Self-assembled peptido-nanomicelles as an engineered formulation for synergy-enhanced combinational SDT, PDT and chemotherapy to nasopharyngeal carcinoma†
Abstract
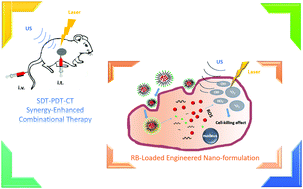
A formulation of self-assembled peptido-nanomicelles has been developed for a combinational treatment of SDT, PDT and chemotherapy to nasopharyngeal carcinoma. In vitro cellular tests and in vivo mice therapy proved effective for targeted tumor growth inhibition. These merits provided a novel approach to non-invasive cancer treatments.


 Please wait while we load your content...
Please wait while we load your content...