Open Access Article
Open Access ArticleEvidence of chemical-bond formation at the interface between an epoxy polymer and an isocyanate primer†
Kazuhiro
Sensui
 a,
Taishi
Tarui
a,
Takayuki
Miyamae
a,
Taishi
Tarui
a,
Takayuki
Miyamae
 *b and
Chiaki
Sato
*b and
Chiaki
Sato
 *bc
*bc
aNissan Motor Co., Ltd., 1, Natsushima, Yokosuka, Kanagawa 237-8523, Japan
bNational Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: t-miyamae@aist.go.jp
cTokyo Institute of Technology, Institute of Innovative Research, 4259-G2-20, Nagatsuta-cho, Midori-ku, Yokohama, 226-8503, Japan. E-mail: csato@pi.titech.ac.jp
First published on 25th November 2019
Abstract
For several decades, hydroxyl function containing polymer surfaces have been modified using isocyanate primers to improve the adhesion properties. In this study, sum frequency generation spectroscopy was applied to study epoxy polymer/isocyanate solution interfaces, confirming the presence of chemical bonds at the joining interfaces.
Adhesive bonding is a common joining technology that is applied in various fields. However, despite its popularity, this process has not been sufficiently clarified yet, mostly due to its complexity and the involvement of multiple mechanisms. In the case of adhesion, for instance, mechanical interlocking, physical bonding, interdiffusion at interfaces, thermodynamic diffusion, and chemical bonding have been proposed as representative mechanisms.1–3 Among these, it is believed that adhesion is promoted by introducing highly reactive substituted functional groups that may enhance the formation of chemical bonds, because chemical-bond formation between adhesives and adherends is considered to lead to the highest adhesion strength.4–7 Thus, surface pre-treatments to remove chemically inert materials and create active functional groups at the surfaces are usually recommended.4–7 Alternatively, adhesive promoters for the chemically inert materials have often been used. However, no direct evidence of the chemical reactions occurring at the joining interface has been reported so far for most of the material combinations used. This can be attributed to the technical difficulties associated with determining the nature of chemical bonds at a buried interface between an adherent and an adhesive at the molecular level. Therefore, we had to use an indirect index, such as the adhesion strength, to evaluate the effect of the surface pre-treatment on the process. However, the adhesion strength rarely depends on a single factor; so it is hard to clarify the promotion of chemical-bond formation only based on this parameter.
Sum frequency generation (SFG) spectroscopy is a powerful technique applied for selective structural analyses of surfaces and interfaces at the sub-monolayer level, which makes it a suitable method for probing the interfacial chemical structure of our system.8–15 Since SFG spectroscopy has a high selectivity for either the outermost surface of a material or the interface between different materials, it enabled us to observe the chemical phenomena occurring at the interfaces between adherends and adhesives.
In this study, we demonstrate the molecular behavior at the interface between an epoxy polymer and a primer bearing isocyanate functionalities. It is well known that isocyanate compounds react with hydroxyl groups to form urethane bonds, and this reaction is commonly used for polyurethane polymerization processes. Thus, the progress of the polymerization reaction in the bulk can be monitored directly from the disappearance of the isocyanate band and the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band using infrared spectroscopy. Since the isocyanate groups present on the adhesives are highly reactive to hydroxyl groups, urethane bonds are believed to be also formed on the surface of the epoxy polymer.16 In addition, diisocyanate compounds dissolved in organic solvents are well known to act as good adhesion promoters when applied to the surface of an adherent before adhesion.17,18 However, since the formation of urethane bonds at the interface between the isocyanate groups and the epoxy polymer has not been directly observed so far, we wished to verify this hypothesis.
O band using infrared spectroscopy. Since the isocyanate groups present on the adhesives are highly reactive to hydroxyl groups, urethane bonds are believed to be also formed on the surface of the epoxy polymer.16 In addition, diisocyanate compounds dissolved in organic solvents are well known to act as good adhesion promoters when applied to the surface of an adherent before adhesion.17,18 However, since the formation of urethane bonds at the interface between the isocyanate groups and the epoxy polymer has not been directly observed so far, we wished to verify this hypothesis.
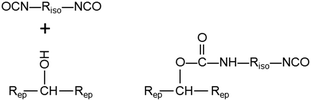
Bisphenol A diglycidyl ether and triethylenetetramine—the precursors of the epoxy polymer—were acquired from Mitsubishi Chemical Corporation and Kanto Chemical Co., Inc., respectively. 4,4-Methylene diphenyl diisocyanate (MDI) was purchased from Kanto Chemical Co., Inc. A mixture of the precursors was diluted with chloroform and then spin-cast on a calcium fluoride substrate coated with a 100 nm thick silica film. After spin-casting, the films were cured at 80 °C for 18 h. Next, 5 wt% MDI butyl acetate or chloroform solutions were prepared as a model isocyanate primer compound, since MDI is the main component of the commercial isocyanate primers. The chemical structures of the epoxy polymer precursors and MDI are shown in Fig. 1.
Attenuated total internal reflection infrared spectroscopy (ATR-IR) measurements were performed using a Spectrum Two apparatus (PerkinElmer Co., Ltd) with a germanium prism to confirm the presence of chemical bonds close to the interface between the epoxy polymer and the isocyanate compounds. The incident angle of the IR beam was 45°. The details of the SFG system utilized in this study have been reported previously.15 Details of the experimental setup for the SFG are described in the ESI.† All the SFG spectra were collected in SSP (s-polarized SF signal, s-polarized visible beam, and p-polarized infrared beam) polarization combinations.
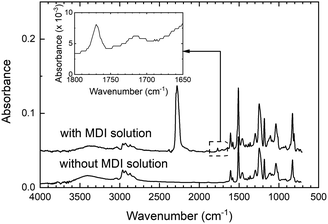
Fig. 2 shows the ATR-IR spectra of the epoxy polymer thin film with and without the butyl acetate solution of MDI. In the IR spectrum of the MDI-treated epoxy polymer, a tiny C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band is observed at 1780 cm−1, which can be attributed to uretdione, an isocyanate dimer.19–23 The weak feature observed at around 1720 cm−1 must be due to the residual butyl acetate. If urethane bonds are formed between the hydroxyl groups of the epoxy polymer and the isocyanate groups of MDI, a C
O band is observed at 1780 cm−1, which can be attributed to uretdione, an isocyanate dimer.19–23 The weak feature observed at around 1720 cm−1 must be due to the residual butyl acetate. If urethane bonds are formed between the hydroxyl groups of the epoxy polymer and the isocyanate groups of MDI, a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band originating from the urethane bond is expected to appear around 1730 cm−1.16 However, despite the high reactivity of the isocyanate groups to the hydroxyl groups, the IR band corresponding to the urethane bonds was too weak and indistinguishable from the C
O band originating from the urethane bond is expected to appear around 1730 cm−1.16 However, despite the high reactivity of the isocyanate groups to the hydroxyl groups, the IR band corresponding to the urethane bonds was too weak and indistinguishable from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of residual butyl acetate in the ATR-IR spectrum. It should be noted that an IR band at 2270 cm−1, which could be attributed to the isocyanate groups, was also observed. These results indicate that most of the MDI molecules are in the unreacted state with tiny amounts of the dimer being present in the vicinity of the epoxy-polymer surface. The expected urethane bond formation was not clearly detected in the IR measurements, which may be due to the large penetration depth of ATR-IR, which (using a germanium prism at 1730 cm−1) is approximately 400 nm. Therefore, if urethane C
O of residual butyl acetate in the ATR-IR spectrum. It should be noted that an IR band at 2270 cm−1, which could be attributed to the isocyanate groups, was also observed. These results indicate that most of the MDI molecules are in the unreacted state with tiny amounts of the dimer being present in the vicinity of the epoxy-polymer surface. The expected urethane bond formation was not clearly detected in the IR measurements, which may be due to the large penetration depth of ATR-IR, which (using a germanium prism at 1730 cm−1) is approximately 400 nm. Therefore, if urethane C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are formed only on the surface of the epoxy polymers, the corresponding C
O bonds are formed only on the surface of the epoxy polymers, the corresponding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands will be buried in the noise signals and may be hard to detect.
O bands will be buried in the noise signals and may be hard to detect.
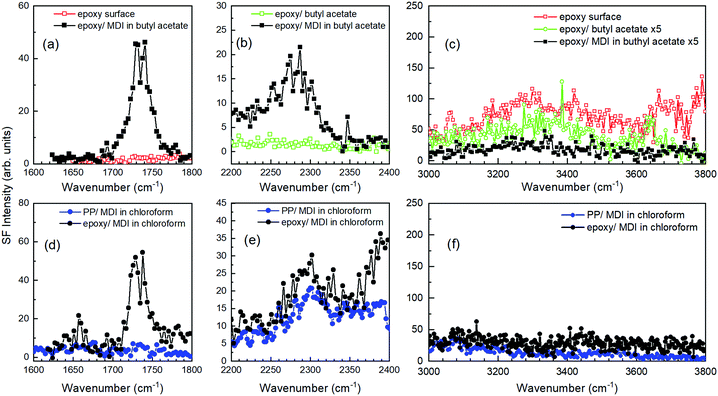
We then applied the SFG technique to investigate the interfacial chemical states as well as the functional groups and chemical bonds at the interface between the epoxy polymer and the MDI solution. Fig. 3a and d show the SFG spectra of the epoxy polymer in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching region in contact with MDI butyl acetate and chloroform solutions, respectively. Previous to the contact with the MDI solution, no C
O stretching region in contact with MDI butyl acetate and chloroform solutions, respectively. Previous to the contact with the MDI solution, no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak was observed at the epoxy surface, as shown in Fig. 3a. However, a C
O peak was observed at the epoxy surface, as shown in Fig. 3a. However, a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band appeared when the epoxy polymer was in contact with the MDI solution. In the case of the SFG results obtained from the interface between the epoxy polymer and the butyl acetate solution of MDI, the contribution from the C
O stretching band appeared when the epoxy polymer was in contact with the MDI solution. In the case of the SFG results obtained from the interface between the epoxy polymer and the butyl acetate solution of MDI, the contribution from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of butyl acetate should be taken into account as in the case of the ATR-IR. However, the appearance of a C
O stretching of butyl acetate should be taken into account as in the case of the ATR-IR. However, the appearance of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peak was also observed in the SFG spectrum taken from the interface between the epoxy polymer and the chloroform solution of MDI, as shown in Fig. 3d. Thus, we conclude that the SFG spectrum peak at 1730 cm−1 originates from the C
O stretching peak was also observed in the SFG spectrum taken from the interface between the epoxy polymer and the chloroform solution of MDI, as shown in Fig. 3d. Thus, we conclude that the SFG spectrum peak at 1730 cm−1 originates from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of urethane bonds formed at the epoxy surfaces. Since the urethane bonds are formed by the reaction between the isocyanate groups of MDI and the surface hydroxyl groups of the epoxy polymer, the SFG spectra of the hydroxyl groups of the epoxy polymer should disappear upon reaction with the isocyanate of the MDI. Fig. 3c and f show the SFG spectra of the epoxy-polymer interfaces before and after being in contact with the butyl acetate and chloroform solutions of MDI in the OH-stretching region. Although the hydrogen-bonded OH bands are clearly visible in the SFG spectra of the epoxy polymer before contact with the MDI solutions, they disappear completely by contacting the MDI solution. In contrast, OH bands remain in the SFG spectra of the epoxy/butyl acetate solvent interface. The fact that the OH bands completely disappear by contacting with MDI–butyl acetate solution and do not disappear only by contacting with butyl acetate solvent without MDI may support the conclusion that the OH group on the epoxy surface was used for the reaction with MDI. Even if not, there is certainly an interaction between MDI and OH in the epoxy polymer. Note that the intensity of the OH stretching band became weak in the SFG spectrum in contact with liquids, as depicted in Fig. 3c. This must be due to the Fresnel factor difference between solid/air and solid/liquid interfaces. Although the SFG spectrum peak intensity does not only represent the amount of surface functional groups, this result suggests that the hydroxyl groups on the epoxy polymer surfaces have reacted with the isocyanate groups on the MDI. For comparison, we also show the polypropylene/MDI chloroform solution in Fig. 3d–f. Since polypropylene does not have a hydroxyl group, it can be found that no C
O stretching of urethane bonds formed at the epoxy surfaces. Since the urethane bonds are formed by the reaction between the isocyanate groups of MDI and the surface hydroxyl groups of the epoxy polymer, the SFG spectra of the hydroxyl groups of the epoxy polymer should disappear upon reaction with the isocyanate of the MDI. Fig. 3c and f show the SFG spectra of the epoxy-polymer interfaces before and after being in contact with the butyl acetate and chloroform solutions of MDI in the OH-stretching region. Although the hydrogen-bonded OH bands are clearly visible in the SFG spectra of the epoxy polymer before contact with the MDI solutions, they disappear completely by contacting the MDI solution. In contrast, OH bands remain in the SFG spectra of the epoxy/butyl acetate solvent interface. The fact that the OH bands completely disappear by contacting with MDI–butyl acetate solution and do not disappear only by contacting with butyl acetate solvent without MDI may support the conclusion that the OH group on the epoxy surface was used for the reaction with MDI. Even if not, there is certainly an interaction between MDI and OH in the epoxy polymer. Note that the intensity of the OH stretching band became weak in the SFG spectrum in contact with liquids, as depicted in Fig. 3c. This must be due to the Fresnel factor difference between solid/air and solid/liquid interfaces. Although the SFG spectrum peak intensity does not only represent the amount of surface functional groups, this result suggests that the hydroxyl groups on the epoxy polymer surfaces have reacted with the isocyanate groups on the MDI. For comparison, we also show the polypropylene/MDI chloroform solution in Fig. 3d–f. Since polypropylene does not have a hydroxyl group, it can be found that no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch appears at the interface, even when it contacts with the MDI solution. It should be noted that the peak due to the hydroxyl groups on the epoxy-polymer surface may be derived not only from the OH groups on the polymer surface but also from the water molecules adsorbed on the epoxy polymer surface.
O stretch appears at the interface, even when it contacts with the MDI solution. It should be noted that the peak due to the hydroxyl groups on the epoxy-polymer surface may be derived not only from the OH groups on the polymer surface but also from the water molecules adsorbed on the epoxy polymer surface.
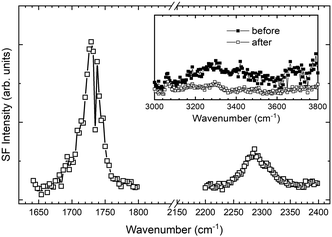
Fig. 3b and e show the SFG spectra of the interfaces between the epoxy polymer and the MDI solutions in the NCO-stretching region. Interestingly, the asymmetric stretching mode of the isocyanate groups in the MDI molecule was observed at 2270 cm−1 for both the butyl acetate and chloroform solutions of MDI epoxy interfaces. This peak could be derived from unreacted isocyanate groups of MDI, and this peak is also observed in the ATR-IR. Actually, this unreacted NCO peak is also observed in the SFG spectrum of the polypropylene/MDI solution interface. To remove any unreacted MDI molecules adsorbed at the epoxy interfaces, the epoxy polymers were rinsed with acetone after being in contact with the MDI–butyl acetate solution. To avoid the influence of residual butyl acetate and acetone, the samples were then heated at 80 °C. Even after rinsing and heating, peaks derived from both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NCO stretching could be observed at the epoxy polymer surface, as shown in Fig. 4. We also show the SFG spectra of the epoxy surface in the OH stretching region before and after MDI solution treatment in Fig. 4. The presence of C
O and NCO stretching could be observed at the epoxy polymer surface, as shown in Fig. 4. We also show the SFG spectra of the epoxy surface in the OH stretching region before and after MDI solution treatment in Fig. 4. The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NCO peaks clearly indicates that one of the isocyanate groups present in MDI reacts with the hydroxyl groups of the epoxy polymers. Although the SFG spectrum peak intensity does not represent only the amount of the surface functional groups, decrease in intensity of the SFG spectrum peaks derived from OH bands after the MDI treatment may support this hypothesis. Since the MDI molecule has two isocyanates, the other side of the isocyanate group remains in the unreacted state. It should be noted that the C
O and NCO peaks clearly indicates that one of the isocyanate groups present in MDI reacts with the hydroxyl groups of the epoxy polymers. Although the SFG spectrum peak intensity does not represent only the amount of the surface functional groups, decrease in intensity of the SFG spectrum peaks derived from OH bands after the MDI treatment may support this hypothesis. Since the MDI molecule has two isocyanates, the other side of the isocyanate group remains in the unreacted state. It should be noted that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak is not derived from the acetone molecules adsorbed on the polymer surface because the C
O peak is not derived from the acetone molecules adsorbed on the polymer surface because the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of acetone appears at 1700 cm−1 (see ESI†), and the C
O peak of acetone appears at 1700 cm−1 (see ESI†), and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of acetone completely disappears after heating. Therefore, we conclude that one side of the isocyanates of MDI reacts with the surface hydroxyl group of the epoxy to form the urethane bond upon contact with the MDI solution.
O peak of acetone completely disappears after heating. Therefore, we conclude that one side of the isocyanates of MDI reacts with the surface hydroxyl group of the epoxy to form the urethane bond upon contact with the MDI solution.
From these observations, a possible chemical reaction occurring at the epoxy-polymer interface was postulated, as shown in Fig. 5. When the epoxy-polymer surface is in contact with the MDI solution, the isocyanate groups at one side of the MDI molecules react promptly with the hydroxyl groups on the epoxy-polymer surface. On the other hand, the residual isocyanate groups on the opposite side of the MDI molecule remain in the unreacted state. Since isocyanate groups show a high reactivity toward polyol groups, which are the components of polyurethane adhesives, the residual isocyanate functions of MDI may react with the polyol groups in the adhesive. Actually, the SFG spectrum signals of the residual isocyanate completely disappeared when the MDI-treated epoxy surface was in contact with polyol (data not shown). As a result, chemical bridges between the epoxy polymer and the adhesive are formed by the MDI promoters. This shall improve the adhesion strength between the epoxy polymers and the polyurethane adhesives. In fact, primers containing molecules that have isocyanate groups have been used to promote the adhesion between clear epoxy coatings and polyurethane adhesives for several decades.24,25
In conclusion, we have successfully analysed the interface between an epoxy polymer and an MDI solution by ATR-IR and SFG spectroscopy. ATR-IR experiments demonstrated that most of the MDI dissolved in butyl acetate was in an unreacted state. On the other hand, the SFG spectral analysis indicated the presence of urethane bonds derived from the reaction between the isocyanate groups of MDI and the hydroxyl groups of the epoxy polymer. Considering that it was not possible to detect these urethane bonds by ATR-IR, it could be concluded that the chemical reaction between the isocyanate primer and the epoxy polymer occurred only on the outermost surface of the epoxy polymer. Furthermore, the SFG spectra of the rinsed epoxy-polymer surface (after being in contact with the MDI solution) indicated the presence of unreacted isocyanate functions after the formation of the urethane bonds. These results suggest that MDI primers applied to epoxy polymers can serve as a chemical bridge for urethane adhesives. Although this mechanism has already been proposed, this study represents the first direct evidence of chemical-bond formation occurring only at the adherent surfaces. Further experiments to investigate the adhesive and polymer interfaces are currently in progress.
KS sincerely thanks Kazuomi Wakabayashi for fruitful advice about selecting the materials and Dr Kozo Miyoshi for the great help in the preparation of the substrates. Part of this work was supported by a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Conflicts of interest
The authors declare that there are no conflicts of interest.Notes and references
- G. Meschut, V. Janzen and T. Olfermann, J. Mater. Eng. Perform., 2014, 23, 1515 CrossRef CAS.
- F. Awaja, M. Gilbert, G. Kelly, B. Fox and P. Pigram, Prog. Polym. Sci., 2009, 34, 948 CrossRef CAS (and references therein).
- A. Baldan, Int. J. Adhes. Adhes., 2012, 38, 95 CrossRef CAS.
- S. Ebnesajjad and A. H. Landrock, Adhesion Technology Handbook, Willian Andrew Publisher, New York, 2009 Search PubMed.
- K. Jub, Chimia, 1990, 44, 321 Search PubMed.
- J. R. J. Wingfield, Int. J. Adhes. Adhes., 1993, 13, 151 CrossRef CAS.
- R. Snyders, O. Zabeida, C. Roberges, K. I. Shingel, M.-P. Faure, L. Marinu and J. E. Klemberg-Sapieha, Surf. Sci., 2007, 601, 112 CrossRef CAS.
- X. Lu, C. Zhang, N. Ulrich, M. Xiao, Y. H. Ma and Z. Chen, Anal. Chem., 2017, 89, 466 CrossRef CAS PubMed.
- C. Zhang, Appl. Spectrosc., 2017, 71, 1717 CrossRef CAS PubMed.
- X. Zhuang, P. B. Miranda, D. Kim and Y. R. Shen, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 12632 CrossRef CAS.
- T. Miyamae, Y. Yamada, H. Uyama and H. Nozoye, Appl. Surf. Sci., 2001, 180, 126 CrossRef CAS.
- T. Miyamae and H. Nozoye, Appl. Phys. Lett., 2004, 85, 4373 CrossRef CAS.
- T. Miyamae, H. Akiyama, M. Yoshida and N. Tamaoki, Macromolecules, 2007, 40, 4601 CrossRef CAS.
- C. Urata, B. Masheder, D. F. Cheng, D. F. Miranda, G. J. Dunderdale, T. Miyamae and A. Hozumi, Langmuir, 2014, 30, 4049 CrossRef CAS PubMed.
- T. Sato, H. Akiyama, S. Horiuchi and T. Miyamae, Surf. Sci., 2018, 677, 93 CrossRef CAS.
- R. D. Adams, Adhesive Bonding, Science, Technology and Applications, Woodhead Pub, Cambridge, 2005 Search PubMed.
- G. L. Schneberger, Adhesives in Manufacturing, Tayler & Francis Inc., Oxfordshire, 1983 Search PubMed.
- S. R. Hartshorn, Structural Adhesives Chemistry and Technology, Springer Publishing, New York, 1986 Search PubMed.
- H. L. Lee, A. L. Cupples, R. J. Schubert and M. L. Swartz, J. Dent. Res., 1971, 50, 125 CrossRef CAS PubMed.
- M. Furukawa, Nippon Gomu Kyokaishi, 2011, 84, 125 Search PubMed.
- H. Ulrich, J. Cell. Plast., 1981, 17, 31 CrossRef CAS.
- J. N. Gibb and J. M. Goodman, Org. Biomol. Chem., 2013, 11, 90 RSC.
- P. I. Kordomenos and J. E. Kresta, Macromolecules, 1981, 14, 1434 CrossRef CAS.
- H. Okamoto, J. Adhes. Soc. Jpn., 2006, 42, 450 CrossRef CAS.
- Y. Nakata, J. Adhes. Soc. Jpn., 2003, 39, 455 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental set ups and SFG spectra of the epoxy polymer surface just after contacting MDI solution and after annealing. See DOI: 10.1039/c9cc05911c |
| This journal is © The Royal Society of Chemistry 2019 |