complex structures. The streamlined, automated production of oligosaccharides presents a far tougher challenge. With multiple reactive sites and potential branching points and the need for complex protection strategies, the chemistry that underpins the synthesis of oligosaccharides is non-trivial. The potential prize, however, is at least as enticing.The Seeberger group has been continually refining their processes to broaden the scope and improve the yields of oligosaccharides prepared through automated assembly. Their success has translated to a commercially available automated oligosaccharide synthesizer, the Glyconeer, designed to allow in-house production of bespoke glycans with minimal synthetic manipulation. As Peter told us, these days the Glyconeer can achieve the synthesis of a 100-mer polysaccharide using 9 g of building blocks with 30 minute coupling reactions. Already, the Seeberger group has explored a vast scope of applications for these glycans, including the production of glycan microarrays, as candidates for vaccine development and providing building blocks for the development of new materials.3
Session 1: Multidimensional micro- and nano-printing technologies
The development of printing technologies has been critical to the advancement of microarrays in biology – starting from the use of inkjet methods to transfer DNA molecules4 in gene chips and into glycan microarrays to mimic cell surfaces. Parameters such as throughput, immobilization efficiency, spatial resolution and bioactivity are important to consider when fabricating such surfaces. Particularly in glycoscience, the invention of high-precision printing technologies has been highly enabling for the study of multivalency and three-dimensional presentation in glycan binding interactions. To kick off the first session chaired by Adam Braunschweig, Elisa Riedo (New York University) described the use of thermochemical scanning probe lithography to pattern enzymes in high-throughput and sub-10 nm resolution (DOI:10.1039/C9FD000025A, Fig. 3A). In this technique, the scanning probe lithography method is integrated with thermochemistry to immobilize and pattern an anionic enzyme onto cationic amino-functionalized surfaces via electrostatic interactions. In this example, the authors generated a sulfonate-conjugated thermolysin enzyme to generate an anionic protein. During the discussion, we learned that the curvature of the tip and the thermal conductivity of the substrate were important to achieve the high-resolution patterns and, that after two days of use, the heat does start to wear-off the performance of the tip. Nevertheless, the enzymes in this platform remain active in this setting.This talk was followed by Nathan Gianneschi (Northwestern University), whose group is using cyclic peptides as “biological inks” to create artificial tissues (DOI: 10.1039/C9FD00026G, Fig. 3B). In this platform, the cyclic peptides are immobilized using a nanoprinter. The macrocyclic constraint of these molecules prevents them from gelling, such that incubation with light and a matrix metalloproteinase enzyme results in the cleavage of the macrocyclic amide bond and converts the molecule into a linear format from which they can assemble and form hydrogels. These stimuli-responsive materials can be integrated with state-of-the art techniques such as liquid TEM and MALDI imaging mass spectrometry.
The third speaker was Alshakim Nelson (University of Washington). Prior to starting his independent laboratory, Alshakim spent ten years at IBM technologies. Now, aiming to recreate functionalities derived from cells using synthetic systems, he leverages his background to create poly(alkyl glycidyl ether) hydrogels that can immobilize engineered yeast cells for whole-cell catalysis (DOI: 10.1039/C9FD00019D, Fig. 3C). His group is deriving the ability of yeast cells to ferment (i.e. convert sugars to ethanol and energy) to generate “additive manufacturing” where a yeast mating pheromone called α-factor, is manufactured in a continuous flow format.
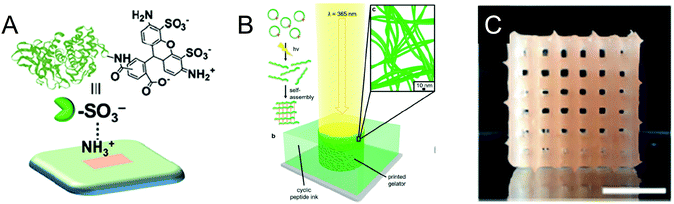 | ||
| Fig. 3 Summary of Session 1 papers. (A) Scanning probe lithography and thermochemistry were used to immobilize a sulfonate-conjugated thermolysin enzyme to cationic amine-functionalized surfaces in sub-10 nm resolution (DOI:10.1039/C9FD000025A). (B) UV-responsive cyclic peptides linearize and self-assemble to form inks that can gelate upon printing (DOI: 10.1039/C9FD00026G). (C) 3D-printed yeast-laden hydrogel generated from poly(alkyl glycidyl ether) polymers (DOI: 10.1039/C9FD00019D). | ||
Session 2: Preparation of multivalent glycan micro- and nano-arrays
In this session chaired by Laura Hartmann (Heinrich-Heine-Universitat, Germany), we dig deeper into the factors contributing to array performance and variability. Following the initial wave of publications describing glycan microarrays and the demand for the CFG (Consortium for Functional Glycomics) array,5 the (inter)national resource for glycan arrays, many groups started fabricating their own. Over a hundred combinations of immobilization chemistries, linker moieties and solid supports were used, and as a result, investigators started observing differences in their glycan binding data, and the systematic comparison of different formats has started to shed light onto what factors might be contributing to these variances. In a surprising observation, the density of glycan patterning onto the solid supports, which affects glycan multivalency, was among the most important contributors. Daniel Valles (City University of New York), a graduate student from the Braunschweig Laboratory, first presented the use of variable-density glycan microarrays to interrogate the binding characteristics of the plant lectin, Concanavalin A (Con A) (DOI: 10.1039/C9FD00028C). The variable density arrays were created using photo-induced printing of monosaccharides harboring alkene moieties onto thiol-terminating substrates via a thiol–ene click reaction (Fig. 4A). Remarkably, the printing device consisted of 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mirrors and microfluidic flow was used to introduce the alkene monosaccharides. The spots were printed with 7 nm distances between each other.
000 mirrors and microfluidic flow was used to introduce the alkene monosaccharides. The spots were printed with 7 nm distances between each other.
Jeffrey Gildersleeve (National Cancer Institute) then followed to discuss the factors contributing to variable measurements observed by different groups using glycan microarrays (DOI: 10.1039/C9FD00021F). The group performed a systematic comparison of microarray data using eight different lectins between the CFG array and a neoglycoprotein array platform, which the Gildersleeve group uses (Fig. 4B). The CFG array uses N-hydroxysuccinimide functionalized slides to immobilize amine-terminated glycans, whereas the neoglycoprotein array consists of glycans covalently conjugated onto the bovine serum albumin protein, and it is this protein-glycoconjugate that is immobilized onto surfaces. In summary, the group found that differences in glycan density and linker composition were highly contributing factors towards binding variability. Their results will provide context for the interpretation of microarray data between these two platforms and will instruct the design of future glycan microarrays.
Clare Mahon (University of Leeds) then presented her paper on the use of reversible glycopolymers that ‘catch’ the cholera toxin proteins from solution and ‘release’ them following stimulation, for applications in water purification (DOI: 10.1039/C9FD00017H, Fig. 4C). The cholera toxin B subunit (CTB) exhibits high affinity for the glycan pentasaccharide GM1os. Leveraging this interaction, the authors used the reducing ends of GM1os to covalently attach them to a co-polymer bearing side chains of hydrazine and N-isopropylacrylamide (NIPAm) groups. The NIPAm domains endow the polymer with thermoresponsive characteristics as these polymers exhibit a lower critical solution temperature (LCST). At temperatures below the LCST the polymer exposes its glycan groups to facilitate high-affinity binding with CTB in solution. Above the LCST polymers undergo hydrophobic collapse and cluster, sequestering recognition motifs away from the surface of the globule, leading to a drop in avidity of interaction with CTB. Beads formulated from these multi-responsive polymers were able to isolate CTB from a complex mixture of Vibrio conditioned medium upon treatment at 60 °C.
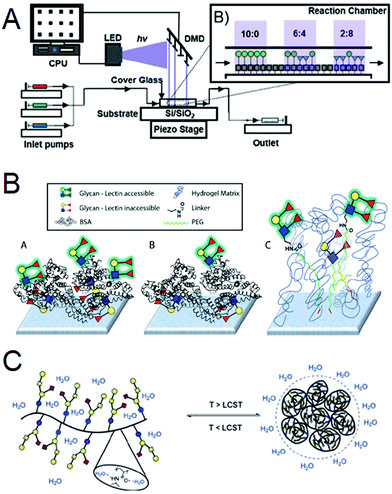 | ||
| Fig. 4 Summary of Session 2 papers. (A) A photochemical printer uses digital micromirror devices and microfluidics to immobilize alkene-functionalized mannosides in varying densities onto thiol-terminated surfaces (DOI: 10.1039/C9FD00028C). (B) Comparison of the high-density (A′) and low density (B′) neoglycoprotein microarrays generated by the Gildersleeve laboratory and the Consortium for Functional Glycomics microarray (C′) (DOI: 10.1039/C9FD00021F). (C) Thermal-responsive GM1os-pentasaccharide bearing glycopolymers collapse above a defined LCST (lower critical solution temperature) and can be used for the reversible capture of cholera-toxin-B from complex biological mixtures (DOI: 10.1039/C9FD00017H). | ||
Session 3: Glycan interactions on glycocalyx mimetic surfaces
This session started with Prof. Kamil Godula (University of California San Diego) telling the audience about the ‘spectator glycocalyx’ (DOI: 10.1039/C9FD00024K, Fig. 5A). In addition to enabling the presentation of carbohydrate motifs on cellular surfaces, the glycocalyx provides an important physical barrier which regulates cell–cell interactions and mediates exchange across the cell membrane. In order to explore how the physical properties of the cellular interface effect carbohydrate recognition his group synthesized a series of PEG-based analogues of mucin glycoproteins with variable glycan composition. These polymers were incorporated within the plasma membranes of red blood cells to provide an artificial extended glycocalyx. The interactions of these modified cells with lectins Con A and the soybean agglutinin (SBA) were investigated using agglutinnation studies and flow-cytometry assays. The presence of an extended physical barrier ultimately exerted a protective effect against lectin-promoted agglutination, but interesting lectin-specific effects on binding were observed. Con A, which recognizes mannose-terminated glycans typically displayed deep within the glycocalyx, suffered greater suppression of binding to cells modified with an extended “spectator” glycocalyx than that observed with SBA, which recognizes sialic acid terminated glycans displayed primarily on the outer surface and central regions of the glycocalyx. These observations highlight that differences in the spatial orientation of binding motifs can effect recognition at cellular interfaces.The next paper, presented by Yoshiko Miura (Kyushu University), detailed the preparation of porous glycopolymer matrices termed “glycomonoliths” by copolymerization of an α-mannose acrylamide derivative within an acrylamide/bis-acrylamide mixture in a porogenic solvent (DOI: 10.1039/C9FD00018F, Fig. 5B). The pore morphology and volume could be controlled through variation in the monomer feed ratio and solvent choice. Mannose-functionalised glycomonoliths were found to selectively bind the complementary lectin Con A, whilst demonstrating low absorption of both bovine serum albumin and the non-complementary peanut agglutinin, suggesting potential applications in the separation of proteins.
Helena Azevedo (Queen Mary University of London) then detailed the development of a strategy for the production of micropatterned hyaluronic acid (HA) surfaces (DOI: 10.1039/C9FD00015A, Fig. 5C). HA is an important constituent of the endothelial glycocalyx and plays a key role in the maintenance of vascular integrity. A peptide which had previously been shown to bind specifically to HA (Pep-16) was modified to contain a thiol functionality and applied to gold surfaces using a micropatterned stamp. Subsequent immersion in a solution of HA yielded surfaces with similar glycosaminoglycan (GAG) presentation to that observed in the endothelial glycocalyx. These synthetic surfaces were used to investigate the effects of HA molecular weight on cellular adhesion, with smaller HA polysaccharides (5/60 kDa) shown to promote improvements in cell spreading, migration and viability when compared to high molecular weight HA (700 kDa).
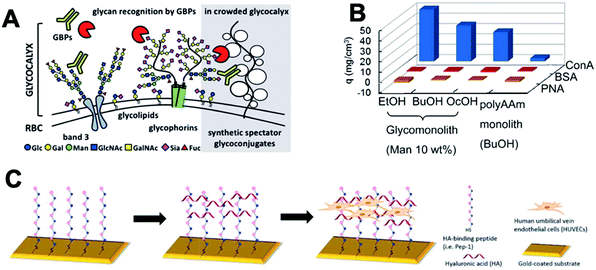 | ||
| Fig. 5 Summary of Session 3 papers. (A) The effects of non-binding ‘spectator’ glycoconjugates on the recognition of glycans at cellular surfaces has been investigated through extending the glycocalyx of red blood cells with synthetic glycopolymers (DOI: 10.1039/C9FD00024K). (B) Mannose-functionalized ‘glycomonoliths’ can selectively bind Con A, with minimal absorption of other proteins (DOI: 10.1039/C9FD00018F). (C) Hyaluronic acid (HA) coated surfaces for cell culture can be fabricated using self-assembled monolayers of a HA-binding peptide (DOI: 10.1039/C9FD00015A). | ||
Session 4: New directions in surface functionalization and characterization
This last session consisted of two papers presented on the afternoon of July 4th and another three the following day, which preceded the concluding remarks lecture and the close of the meeting. Lee Cronin (University of Glasgow) proved to be an excellent moderator for this session. Zhijian Zhen (The Hong Kong Polytechnic University) discussed the use of multilayer polymer brushes generated using surface-initiated atom transfer radical polymerization (SI-ATRP) to yield micropatterned surfaces for cell culture applications (DOI: 10.1039/C9FD00013E, Fig. 6A). Initially, an inert ‘hosting’ polymer brush layer was grown on gold surfaces, before application of an ATRP initiator by microcontact printing enabled the growth of a second polymer brush layer to yield 3D-patterned surfaces. These surfaces were chemically modified with gelatin proteins and used as scaffolds for the culture of mammalian cells, enabling the production of well-defined micropatterned cultures.The theme of patterning surfaces for cell culture was continued by Matteo Palma (Queen Mary University of London), who presented a paper detailing the production of biomimetic nanoarray platforms through a combination of top-down and bottom-up assembly strategies (DOI: 10.1039/C9FD00023B, Fig. 6B). DNA nanostructures, fabricated through origami methods, were decorated with recognition motifs including epidermal growth factor and integrin binding peptides. These conjugates were then introduced onto patterned surfaces generated using surface lithography techniques, allowing for their selective attachment in defined positions upon the surfaces. This approach provides a customizable platform to study the effects of spatial positioning and receptor density on the growth of cells on patterned surfaces, yielding new insights into receptor mediated adhesion and clustering behaviour.
On Friday morning the discussion continued in a session opened with Bart Jan Ravoo describing a modular co-assembly strategy for the preparation of dual-responsive hydrogels (DOI: 10.1039/C9FD00012G, Fig. 6C). A peptide-based low molecular weight gelator was modified with a photoresponsive arylazopyrazole group, in which the stereochemistry of the azo unit can be reversed by application of light of specific wavelengths. This gelator was co-assembled with cyclodextrin vesicles displaying membrane-embedded superparamagnetic CoFe2O4 nanoparticles. The mechanical properties of the resulting hydrogels could be modulated by application of an external magnetic field, with the alignment of nanoparticles with the applied field inducing an increase in the storage modulus. In contrast, irradiation-induced isomerism disrupts interactions between the arylazopyrazole moiety and cyclodextrin, which decreases crosslinking density and, consequently, gel stiffness. These materials offer scope for the incorporation of glycans or other recognition motifs, presenting exciting opportunities to generate materials in which biorecognition properties could be tuned both through compositional factors such as choice of recognition element, and physical factors including the application of orthogonal stimuli.
Inspired by the distribution of receptor ligands on cellular surfaces, Shelley Claridge (Purdue University) presented a route to controlling the surface presentation of amphiphilic ligands through the production of striped-phase monolayers (DOI: 10.1039/C9FD00022D, Fig. 6D). Microcontact printing was used to deposit stripes of amphiphiles including phospholipids and a phosphatidylinositol, generating lamellar structures with nanometer spacings. The separation between the headgroups of the amphiphiles within the monolayers can be controlled by adjusting the length of the corresponding hydrocarbon chain, allowing for the spatially controlled presentation of ligands on surfaces.
The presentations were concluded by Carsten Werner (Leibniz Institute of Polymer Research), who described the preparation of GAG-based biohybrid hydrogels (DOI: 10.1039/C9FD00016J, Fig. 6E). Thiol- and maleimide- terminated star-PEGs were crosslinked with co-incorporation of maleimide-functionalised heparin derivatives to yield hydrogels with varied GAG-sulfation patterns and charge densities. The effects of these parameters on the ability of the hydrogels to sequester cytokines was investigated, providing insights to enable the design of gel systems for tailored protein sequestration, and provide a platform to study the association of cytokines with the extracellular matrix.
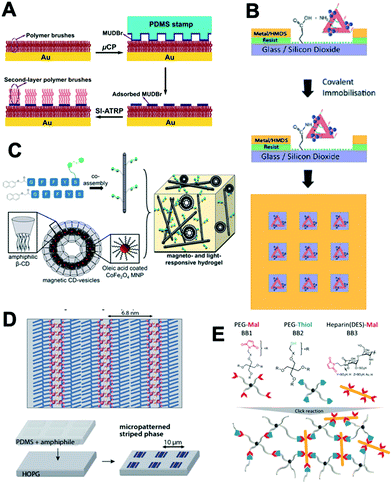 | ||
| Fig. 6 Summary of Session 4 papers. (A) The growth of multilayer polymer brushes from gold substrates can be used to generate micropatterned surfaces for cell culture applications (DOI: 10.1039/C9FD00013E). (B) DNA nanostructures decorated with cellular recognition motifs can be attached to patterned surfaces with control over their spatial positioning and density (DOI: 10.1039/C9FD00023B). (C) A modular co-assembly strategy generates hydrogels which respond to photo- and magnetic stimuli (DOI: 10.1039/C9FD00012G). (D) Striped-phase monolayers can be used to control the spatial positioning of functional ligands, including carbohydrates, on surfaces (DOI: 10.1039/C9FD00022D). (E) Glycosaminoglycan (GAG)-based biohybrid gels have been used to explore the effects of varying GAG composition on the ability of the materials to sequester cytokines (DOI: 10.1039/C9FD00016J). | ||
Posters
A total of fourteen posters were presented in this meeting over lunch on July 4th. Before commencing the session, each poster presenter also gave strictly-timed, one-minute lightning presentations to briefly describe each project. The topics represented ranged from devices and diagnostics, polymers and platforms to chemically editing proteoglycan macromolecules. Discussions around the posters continued beyond the allocated session, with the poster area frequently playing host to enthusiastic discussions throughout the duration of the meeting.Closing lecture
In the closing lecture delivered by Prof. Ten Feizi (Imperial College, London), we were reminded of the history and impact of the glycan microarray. Prof. Feizi is regarded as having created the first glycan microarray, and as relative newcomers to the field, we were honoured to hear her account of her journey through glycoscience. Prof. Feizi recapitulated the talks and discussions covered during the meetings and provided her own insights on the research presented and discussed as well. Ten's journey with glycan microarrays began with her investigation of the pathogen Mycoplasma pneumoniae, which causes respiratory infections. Interestingly, this pathogen causes a transient autoimmune response that is characterized by the presence of autoantibodies that agglutinate with host erythrocytes.7 Notably, it is the reaction product of the I antigen with the pathogen (and not the pathogen alone) that is responsible for the production of these agglutinins. Not long after she began her studies, she surmised that erythrocyte glycans were used by this pathogen to initiate infections, which were determined to be sialic acid glycans in α(2–3) linkages to terminal galactose residues.8 These observations highlighted the need for a micromethod to study glycan recognition patterns. The following year, neoglycolipid technology was introduced as a method to systematically interrogate glycan-binding preferences using lipid-linked glycans. In 2002, the neoglycolipid platform became the first arrayed system of glycans intended to encompass entire glycomes. Ten also recapitulated a recurring theme of this meeting, which is the need to corroborate the observations determined using screening glycan arrays with in vivo studies.Final remarks and thoughts
The 2019 Faraday Discussion on the Nanolithography of Biointerfaces has been a vibrant and stimulating meeting of the minds. We were told that our coming to London during this time was quite fortuitous. Indeed, the London that we had come to see was very different from the city in movies. London was bright, warm and happy. True to this rare weather occasion was this gathering of scientists that would normally never see each other. Nowadays, scientists are becoming ever more specialized and seek audiences similar to their own work; this meeting offers an entirely distinct experience and presents a different set of opportunities. As Adam remarks, not one group has all the expertise necessary to build the new-age biointerface, replete with the spatial orientation and the complex information encoded by cells. Only through the combined expertise of researchers from a range of disciplines, working together, can we truly interrogate the function of nature's complex interfaces.Whereas in most scientific conferences, speakers have close to an hour to present their work and five minutes for questions at the end, the Faraday Discussions implement a flipped format, where five minutes are allocated for presentations and there are close to ninety minutes for debate and discussion. Being integrated in the field, we follow publications thoroughly and often ask what new information we have learned from an oral presentation that we have not already read in the publication. What is most valuable from the Faraday Discussion setting is indeed the practice of conversation and debate. We found ourselves, along with the rest of the audience, thoroughly engaged throughout the entirety of the sessions. Notably, the now traditional dissemination of events at the meeting via Twitter was subdued, as the audience was fully immersed in stimulating face-to-face exchanges. Most importantly, as newly independent academic researchers, we were thrilled to engage in discussion and debate with the biggest names in the field. The format of the conference was very conducive to catalyzing collaborations and identifying the directions the field is going and where else it should go. After a last chance to discuss talks with other delegates over morning tea, everybody then dispersed into a sunny London. We would like to thank the scientific committee for all their hard work to bring us all together: Adam Braunschweig (Chair), Laura Hartmann, Ryan Chiechi, Lee Cronin, Stephan Schmidt and Sebastien Vidal.
Acknowledgements
MLH acknowledges support from Scripps Research and the NIH Pathway to Independence Award (5R00HD090292-03). This meeting was generously sponsored by The US Army and Swiss Litho AG (Innovative Fabrication Tools).References
- Royal Society of Chemistry (11 June 2019) Driving Discovery through Debate: Celebrating more than a century of the Faraday Discussions.
- Letter to J.J. Berzelius (28 Jan 1835). In Bulletin of the Atomic Scientists (Nov 1949), 310. Date of letter as given in Alan L. Mackay, A Dictionary of Scientific Quotations (1991), 267.
- M. Guberman and P. H. Seeberger, Automated Glycan Assembly: A Perspective, J. Am. Chem. Soc., 2019, 141, 5581–5592 CrossRef CAS PubMed.
- T. Goldmann and J. S. Gonzalez, DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports, J. Biochem. Biophys. Methods, 2000, 42, 105–110 CrossRef CAS PubMed.
- J. C. Paulson, O. Blixt and B. E. Collins, Sweet spots in functional glycomics, Nat. Chem. Biol., 2006, 2, 238–248 CrossRef CAS PubMed.
- M. E. Mummert, M. Mohamedzadeh, D. I. Mummert, N. Mizumoto and A. Takashima, Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking, J. Exp. Med., 2000, 192, 769–779 CrossRef CAS PubMed.
- T.-R. D. Feizi, M. D. Shields and R. A. Carter, Production of cold agglutinins in rabbits immunized with human erythrocytes treated with Mycoplasma pneumoniae, Nature, 1969, 222, 1353–1356 Search PubMed.
- L. M. Loomes, K. Uemura, R. A. Childs, J. C. Paulson, G. N. Rogers, P. R. Scudder, J. C. Michalski, E. F. Hounsell, D. Taylor-Robinson and T. Feizi, Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosachharides of Ii antigen type, Nature, 1984, 307, 560–563 CrossRef CAS PubMed.
- R. K. Letsinger and V. Mahadevan, Oligonucleotide synthesis on a polymer support, J. Am. Chem. Soc., 1965, 87, 3526–3527 CrossRef CAS PubMed; S. L. Beaucage and M. H. Caruthers, Deoxynucleotide phosphoramidites – A new class of key intermediates for deoxypolynucleotide synthesis, Tetrahedron Lett., 1981, 22, 1859–1862 CrossRef.
- R. B. Merrifield, Solid phase peptide synthesis I. The synthesis of a tetrapeptide, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS.
- O. K. Plante, E. R. Palmacci and P. H. Seeberger, Automated solid-phase synthesis of oligosaccharides, Science, 2001, 291, 1523–1527 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |


