SO2 conversion to sulfones: development and mechanistic insights of a sulfonylative Hiyama cross-coupling†
Abstract
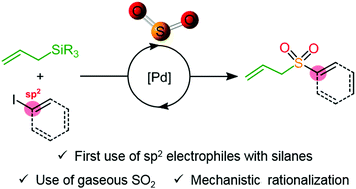
A Pd-catalyzed Hiyama cross-coupling reaction using SO2 is described. The use of silicon-based nucleophiles leads to the formation of allyl sulfones under mild conditions with a broad functional group tolerance. Control experiments coupled with DFT calculations shed light on the key steps of the reaction mechanism, revealing the crucial role of a transient sulfinate anion.


 Please wait while we load your content...
Please wait while we load your content...