Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly chemoselective difluoromethylative homologation of iso(thio)cyanates: expeditious access to unprecedented α,α-difluoro(thio)amides†
Margherita
Miele
a,
Rosarita
D’Orsi
b,
Vellaisamy
Sridharan
 c,
Wolfgang
Holzer
a and
Vittorio
Pace
c,
Wolfgang
Holzer
a and
Vittorio
Pace
 *a
*a
aDepartment of Pharmaceutical Chemistry, University of Vienna, Althanstrasse 14, A-1090, Vienna, Austria. E-mail: vittorio.pace@univie.ac.at; Web: http://drugsynthesis.univie.ac.at/
bDepartment of Sciences, University of Basilicata, Via dell’Ateneo Lucano 10, 85100 Potenza, Italy
cDepartment of Chemistry and Chemical Sciences, Central University of Jammu, Rahya-Suchani (Bagla), 181143, India
First published on 11th October 2019
Abstract
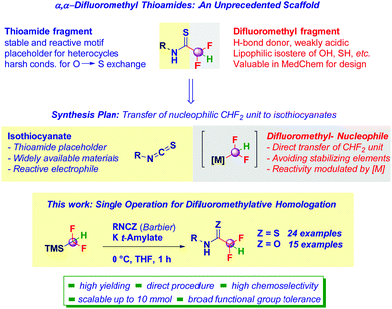
The new motif – α,α-difluoromethyl thioamide – has been assembled starting from isothiocyanate (as thioamide precursor) and a formal difluoromethyl-carbanion generated from commercially available TMSCHF2. Upon proper activation of this reagent with potassium tert-amylate, the high-yielding transfer of the difluorinated nucleophile takes place under high chemocontrol. Various sensitive functionalities (e.g. ester, nitrile, nitro, azido groups) can be accommodated across the isothiocyanate core, thus allowing a wide scope. The methodology is highly flexible and adaptable to prepare analogous α,α-difluoromethyl oxoamides by conveniently using isocyanates as the electrophilic building-blocks.
Chemical entities featuring a difluoromethyl substituent (CHF2) are receiving prominent interest within different audiences because of the fine modulation of physico-chemical parameters achievable by its introduction into organic skeletons.1 The relatively acidic proton confers a unique capability to establish H-bond phenomena;2 moreover, the CHF2-group acts as a valuable – more lipophilic isostere – of important moieties such as OH or SH, inter alia.3 These properties are regarded as highly valuable during drug optimization processes, in which the continued demand for CHF2-containing scaffolds has boosted the design of novel tactics for their preparation.4 In the frame of a medicinal chemistry project, we became interested in preparing α,α-difluoromethyl thioamides – completely unprecedented scaffolds – conjugating the versatility of the CHF2 group with unique structural/chemical aspects (stability, crystallizability, good reactivity towards nucleophiles or placeholders for heterocycles) conferred by the formal replacement of oxygen (in oxoamides) with sulfur (Scheme 1).5
At the outset of our investigations, we were cognizant of the stability risk of α-halogenated thioamides,6 thus requiring a straightforward synthetic route – ideally high yielding – to be adopted, in which critical steps such as the formal thionation (O → S substitution) could be advantageously skipped. For this purpose, we conceived an approach dealing with the transfer of a putative nucleophilic CHF2-unit to isothiocyanates – as (thio)amides precursors – which proved to be highly effective in addition processes,6b,7 thus enabling the limitations observed with thionating reagents (e.g. Lawesson) to be circumvented.8 Because of the excellent electrophilicity of the heterocumulene carbon a wide array of nucleophilic elements could be installed, thus providing a reliable technique operating under chemocontrol and mild reaction conditions.9 However, the well-known instability of F-containing carbanions10 posed important risks on the success of the technique. In fact, to make the strategy productive, two key requirements had to be fulfilled: (1) the conditions to generate the formal CHF2-carbanion must ensure its (limited) chemical integrity and, (2) the per se reactive isothiocyanate had to be fully preserved during the carbanion generation event.
The direct use of MCHF2 reagents (M = metal), limited in terms of versatility and toxicity, stimulated the development of alternative pro-nucleophilic sources for the difluoromethyl anion.10a In fact, through the positioning of the TMS-group on the putative carbanion,11 Hu and co-workers demonstrated the effectiveness of employing TMSCHF2 in difluoromethylation under nucleophilic regime of carbonyls (ketones and aldehydes) and imines.12 Remarkably, it manifests a series of positive features including easy manipulability (bp 65 °C) and, the protecting TMS group is advantageously cleaved during the activation event imposed by the presence of the strong Si–CF2H bond. This is a particularly attractive characteristic compared to the use of different protecting elements, mainly strong electron-withdrawing groups,13 which would have required two additional undesirable operations: the installation and the removal. An analogous approach has been documented by Dilman who demonstrated the effectiveness of difluorinated phosphorus ylides as nucleophilic reagents.14 Herein, we present a straightforward preparation of α,α-difluoromethyl thioamides consisting of the nucleophilic CHF2-transfer agent generation/chemoselective addition to isothiocyanates. We anticipate that the strategy is modular and adaptable to the synthesis of analogous α,α-difluoromethyl oxoamide derivatives by simply switching to isocyanates as the starting materials.7a,15
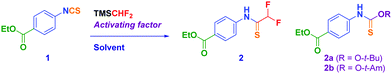
With the aim of verifying the initial hypothesis and evaluating the chemoselectivity of the transformation, the challenging ester-substituted isothiocyanate 1 – potentially susceptible of nucleophilic addition at the carbonyl – was selected as the model substrate (Table 1). The activation of TMSCHF2 with CsF was inefficient regardless of the solvent used, thus indicating a distinct behaviour – governed by the nature of the electrophile – compared to Hu's work on carbonyls (entries 1 and 2).12 Potassium tert-butoxide activated the pro-nucleophile enabling the desired transformation, though significant attack of the t-BuO anion on the isothiocyanate was observed (entry 3). Unfortunately, cooling the reaction at −78 °C did not suppress the side product formation (entry 4); interestingly, by switching the solvent to toluene or CPME an increase of the undesired thiocarbamate was noticed (entries 5 and 6). These initial indications suggested us to activate the donor with a more sterically hindered alkoxide, the commercially available tert-amylate being a valid alternative.16 Pleasingly, the desired difluoromethylthioamide 2 was obtained in high yield as the major product together with a minimal amount of thiocarbamate 2b (entry 7). Additional considerations merit mention: (1) by decreasing the nucleophile loading to 1.2 equiv. complete suppression of the undesired product was achieved, thus allowing 2 to be prepared with high chemocontrol (entry 8); (2) the putative carbanion needs to be generated under Barbier-type conditions, since late addition (even after 1 min at −78 °C) of the electrophile results in destruction of the species (entries 9 and 10); (3) the reaction is quite fast reaching completion within 1 h at 0 °C. Moreover, the use of a small excess of TMSCHF2 compared to t-AmOK (0.3 equiv.) guarantees the nucleophile generation event to proceed quantitatively (entry 8 vs. 11).
| Entry | Activator (equiv.) | TMSCHF2 (equiv.) | Solvent | Temperature [°C] | Ratio 2/2aba | Yield of 2b (%) |
|---|---|---|---|---|---|---|
| a The ratio has been calculated by 1H-NMR analysis using 1,3,5-trimethylbenzene as an internal standard. b Isolated yield. c 2a (20%). d 2a (14%). e 2a (31%). f 2a (32%). g 2b (9%). h Non-Barbier conditions were used (i.e. the nucleophile was generated 1 min prior to the addition of 1). i 2b (4%). | ||||||
| 1 | CsF (2.0) | 1.8 | DMF | rt | — | — |
| 2 | CsF (2.0) | 1.8 | THF | rt | — | — |
| 3 | t-BuOK (1.9) | 2.0 | THF | 0 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
61c |
| 4 | t-BuOK (1.9) | 2.0 | THF | −78 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
66d |
| 5 | t-BuOK (1.9) | 2.0 | Toluene | 0 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
47e |
| 6 | t-BuOK (1.9) | 2.0 | CPME | 0 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
41f |
| 7 | t-AmOK (1.9) | 2.0 | THF | 0 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
72g |
| 8 | t-AmOK (1.2) | 1.5 | THF | 0 |
>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
83 |
| 9h | t-AmOK (1.2) | 1.5 | THF | 0 | — | — |
| 10h | t-AmOK (1.2) | 1.5 | THF | −78 | — | — |
| 11 | t-AmOK (1.2) | 1.2 | THF | 0 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7i 7i |
67 |
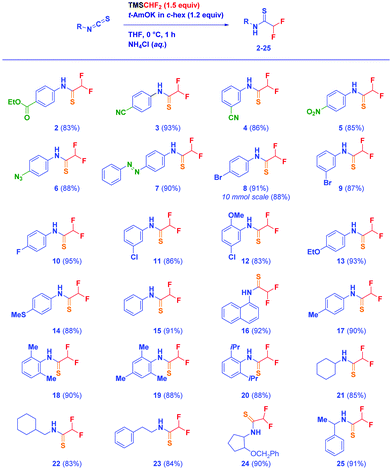
With the optimized conditions in hand (entry 8 – Table 1), we then studied the scope of the reaction (Scheme 2). Not only a sensitive ester (2) could be placed on the reactive aryl isothiocyanate core, but also additional susceptible electrophilic functionalities such as nitrile (3–4) and nitro (5) which remained completely untouched during the nucleophile formation/homologation sequence. Moreover, nitrogen-containing groups did not interfere with the transformation, as deduced in the case of azide (6) and diazo (7). Different halogens – bromine (8–9 – eventually performing the reaction in 10 mmol scale), fluorine (10), chlorine (11–12) – decorating the aryl nucleus at diverse positions are tolerated, thus allowing their reactivity in late transformations (vide infra) to be exploited. Moving to substituents of opposite electronic behaviour (electron donating groups) – alkoxy (12–13) or alkylmercapto (14) – resulted in clean reactions of comparable efficiency. Moreover, simple aromatic groups – phenyl, p-tolyl (15, 17) or 1-naphthyl (16) – provide the expected difluoromethyl thioamides in excellent yields. An intriguing and significantly valuable opportunity of the methodology is the one-step construction of highly sterically demanding scaffolds: accordingly, the introduction of methyl groups at the critical 2 and 6 positions of the phenyl moiety documents the validity of the tactic giving analogues 18 and 19. This aspect is further showcased in the case of the more crowded 2,6-di-i-propylphenyl analogue 20. The nature of the isothiocyanate does not influence the effectiveness of the technique: also aliphatic materials undergo the difluoromethylation giving the corresponding thioamides in high yields (21–25), thus confirming the positive trend manifested by aromatic counterparts.
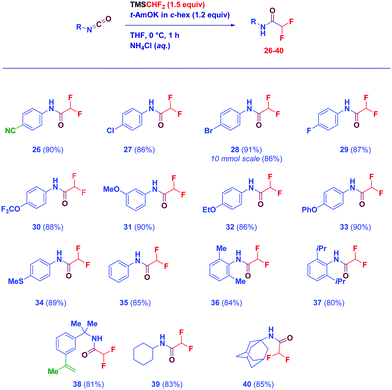
The success of the procedure motivated us to evaluate the reactivity of oxo-analogues (isocyanates) en route to valuable α,α-difluoromethyl amides, synthetically and structurally relevant scaffolds,17 previously prepared via classical amide-linkage forging chemistry.18 The transformation is perfectly flexible affording the targeted motifs in high yield under full chemocontrol (Scheme 3). A series of functionalized aryl isocyanates are amenable for the direct difluoromethylation, including cyano (26), halogen-containing (27–29), alkoxy (30–33), and alkylthio (34). Again, scaling up to 10 mmol is compatible with the protocol (28). Clear confirmation of the suitability of the methodology for preparing challenging sterically demanding substrates arises from the outcome noticed with examples 36 and 37 (aromatic). Additionally, aliphatic congeners afford difluoromethyl amides with similar efficiency, it being worth noting the congested vinyl-substituted (38) and the adamantyl derivative 40.
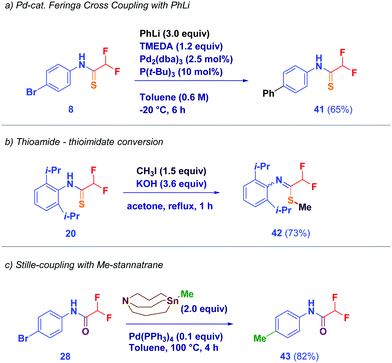
With the aim to explore the potential behaviour of the α,α-difluoromethyl thioamide motif, we realized a series of manipulations on the synthesized scaffolds (Scheme 4). Notably, the new motif withstands the Feringa Pd-catalyzed cross-coupling19 of PhLi, thus allowing the phenylation on the bromine-containing nucleus 8 to be selectively conducted giving the bis-phenylated structure 41 (path a). Under alkylative conditions (MeI, KOH), the difluoromethyl thioamide group of 20 is conveniently converted into the thioimidate 42 (path b). Because of our interest towards C1–Sn reagents,20 we were pleased to use a stannatrane21 for the transfer of the methyl unit to oxoamide 28 under Stille-coupling conditions, thus evidencing the stability of the difluoromethyl amide 43 (path c).
In summary, we have developed a straightforward synthesis of previously undisclosed α,α-difluoromethyl thioamides via a tactic based on the nucleophilic transfer of a difluoromethyl carbanion equivalent to isothiocyanates. The treatment of the commercially available and experimentally convenient reagent TMSCHF2 with potassium tert-amylate allows the efficient formation of the difluorinated nucleophile. Full chemocontrol is uniformly manifested as indicated in the cases of challenging isothiocyanates embodying chemical moieties susceptible of nucleophilic attack. This high yielding methodology fully preserves its effectiveness when applied to the synthesis of α,α-difluoromethyl oxoamide using isocyanates as amide precursors.
We thank the University of Vienna and the OEAD for generous support. Dr R. Bartolotta is acknowledged for initial contributions and D. Dobusch for HRMS data. R. D’O. thanks the University of Basilicata for a visiting doctoral fellowship. This paper is dedicated to Professor Marko Mihovilovic in the occasion of his 50th birthday.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For excellent reviews, see: (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (b) R. Ragni, A. Punzi, F. Babudri and G. M. Farinola, Eur. J. Org. Chem., 2018, 3500 CrossRef CAS.
- C. D. Sessler, M. Rahm, S. Becker, J. M. Goldberg, F. Wang and S. J. Lippard, J. Am. Chem. Soc., 2017, 139, 9325 CrossRef CAS PubMed.
- (a) Y. Zafrani, G. Sod-Moriah, D. Yeffet, A. Berliner, D. Amir, D. Marciano, S. Elias, S. Katalan, N. Ashkenazi, M. Madmon, E. Gershonov and S. Saphier, J. Med. Chem., 2019, 62, 5628 CrossRef CAS PubMed; (b) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797 CrossRef CAS PubMed.
- For excellent reviews, see: (a) D. E. Yerien, S. Barata-Vallejo and A. Postigo, Chem. – Eur. J., 2017, 23, 14676 CrossRef CAS PubMed; (b) M.-C. Belhomme, T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2015, 21, 12836 CrossRef CAS PubMed; (c) J. Rong, C. Ni and J. Hu, Asian J. Org. Chem., 2017, 6, 139 CrossRef CAS . For outstanding recent contributions in the area, see: ; (d) C. F. Meyer, S. M. Hell, A. Misale, A. A. Trabanco and V. Gouverneur, Angew. Chem., Int. Ed., 2019, 58, 8829 CrossRef CAS PubMed; (e) Q. Xie, Z. Zhu, L. Li, C. Ni and J. Hu, Angew. Chem., Int. Ed., 2019, 58, 6405 CrossRef CAS PubMed; (f) F. Scheidt, M. Schäfer, J. C. Sarie, C. G. Daniliuc, J. J. Molloy and R. Gilmour, Angew. Chem., Int. Ed., 2018, 57, 16431 CrossRef CAS PubMed; (g) F. Pan, G. B. Boursalian and T. Ritter, Angew. Chem., Int. Ed., 2018, 57, 16871 CrossRef CAS PubMed; (h) C. Xu, W.-H. Guo, X. He, Y.-L. Guo, X.-Y. Zhang and X. Zhang, Nat. Commun., 2018, 9, 1170 CrossRef PubMed.
- (a) T. S. Jagodziński, Chem. Rev., 2003, 103, 197 CrossRef PubMed; (b) T. Murai, Top. Curr. Chem., 2005, 251, 247 CrossRef CAS; (c) Chemistry of Thioamides, ed. T. Murai, Springer Nature, Singapore, 2019 Search PubMed; (d) K.-J. Xiao, J.-M. Luo, K.-Y. Ye, Y. Wang and P.-Q. Huang, Angew. Chem., 2010, 122, 3101 CrossRef; (e) K.-J. Xiao, A.-E. Wang and P.-Q. Huang, Angew. Chem., 2012, 124, 8439 CrossRef; (f) V. Pace, W. Holzer and B. Olofsson, Adv. Synth. Catal., 2014, 356, 3697 CrossRef CAS; (g) V. Pace and W. Holzer, Aust. J. Chem., 2013, 66, 507 CrossRef CAS.
- (a) B. Yde, N. M. Yousif, U. Pedersen, I. Thomsen and S. O. Lawesson, Tetrahedron, 1984, 40, 2047 CrossRef CAS; (b) L. Castoldi, S. Monticelli, R. Senatore, L. Ielo and V. Pace, Chem. Commun., 2018, 54, 6692 RSC.
- (a) V. Pace, S. Monticelli, K. de la Vega-Hernández and L. Castoldi, Org. Biomol. Chem., 2016, 14, 7848 RSC; (b) V. Pace, L. Castoldi, S. Monticelli, M. Rui and S. Collina, Synlett, 2017, 879 CrossRef CAS . For additional studies from our group on the addition of α-substituted organometallic reagents, see for example: ; (c) R. Senatore, L. Castoldi, L. Ielo, W. Holzer and V. Pace, Org. Lett., 2018, 20, 2685 CrossRef CAS PubMed; (d) R. Senatore, L. Ielo, E. Urban, W. Holzer and V. Pace, Eur. J. Org. Chem., 2018, 2466 CrossRef CAS; (e) L. Castoldi, L. Ielo, P. Hoyos, M. J. Hernáiz, L. De Luca, A. R. Alcántara, W. Holzer and V. Pace, Tetrahedron, 2018, 74, 2211 CrossRef CAS; (f) R. Senatore, L. Ielo, S. Monticelli, L. Castoldi and V. Pace, Synthesis, 2019, 2792 CAS.
- T. Ozturk, E. Ertas and O. Mert, Chem. Rev., 2007, 107, 5210 CrossRef CAS PubMed.
- (a) V. Pace, L. Castoldi, S. Monticelli, S. Safranek, A. Roller, T. Langer and W. Holzer, Chem. – Eur. J., 2015, 21, 18966 CrossRef CAS PubMed; (b) K. de la Vega-Hernández, R. Senatore, M. Miele, E. Urban, W. Holzer and V. Pace, Org. Biomol. Chem., 2019, 17, 1970 RSC.
- (a) J. Hu, W. Zhang and F. Wang, Chem. Commun., 2009, 7465 RSC; (b) A. D. Dilman and V. V. Levin, Acc. Chem. Res., 2018, 51, 1272 CrossRef CAS PubMed . For uses of fluorinated carbenoids (LiCH2F and LiCHIF) from our group, see: ; (c) S. Monticelli, M. Colella, V. Pillari, A. Tota, T. Langer, W. Holzer, L. Degennaro, R. Luisi and V. Pace, Org. Lett., 2019, 21, 584 CrossRef CAS PubMed; (d) G. Parisi, M. Colella, S. Monticelli, G. Romanazzi, W. Holzer, T. Langer, L. Degennaro, V. Pace and R. Luisi, J. Am. Chem. Soc., 2017, 139, 13648 CrossRef CAS PubMed; (e) L. Ielo, S. Touqeer, A. Roller, T. Langer, W. Holzer and V. Pace, Angew. Chem., Int. Ed., 2019, 58, 2479 CrossRef CAS PubMed.
- T. Hagiwara and T. Fuchikami, Synlett, 1995, 717 CrossRef CAS.
- Y. Zhao, W. Huang, J. Zheng and J. Hu, Org. Lett., 2011, 13, 5342 CrossRef CAS PubMed.
- G. K. S. Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921 CrossRef CAS PubMed.
- (a) S. S. Ashirbaev, V. V. Levin, M. I. Struchkova and A. D. Dilman, J. Org. Chem., 2018, 83, 478 CrossRef CAS PubMed; (b) A. L. Trifonov, V. V. Levin, M. I. Struchkova and A. D. Dilman, Org. Lett., 2017, 19, 5304 CrossRef CAS PubMed.
- For an excellent review, see: (a) E. Serrano and R. Martin, Eur. J. Org. Chem., 2018, 3051 CrossRef CAS . For recent outstanding contributions in the area, see: ; (b) G. Schäfer, C. Matthey and J. W. Bode, Angew. Chem., Int. Ed., 2012, 51, 9173 CrossRef PubMed; (c) X. Wang, M. Nakajima, E. Serrano and R. Martin, J. Am. Chem. Soc., 2016, 138, 15531 CrossRef CAS PubMed; (d) S. Zheng, D. N. Primer and G. A. Molander, ACS Catal., 2017, 7, 7957 CrossRef CAS PubMed; (e) J. D. Williams, W. J. Kerr, S. G. Leach and D. M. Lindsay, Angew. Chem., Int. Ed., 2018, 57, 12126 CrossRef CAS PubMed; (f) D. E. Polat, D. D. Brzezinski and A. M. Beauchemin, Org. Lett., 2019, 21, 4849 CrossRef CAS PubMed; (g) J. S. Derasp and A. M. Beauchemin, ACS Catal., 2019, 8104 CrossRef CAS; (h) V. Pace, L. Castoldi and W. Holzer, Chem. Commun., 2013, 49, 8383 RSC; (i) V. Pace, K. de la Vega-Hernández, E. Urban and T. Langer, Org. Lett., 2016, 18, 2750 CrossRef CAS PubMed.
- (a) D. D. Caspi and S. M. Richter, J. Org. Chem., 2019, 84, 8256 CrossRef CAS PubMed . Recently, we observed an analogous beneficial effect of K t-amylate in the synthesis of difluoromethyl ketones, see: ; (b) M. Miele, A. Citarella, N. Micale, W. Holzer and V. Pace, Org. Lett., 2019 DOI:10.1021/acs.orglett.9b03024.
- (a) P. J. Czerwiński and B. Furman, Chem. Commun., 2019, 55, 9436 RSC; (b) C. R. Jones, P. K. Baruah, A. L. Thompson, S. Scheiner and M. D. Smith, J. Am. Chem. Soc., 2012, 134, 12064 CrossRef CAS PubMed.
- (a) S. S. Biechler and R. W. Taft, J. Am. Chem. Soc., 1957, 79, 4927 CrossRef CAS . For the use of amides in nucleophilic additions, see: ; (b) M. Nakajima, Y. Oda, T. Wada, R. Minamikawa, K. Shirokane, T. Sato and N. Chida, Chem. – Eur. J., 2014, 20, 17565 CrossRef CAS PubMed.
- (a) M. Giannerini, M. Fañanás-Mastral and B. L. Feringa, Nat. Chem., 2013, 5, 667 CrossRef CAS PubMed . For a highlight, see: ; (b) V. Pace and R. Luisi, ChemCatChem, 2014, 6, 1516 CrossRef CAS.
- S. Touqeer, L. Castoldi, T. Langer, W. Holzer and V. Pace, Chem. Commun., 2018, 54, 10112 RSC.
- (a) E. Vedejs, A. R. Haight and W. O. Moss, J. Am. Chem. Soc., 1992, 114, 6556 CrossRef CAS; (b) L. Li, C.-Y. Wang, R. Huang and M. R. Biscoe, Nat. Chem., 2013, 5, 607 CrossRef CAS PubMed; (c) C.-Y. Wang, G. Ralph, J. Derosa and M. R. Biscoe, Angew. Chem., Int. Ed., 2017, 56, 856 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc06929a |
| This journal is © The Royal Society of Chemistry 2019 |