Open Access Article
Open Access ArticleStructure and thermal expansion of the distorted Prussian blue analogue RbCuCo(CN)6†
Hanna L. B.
Boström
 *ab and
Ronald I.
Smith
*ab and
Ronald I.
Smith
 c
c
aDepartment of Chemistry, Ångström Laboratory, Uppsala University, Box 538, 751 21 Uppsala, Sweden. E-mail: hanna.bostrom@kemi.uu.se
bDepartment of Chemistry, Inorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QR, UK
cISIS Facility, Rutherford Appleton Laboratory, Harwell Campus, Didcot OX11 0QX, UK
First published on 30th July 2019
Abstract
The structure and thermal expansion of the Prussian blue analogue RbCuCo(CN)6 has been determined via neutron and X-ray powder diffraction. The system crystallises in Cccm and harbours three coexisting distortions relative to the parent Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure, which leads to anisotropic thermal expansion with a near-zero component in one direction. The difficulties associated with determining octahedral tilt systems in Prussian blue analogues and related double molecular perovskites are discussed.
m structure, which leads to anisotropic thermal expansion with a near-zero component in one direction. The difficulties associated with determining octahedral tilt systems in Prussian blue analogues and related double molecular perovskites are discussed.
Prussian blue analogues (PBAs) are a class of cyanide-linked coordination polymers with general formula AxM[M′(CN)6]y and high-symmetry parent space group Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The composition is variable and ranges from stoichiometric, A-site deficient M[M′(CN)6] to defective M[M′(CN)6]y (y < 1) or cation-containing AM[M′(CN)6], depending on the charges on the metals.1–3 The two transition metals M and M′ are both octahedrally coordinated and form a 3D framework analogous to double perovskites (A2BB′X6). This article focuses on the composition AMM′(CN)6, where only half of the cubic interstices (“A-sites” in perovskite terminology) are occupied by cations. PBAs can be considered a subset of so-called molecular or hybrid perovskites,4,5 which are characterised by high flexibility and additional degrees of freedom in comparison to conventional oxide perovskites.6–8 For example, the octahedra can rotate in phase in directions perpendicular to the rotation axis or translate cooperatively in columns or layers, denoted “unconventional” tilting and columnar shifts, respectively.6,7,9 Many molecular perovskites—e.g. azides,10 hypophosphites,11 or formates12—are very flexible and predominantly adopt distorted low-symmetry (hettotype) structures. Although cubic space groups dominate for PBAs,13,14 symmetry-lowering distortions occur under certain conditions and can act as useful design elements for crystal engineering. For example, the inclusion of Jahn–Teller active cations in CuPt(CN)6 lowers the symmetry to tetragonal I4/mmm, which significantly alters the thermal expansion and compressibility.15,16 Moreover, octahedral tilting—as is well known in perovskites—can be induced by external or chemical pressure and has been shown to both improve the ionic transport properties and enhance the magnetic ordering temperature.16–19
m. The composition is variable and ranges from stoichiometric, A-site deficient M[M′(CN)6] to defective M[M′(CN)6]y (y < 1) or cation-containing AM[M′(CN)6], depending on the charges on the metals.1–3 The two transition metals M and M′ are both octahedrally coordinated and form a 3D framework analogous to double perovskites (A2BB′X6). This article focuses on the composition AMM′(CN)6, where only half of the cubic interstices (“A-sites” in perovskite terminology) are occupied by cations. PBAs can be considered a subset of so-called molecular or hybrid perovskites,4,5 which are characterised by high flexibility and additional degrees of freedom in comparison to conventional oxide perovskites.6–8 For example, the octahedra can rotate in phase in directions perpendicular to the rotation axis or translate cooperatively in columns or layers, denoted “unconventional” tilting and columnar shifts, respectively.6,7,9 Many molecular perovskites—e.g. azides,10 hypophosphites,11 or formates12—are very flexible and predominantly adopt distorted low-symmetry (hettotype) structures. Although cubic space groups dominate for PBAs,13,14 symmetry-lowering distortions occur under certain conditions and can act as useful design elements for crystal engineering. For example, the inclusion of Jahn–Teller active cations in CuPt(CN)6 lowers the symmetry to tetragonal I4/mmm, which significantly alters the thermal expansion and compressibility.15,16 Moreover, octahedral tilting—as is well known in perovskites—can be induced by external or chemical pressure and has been shown to both improve the ionic transport properties and enhance the magnetic ordering temperature.16–19
However, unlike many molecular perovskites, relatively few PBAs exhibit more than one structural distortion. Coexistence of distortions is important, as it provides a potential avenue for the development of polar structures by improper ferroelectricity, as reported for other molecular perovskites.20–22 Yet, some multiply distorted PBAs are known and they often show interesting features. To illustrate, the charge-transfer system RbMnFe(CN)6 contains both alternating occupational Rb order and Jahn–Teller distortions, which reduces the symmetry to the piezoelectric space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2.23 Ferromagnetism and ferroelectricity have been demonstrated for a similar composition.24 A related, distorted PBA is RbCuFe(CN)6, where the departure from Fm
m2.23 Ferromagnetism and ferroelectricity have been demonstrated for a similar composition.24 A related, distorted PBA is RbCuFe(CN)6, where the departure from Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry is described by three distortions: octahedral tilting, Rb order, and Jahn–Teller distortions.25 Structure determination is typically performed using powder X-ray diffraction (PXRD), but this does not accurately probe Jahn–Teller distortions and tilts, as these modes only manifest in the positions of the weakly scattering cyanide ions. This motivates the present study, where we use X-ray and neutron powder diffraction to study the previously unreported RbCuCo(CN)6, which is presumably isostructural to the hexacyanoferrate mentioned above. This manuscript discusses the structural distortions and their effect on the thermal expansion followed by a discussion about the difficulties encountered when determining tilt systems for PBAs.
m symmetry is described by three distortions: octahedral tilting, Rb order, and Jahn–Teller distortions.25 Structure determination is typically performed using powder X-ray diffraction (PXRD), but this does not accurately probe Jahn–Teller distortions and tilts, as these modes only manifest in the positions of the weakly scattering cyanide ions. This motivates the present study, where we use X-ray and neutron powder diffraction to study the previously unreported RbCuCo(CN)6, which is presumably isostructural to the hexacyanoferrate mentioned above. This manuscript discusses the structural distortions and their effect on the thermal expansion followed by a discussion about the difficulties encountered when determining tilt systems for PBAs.
It is convenient to describe the symmetry-lowering distortion by means of an irreducible representation (irrep) with respect to parent space group. Irreps in cubic periodic structures assume the form k±#, where k denotes the periodicity of the distortion. The exact form of the irrep is dependent on the origin choice and here, the origin is located at one of the transition metals (B-site in perovskite terminology) throughout. For a conversion to other settings, see ref. 22.
RbCuCo(CN)6 was prepared by carefully placing 2 ml of an aqueous solution of CuCl2 (67.2 mg, 0.5 mmol) on top of 2 ml of an aqueous solution of K3Co(CN)6 (166.2 mg, 0.5 mmol) saturated with RbCl. The reaction was left undisturbed for 1 day, followed by vigorous mixing and the pale blue solid collected by filtration in vacuo. Yields were in the range 69–79% based on CuCl2. The sample composition was verified by energy-dispersive X-ray spectroscopy (EDX) to be Rb0.90Cu[Co(CN)6]0.92. The water content was estimated from thermogravimetric analysis as 3–5 water molecules per formula unit. 5 batches were combined for the neutron diffraction measurement and data analysis was carried out by symmetry-mode Rietveld refinement as implemented in the softwares TOPAS and ISODISTORT.26–28 Further details about the characterisation are given in the ESI.†
Indexing of the PXRD pattern collected at ambient conditions suggested the space group Ccc2 with cell parameters a ∼ 10.9 Å, b ∼ 9.99 Å and c ∼ 10.03 Å (note that a and b are interchangeable in this space group). As PBAs are rarely polar, the centrosymmetric minimal subgroup Cccm—which has an identical diffraction pattern—was used for refinement. This differs from the Pmna symmetry reported for RbCuFe(CN)6,25 which is a maximal subgroup of Cccm. Therefore, the structure reported for RbCuFe(CN)6 is possibly solved in a space group with too low symmetry. Group-theoretical analysis using ISODISTORT27 indicates that the symmetry lowering from Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m to Cccm arises from a superposition of three modes: a Jahn–Teller distortion (irrep Γ+3), an octahedral tilt (irrep X+3) and a rod-like occupational order of the Rb cations (irrep X+4). The combination of any two of these distortions gives the third as a secondary order parameter, hence it is sufficient to specify two of these distortion modes. In other words, the cation order can be said to arise from the combination of the Jahn–Teller distortion and the tilt, or vice versa that the tilt results from the Jahn–Teller distortion and the cation order. The extent to which the third mode is activated will depend on the energies of the three modes, but such information is not available using group-theoretical methods. This coupling can also be inferred by comparing RbCuCo(CN)6 to the P
m to Cccm arises from a superposition of three modes: a Jahn–Teller distortion (irrep Γ+3), an octahedral tilt (irrep X+3) and a rod-like occupational order of the Rb cations (irrep X+4). The combination of any two of these distortions gives the third as a secondary order parameter, hence it is sufficient to specify two of these distortion modes. In other words, the cation order can be said to arise from the combination of the Jahn–Teller distortion and the tilt, or vice versa that the tilt results from the Jahn–Teller distortion and the cation order. The extent to which the third mode is activated will depend on the energies of the three modes, but such information is not available using group-theoretical methods. This coupling can also be inferred by comparing RbCuCo(CN)6 to the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 phase of RbMnFe(CN)6.23 The latter has no Jahn–Teller distortion and therefore also lacks the Rb order, despite possessing the X+3 tilt present in RbCuCo(CN)6.
n2 phase of RbMnFe(CN)6.23 The latter has no Jahn–Teller distortion and therefore also lacks the Rb order, despite possessing the X+3 tilt present in RbCuCo(CN)6.
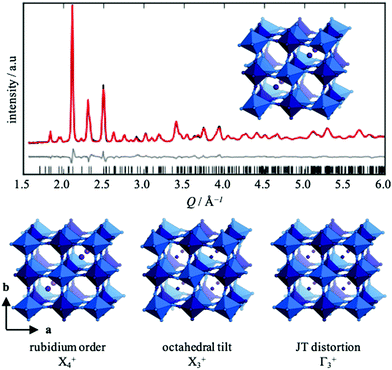
Symmetry-mode refinement against neutron diffraction data [Fig. 1] gives accurate information about the relative magnitude of the three distortions. The most striking distortion is the occupational order of the A-site Rb cations, which also induces a small deformation of the octahedra. This type of order also appears in RbCuFe(CN)6, but is otherwise less prevalent than the alternating pattern (irrep R−2), giving F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m symmetry.23,29,30 The strongest displacive mode—as calculated by the software AMPLIMODES31—is the octahedral tilt described by a0a0c+ in Glazer notation,32i.e. an in-phase tilt polarised along a single axis. This tilt system is relatively infrequent in PBAs, where the dominant tilt patterns are a−a−a− or a−a−c+,16,33–36 yet has a precedent in the metastable P
3m symmetry.23,29,30 The strongest displacive mode—as calculated by the software AMPLIMODES31—is the octahedral tilt described by a0a0c+ in Glazer notation,32i.e. an in-phase tilt polarised along a single axis. This tilt system is relatively infrequent in PBAs, where the dominant tilt patterns are a−a−a− or a−a−c+,16,33–36 yet has a precedent in the metastable P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 phase of RbMnFe(CN)6.23 It is a conventional tilt pattern, unlike the unconventional tilt—where neighbouring octahedra rotate perpendicular to the rotation axis tilt in the same sense7,9—present in the original Pmna structure for RbCuFe(CN)6 suggested by Matsuda and coworkers.25 Finally, the cooperative Jahn–Teller effect manifests as a cooperative elongation of the CuN6 octahedra along a, and mirrors that of CuPt(CN)6, ACuFe(CN)6 (A = Cs or Rb) and the low-temperature form of RbMnFe(CN)6.15,25,37 In Jahn–Teller active formates and perovskite oxides, several distinct periodicities of the cooperative Jahn–Teller order are possible,38,39 whereas defect-free Jahn–Teller-active PBAs always show in-phase arrangements (k = [0, 0, 0]) of the long axes.15,40 This gives a macroscopic symmetry lowering, in contrast to most defective Cu-containing systems, which tend to adopt the cubic aristotype symmetry.41,42 Since A-site cations and vacancies compete, the inclusion of Rb in the title compound prevents the formation of vacancies and so leads to Jahn–Teller order. As these examples highlight, all of the distortions present in RbCuCo(CN)6 are precedented, yet they rarely coexist.
n2 phase of RbMnFe(CN)6.23 It is a conventional tilt pattern, unlike the unconventional tilt—where neighbouring octahedra rotate perpendicular to the rotation axis tilt in the same sense7,9—present in the original Pmna structure for RbCuFe(CN)6 suggested by Matsuda and coworkers.25 Finally, the cooperative Jahn–Teller effect manifests as a cooperative elongation of the CuN6 octahedra along a, and mirrors that of CuPt(CN)6, ACuFe(CN)6 (A = Cs or Rb) and the low-temperature form of RbMnFe(CN)6.15,25,37 In Jahn–Teller active formates and perovskite oxides, several distinct periodicities of the cooperative Jahn–Teller order are possible,38,39 whereas defect-free Jahn–Teller-active PBAs always show in-phase arrangements (k = [0, 0, 0]) of the long axes.15,40 This gives a macroscopic symmetry lowering, in contrast to most defective Cu-containing systems, which tend to adopt the cubic aristotype symmetry.41,42 Since A-site cations and vacancies compete, the inclusion of Rb in the title compound prevents the formation of vacancies and so leads to Jahn–Teller order. As these examples highlight, all of the distortions present in RbCuCo(CN)6 are precedented, yet they rarely coexist.
The thermal dependence of the lattice parameters was monitored in the range 100–500 K using high-resolution PXRD and the thermal expansion coefficients calculated by PASCal [Fig. 2].43 RbCuCo(CN)6 shows an anisotropic thermal response, which can be interpreted in terms of the structural distortions. The expansivity is positive in the ab-plane, with the largest magnitude in the a direction of 31.5(6) M K−1 and a smaller value of 4.29(13) M K−1 along b. These values are within the range observed for the magnitude of the thermal expansion coefficients in other PBAs.44 The thermal expansion along the c direction—where the Cu–N–C–Co linkages are linear on average—is negative and very small (−1.7(3) M K−1). This suggests the presence of a low-energy transverse phonon; a known cause of negative thermal expansion (NTE) in framework materials.45 As a result, further phase transitions may be expected on cooling, as the soft mode condenses. Although NTE is a relatively rare phenomenon in general, it frequently appears in Prussian blue analogues, although there is a strong dependence on the metal composition.15,44 The thermal expansion is consistent with a decrease in the tilting angles upon heating and suggests that the tilting vanishes at even higher temperatures.
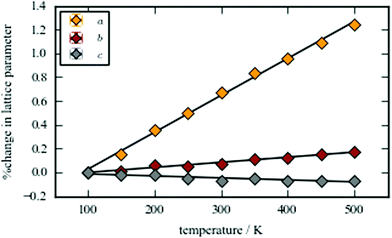 | ||
| Fig. 2 The percentage change of the lattice parameters as a function of temperature, along with lines of best fit. | ||
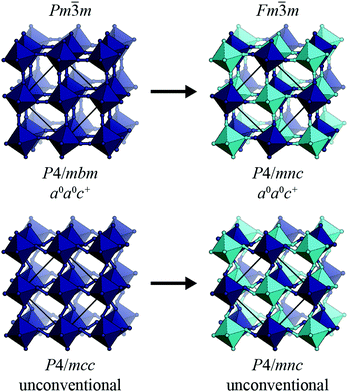
The determination of tilts in double molecular perovskites from the space group symmetry can be challenging. In simple (ABX3) perovskites, distortions with periodicity 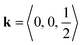 and
and 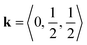 transform as different irreps, labelled X and M, respectively. However, in face-centred double perovskites (A2BB′X6), these points in reciprocal space are equivalent by symmetry and so result in the same space group (see ESI† for further discussion). Thus, determination of the correct space group does not uniquely define the tilt pattern [Fig. 3]. By way of example, the space group P4/mbm is usually a signature of in-phase tilting (a0a0c+) for a simple perovskite.46 In a double perovskite, this tilt system instead drives P4/mnc symmetry,47 yet in molecular double perovskites—which have more degrees of freedom—this space group can also arise from the unconventional tilt system described by
transform as different irreps, labelled X and M, respectively. However, in face-centred double perovskites (A2BB′X6), these points in reciprocal space are equivalent by symmetry and so result in the same space group (see ESI† for further discussion). Thus, determination of the correct space group does not uniquely define the tilt pattern [Fig. 3]. By way of example, the space group P4/mbm is usually a signature of in-phase tilting (a0a0c+) for a simple perovskite.46 In a double perovskite, this tilt system instead drives P4/mnc symmetry,47 yet in molecular double perovskites—which have more degrees of freedom—this space group can also arise from the unconventional tilt system described by
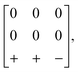 | (1) |
To summarise, the structure of the Prussian blue analogue RbCuCo(CN)6 has been studied with X-ray and neutron powder diffraction. The system exhibits rod-like occupational rubidium order, octahedral tilts and Jahn–Teller distortions, which together reduce the symmetry from the parent space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m to Cccm. Although distorted Prussian blue analogues are known, coexistence of several distortions is less common, but is a prerequisite for e.g. the development of hybrid improper ferroelectricity.20,22 Group-theoretical analysis highlights the coupling of the three distortions, where the presence of two induces the third. The potential problems associated with the determination of tilt systems for Prussian blue analogues highlighted here have implications for research on both PBAs and other systems in a large number of fields. In particular, tilting is often encountered for A-site containing PBAs, which are of currency for their performance as positive electrode materials in Na ion batteries.51,52
m to Cccm. Although distorted Prussian blue analogues are known, coexistence of several distortions is less common, but is a prerequisite for e.g. the development of hybrid improper ferroelectricity.20,22 Group-theoretical analysis highlights the coupling of the three distortions, where the presence of two induces the third. The potential problems associated with the determination of tilt systems for Prussian blue analogues highlighted here have implications for research on both PBAs and other systems in a large number of fields. In particular, tilting is often encountered for A-site containing PBAs, which are of currency for their performance as positive electrode materials in Na ion batteries.51,52
We thank the ISIS neutron and muon source for Xpress beamtime at the GEM diffractometer (data DOI: http://10.5286/ISIS.E.RB1990103).53 High-resolution XRD was carried out at I11, Diamond Light Source, UK under the allocation of a Block Award Group award EE18786. Assistance from W. R. Brant (Uppsala) with synthesis, D. Karlsson (Uppsala) with EDX measurements, A. Gibbs (ISIS) with data collection, and S. J. Cassidy (Oxford) with data analysis is greatly appreciated. M. S. Senn (Warwick) is acknowledged for useful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H.-J. Buser, G. Ron and A. Ludi, J. Chem. Soc., Faraday Trans., 1974, 2473–2474 CAS.
- H. J. Buser, D. Schwarzenbach, W. Petter and A. Ludi, Inorg. Chem., 1977, 16, 2704–2710 CrossRef CAS.
- A. Bleuzen, C. Lomenech, V. Escax, F. Villain, F. Varret, C. Cartier dit Moulin and M. Verdaguer, J. Am. Chem. Soc., 2000, 122, 6648–6652 CrossRef CAS.
- G. Kieslich and A. L. Goodwin, Mater. Horiz., 2017, 4, 362–366 RSC.
- W. Li, Z. Wang, F. Deschler, S. Gao, R. H. Friend and A. K. Cheetham, Nat. Rev. Mater., 2017, 2, 16099 CrossRef.
- H. L. B. Boström, J. A. Hill and A. L. Goodwin, Phys. Chem. Chem. Phys., 2016, 18, 31881–31894 RSC.
- S. G. Duyker, J. A. Hill, C. J. Howard and A. L. Goodwin, J. Am. Chem. Soc., 2016, 138, 11121–11123 CrossRef CAS PubMed.
- W. Zhang, Y. Cai, R.-G. Xiong, H. Yoshikawa and K. Awaga, Angew. Chem., Int. Ed., 2010, 49, 6608–6610 CrossRef CAS PubMed.
- J. A. Hill, A. L. Thompson and A. L. Goodwin, J. Am. Chem. Soc., 2016, 138, 5886–5896 CrossRef CAS PubMed.
- X.-H. Zhao, X.-C. Huang, S.-L. Zhang, D. Shao, H.-Y. Wei and X.-Y. Wang, J. Am. Chem. Soc., 2013, 135, 16006–16009 CrossRef CAS PubMed.
- Y. Wu, T. Binford, J. A. Hill, S. Shaker, J. Wang and A. K. Cheetham, Chem. Commun., 2018, 54, 3751–3754 RSC.
- X.-Y. Wang, L. Gan, S.-W. Zhang and S. Gao, Inorg. Chem., 2004, 43, 4615–4625 CrossRef CAS PubMed.
- J. Balmaseda, E. Reguera, J. Rodríguez-Hernández, L. Reguera and M. Autie, Microporous Mesoporous Mater., 2006, 96, 222–236 CrossRef CAS.
- J. Roque, E. Reguera, J. Balmaseda, J. Rodríguez-Hernández, L. Reguera and L. F. del Castillo, Microporous Mesoporous Mater., 2007, 103, 57–71 CrossRef CAS.
- K. W. Chapman, P. J. Chupas and C. J. Kepert, J. Am. Chem. Soc., 2006, 128, 7009–7014 CrossRef CAS PubMed.
- H. L. B. Boström, I. E. Collings, A. B. Cairns, C. P. Romao and A. L. Goodwin, Dalton Trans., 2019, 48, 1647–1655 RSC.
- X. Liu, Y. Moritomo, T. Matsuda, H. Kamioka, H. Tokoro and S.-I. Ohkoshi, J. Phys. Soc. Jpn., 2009, 78, 013602 CrossRef.
- M. Sugimoto, S. Yamashita, H. Akutsu, Y. Nakazawa, J. G. DaSilva, C. M. Kareis and J. S. Miller, Inorg. Chem., 2017, 56, 10452–10457 CrossRef CAS PubMed.
- Y. Xu, J. Wan, L. Huang, M. Ou, C. Fan, P. Wei, J. Peng, Y. Liu, Y. Qiu, X. Sun, C. Fang, Q. Li, J. Han, Y. Huang, J. A. Alonso and Y. Zhao, Adv. Energy Mater., 2019, 9, 1803158 CrossRef.
- N. A. Benedek and C. J. Fennie, Phys. Rev. Lett., 2011, 106, 107204 CrossRef PubMed.
- A. Stroppa, P. Jain, P. Barone, M. Marsman, J. M. Perez-Mato, A. K. Cheetham, H. W. Kroto and S. Picozzi, Angew. Chem., Int. Ed., 2011, 50, 5847–5850 CrossRef CAS PubMed.
- H. L. B. Boström, M. S. Senn and A. L. Goodwin, Nat. Commun., 2018, 9, 2380 CrossRef PubMed.
- Y. Moritomo, M. Hanawa, Y. Ohishi, K. Kato, M. Takata, A. Kuriki, E. Nishibori, M. Sakata, S. Ohkoshi, H. Tokoro and K. Hashimoto, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 144106 CrossRef.
- S.-i. Ohkoshi, H. Tokoro, T. Matsuda, H. Takahashi, H. Irie and K. Hashimoto, Angew. Chem., Int. Ed., 2007, 46, 3238–3241 CrossRef CAS PubMed.
- T. Matsuda, J. Kim and Y. Moritomo, Dalton Trans., 2012, 41, 7620–7623 RSC.
- H. M. Rietveld, Acta Crystallogr., 1967, 22, 151–152 CrossRef CAS.
- B. J. Campbell, H. T. Stokes, D. E. Tanner and D. M. Hatch, J. Appl. Crystallogr., 2006, 39, 607–614 CrossRef CAS.
- A. A. Coelho, TOPAS-Academic, version 4.1 (computer software), Coelho Software, Brisbane, 2007 Search PubMed.
- E. J. M. Vertelman, T. T. A. Lummen, A. Meetsma, M. W. Bouwkamp, G. Molnar, P. H. M. van Loosdrecht and P. J. van Konigsbruggen, Chem. Mater., 2008, 20, 1236–1238 CrossRef CAS.
- T. Nuida, T. Matsuda, H. Tokoro, S. Sakurai, K. Hashimoto and S.-I. Ohkoshi, J. Am. Chem. Soc., 2005, 127, 11604–11605 CrossRef CAS PubMed.
- D. Orobengoa, C. Capillas, M. I. Aroyo and J. M. Perez-Mato, J. Appl. Crystallogr., 2009, 42, 820–833 CrossRef CAS.
- A. M. Glazer, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 3384–3392 CrossRef CAS.
- C. M. Kareis, S. H. Lapidus, J.-H. Her, P. W. Stephens and J. S. Miller, J. Am. Chem. Soc., 2012, 134, 2246–2254 CrossRef CAS PubMed.
- J.-H. Her, P. W. Stephens, C. M. Kareis, J. G. Moore, K. S. Min, J.-W. Park, G. Ball, B. S. Kennon and J. S. Miller, Inorg. Chem., 2010, 49, 1524–1534 CrossRef CAS PubMed.
- J. Song, L. Wang, Y. Lu, J. Liu, B. Guo, P. Xiao, J.-J. Lee, X.-G. Yang, G. Henkelman and J. B. Goodenough, J. Am. Chem. Soc., 2015, 137, 2658–2664 CrossRef CAS PubMed.
- X. Bie, K. Kubota, T. Hosaka, K. Chihara and S. Komaba, J. Mater. Chem. A, 2017, 5, 4325–4330 RSC.
- H. Tokoro, S.-i. Ohkoshi, T. Matsuda and K. Hashimoto, Inorg. Chem., 2004, 43, 5231–5236 CrossRef CAS PubMed.
- K.-L. Hu, M. Kurmoo, Z. Wang and S. Gao, Chem. – Eur. J., 2009, 15, 12050–12064 CrossRef CAS PubMed.
- E. Sletten and L. H. Jensen, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 1752–1756 CrossRef CAS.
- W. Kosaka, T. Ishihara, H. Yashiro, Y. Taniguchi, K. Hashimoto and S.-I. Ohkoshi, Chem. Lett., 2005, 34, 1278–1279 CrossRef CAS.
- E. Reguera, J. Rodríguez-Hernández, A. Champi, J. G. Duque, E. Granado and C. Rettori, Z. Phys. Chem., 2006, 220, 1609–1619 CrossRef CAS.
- J. Jiménez-Gallegos, J. Rodríguez-Hernández, H. Yee-Madeira and E. Reguera, J. Phys. Chem. C, 2010, 114, 5043–5048 CrossRef.
- M. J. Cliffe and A. L. Goodwin, J. Appl. Crystallogr., 2012, 45, 1321–1329 CrossRef CAS.
- S. Adak, L. L. Daemen, M. Hartl, D. Williams, J. Summerhill and H. Nakotte, J. Solid State Chem., 2011, 184, 2854–2861 CrossRef CAS.
- J. S. O. Evans, J. Chem. Soc., Dalton Trans., 1999, 3317–3326 RSC.
- C. J. Howard and H. T. Stokes, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 782–789 CrossRef.
- C. J. Howard, B. J. Kennedy and P. M. Woodward, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 463–471 CrossRef PubMed.
- M. J. Cliffe, E. N. Keyzer, M. T. Dunstan, S. Ahmad, M. F. L. De Volder, F. Deschler, A. J. Morris and C. P. Grey, Chem. Sci., 2019, 10, 793–801 RSC.
- K.-P. Xie, W.-J. Xu, C.-T. He, B. Huang, Z.-Y. Du, Y.-J. Su, W.-X. Zhang and X.-M. Chen, CrystEngComm, 2016, 18, 4495–4498 RSC.
- M. Mączka, B. Bondzior, P. Dereń, A. Sieradzki, J. Trzmiel, A. Pietraszko and J. Hanuza, Dalton Trans., 2015, 44, 6871–6879 RSC.
- L. Wang, J. Song, R. Qiao, L. A. Wray, M. A. Hossain, Y.-D. Chuang, W. Yang, Y. Lu, D. Evans, J.-J. Lee, S. Vail, X. Zhao, M. Nishijima, S. Kakimoto and J. B. Goodenough, J. Am. Chem. Soc., 2015, 137, 2548–2554 CrossRef CAS PubMed.
- C. Fang, Y. Huang, W. Zhang, J. Han, Z. Deng, Y. Cao and H. Yang, Adv. Energy Mater., 2016, 6, 1501727 CrossRef.
- W. G. Williams, R. M. Ibberson, P. Day and J. E. Enderby, Phys. B, 1998, 241–243, 234–236 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic details, and supplementary group-theoretical discussions. See DOI: 10.1039/c9cc05436g |
| This journal is © The Royal Society of Chemistry 2019 |