Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel ammonium pentaborate – poly(ethylene-glycol) templated polymer-inclusion compound†
Anna R.
Ploszajski
 *a,
Matthew
Billing
*a,
Matthew
Billing
 b,
Neal T.
Skipper
b,
Neal T.
Skipper
 cd and
Jeremy K.
Cockcroft
cd and
Jeremy K.
Cockcroft
 b
b
aDepartment of Mechanical Engineering, University College London, Roberts Building, Torrington Place, London WC1E 7JE, UK. E-mail: anna.ploszajski@ucl.ac.uk
bDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK
cDepartment of Physics and Astronomy, University College London, Gower Street, London, WC1E 6BT, UK
dLondon Centre for Nanotechnology, 17-19 Gordon Street, London, WC1H 0AH, UK
First published on 21st June 2019
Abstract
A new hydrogen-bonded supramolecular framework is reported, consisting of ammonium pentaborate, containing poly(ethylene-glycol) chains extending down tubular cavities in the structure. The crystal architecture is templated by the presence of the polyether chains, analogous to template synthesis of zeolites and metal organic frameworks. The ammonium pentaborate is formed by the thermolysis of ammonia borane, followed by hydrolysis of the dehydrogenation products by ambient water. This structure represents the first known example of a borate-based polymer inclusion compound.
There has been much interest in the use of polymers to template materials such as zeolites, metal organic frameworks (MOFs), and even organic co-crystals. The polymer may form an intrinsic part of a co-crystal structure1 or it may simply act as a template for crystal growth in the synthesis of new polymorphs.2 Alternatively, the polymer may act as a template for the formation of novel supramolecular structures. To the best of our knowledge, the ammonium pentaborate – poly(ethylene-glycol) templated polymer-inclusion compound reported here is the only borate-based inclusion compound to host organic guest molecules.
Poly(ethylene-glycol) (PEG, C2nH4n+2On+1) is known to form co-crystals with a great many guest molecules,3 such as 2-methyl-resorcinol,4para-dibromobenzene,5 mercuric chloride,6,7 resorcinol,8,9p-nitrophenol,10 hydroquinone,11p-dihalogenobenzene,12 various isomers and derivatives of dihydroxybenzene,13 urea and thiourea14 and griseofulvin.15 It was recently shown that it is possible to form a stable co-crystal of PEG with ammonia borane (AB, NH3BH3),1 a material commonly studied as a potential hydrogen storage material.16 Powder X-ray diffraction studies show that this combination is capable of producing at least one other crystalline polymorph.17
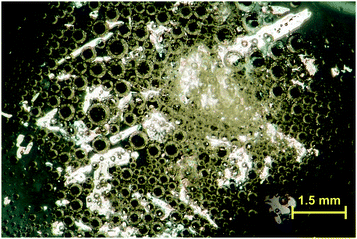
In an attempt to isolate new AB-polyether polymorphs, recrystallisation was attempted from a heated mixture of AB and PEG (Mw 1500 Da) powder. However, gas release was observed from the mixture upon melting (Fig. 1), suggestive of decomposition of AB within the sample to form H2. The sample was cooled and left on the laboratory bench. One week later, square plate-shaped crystals had formed as well as spheroids (Fig. 2). The square plate-shaped crystals were analysed by single-crystal X-ray diffraction. As a test for reproducibility, several crystals were examined and similar unit cells were obtained for each of the crystals with the same morphology. Some crystals were twinned and the best data on a non-twinned crystal is reported here. Given that the bulk sample is a mixture of more than one crystal morphology plus liquid phase, it was not practical to analyse the bulk sample, either via chemical (e.g. mass spectroscopy) or diffraction (e.g. PXRD) methods.
 | ||
| Fig. 1 Optical micrograph of the mixture of AB (bright needles) and PEG (molten liquid) just after heating, showing bubbles of the released gas. | ||
 | ||
| Fig. 2 Optical micrograph of square-shaped crystals observed to form one week after a mixture of AB and PEG was heated and gas release observed. | ||
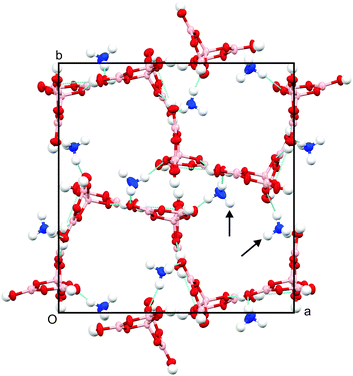
Single-crystal diffraction measurements show that the structure of this material was composed of a hydrogen-bonded network of pentaborate anions [B5O6(OH)4]− and ammonium cations [NH4]+ (Fig. 3) in which there are four large channels per unit cell, which contain disordered PEG molecules. This material may therefore be classified as a polymer inclusion compound. The structure is monoclinic, space group P21/c and unit cell dimensions are a = 9.2922(2) Å, b = 17.3394(5) Å, c = 16.3648(5) Å, β = 91.234(2)°, V = 2636.12(13) Å3 at 150 K. This unit cell does not match other crystalline material derived from combinations of AB and PEO seen in previous work.17 Further details about the structure can be found in the tables of crystallographic coordinates and molecular geometry in the ESI.†
The basic motif forming the framework of the polymer-inclusion compound consists of two ammonium cations and two pentaborate anions (Fig. S1, ESI†). The two crystallographically-distinct anions are structurally similar to each other and are consistent with previously reported structures of the pentaborate anion.18 Similar structures composed of pentaborate anions have been observed for crystal systems containing substituted nitrogen-based non-metal cations.19–25
This supramolecular structure has four channels which run through each unit cell (Fig. 4 and Fig. S2, ESI†). Extended PEG chains exist in these channels, as evidenced by the calculated pore volume (215.7 Å3) and the number of electrons present in each channel (75.4 e−), corresponding to approximately 3.5 monomers per channel. The exact location of the polymer is difficult to determine due to extensive crystallographic disorder. Nevertheless, “squeezing” the structure ensures that the framework may be determined accurately.26 Because of this disorder, the precise crystallographic location of the PEG molecules is unknown.
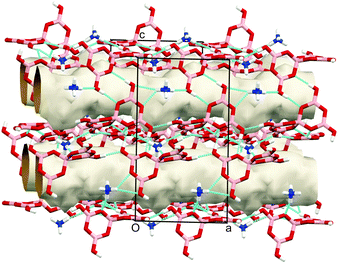 | ||
| Fig. 4 View showing extended channels running through the structure in which the crystallographically disordered PEG molecules sit. | ||
Temporary guest molecules can be used as templates to create large voids in the synthesis of other framework structures such as zeolites27 and metal organic frameworks (MOFs)28 either through hydrogen-bonded or covalent interactions. In this unit cell, two ammonium ions per channel extend towards the centre (indicated with arrows in Fig. 3), forming hydrogen bonds with the ether oxygen atoms in the polymer to provide stability to the structure. It is believed that these interactions play a major role in templating the crystal architecture observed.
Although numerous examples of polymer-inclusion compounds have been reported previously for well-known host materials such as urea29 and cyclodextrins,30 borate-based inclusion compounds have rarely been reported in the literature. The only known examples contain simple inorganic salt guests (e.g. LiF, NaCl).31,32
The first step to creating this inclusion compound is to heat a powdered mixture of AB and PEG powders. The PEG powder melts and the AB molecules decompose to create aminoborane compounds, which rapidly oligomerise to form polyaminoborane compounds. Competing hydrogen transfer reactions and dehydrogenation processes then produce borazine species. These polymeric residues then undergo hydrolysis due to the presence of ambient moisture to form hydrogen gas and ammonium borate. Additional reactions with water form ammonium tetrahydroxyborate, and the condensation reaction between the tetrahydroxyborate anion and the neutral boric acid present from hydrolysis forms the pentaborate anion. A detailed discussion of the potential chemistry of formation may be found in the ESI.†
Demirci et al. previously explored the feasibility of combining thermolysis and hydrolysis to achieve the rapid release of three equivalents of hydrogen from AB at moderate temperatures.33 In their studies, the final products were reported to be amorphous ammonium borate materials, whereas the material reported here is highly crystalline. The well-defined nature of the ammonium pentaborate observed in this work could inform future studies on closing the hydrogen cycle for AB-polyether materials. A number of regeneration protocols are known which reduce borate species to sodium borohydride (an important reagent for the synthesis of AB),34 but, despite recent progress in dramatically reducing the cost of borate reduction,35 no method has yet achieved yields sufficient for AB-polyether materials to become widespread recyclable hydrogen storage solutions for portable applications. This remains an important challenge for AB-polyether materials.
Experimental: ammonia borane (Boron Specialities, 97%) and PEG (Sigma Aldrich, Mw = 1500 Da) were purchased and used without further purification. The two powders were combined with a composition weight ratio of 30% AB to 70% PEG and the mixture was ground using a pestle and mortar for 1 minute. The mixture was aged overnight and then placed onto glass slides. The loaded slides were placed onto a hot plate and heated from room temperature to 45 °C. Upon melting, a gas was released, and the hot plate was subsequently cooled to 35 °C. The samples were left at this temperature exposed to the ambient atmosphere for 1 week. Single crystals were observed under the microscope to have a square plate-like morphology. A suitable crystal was selected and single crystal X-ray diffraction was performed using an Agilent SuperNova diffractometer. Further details of all experiments are available in the ESI.†
In conclusion, the structure of an ammonium pentaborate-polyethylene glycol inclusion compound has been reported which, to the best of our knowledge, is the first example of a borate-based polymer inclusion compound and the first borate inclusion compound hosting organic guest material. In this structure, the pentaborate anions form a hydrogen-bonded supramolecular framework, and extended PEG chains exist within cavities in the structure. The formation of this structure is analogous to synthesis by templating in other materials with porous molecular structures such as zeolites and MOFs. Given recent interest in borate materials for their porous,36 non-linear optical,37 piezoelectric38 and luminescent39 properties, the study of borate-based polymer inclusion compounds such as this may be an interesting new avenue of research.
The authors are grateful for funding from the “EPSRC Centre for Doctoral Training in Molecular Modelling and Materials Science” (EP/L015862/1), the EPSRC for funding the X-ray diffractometers (EP/K03930X/1) and Cella Energy.
Conflicts of interest
There are no conflicts to declare.References
- A. R. Ploszajski, M. Billing, J. K. Cockcroft and N. T. Skipper, CrystEngComm, 2018, 20, 4436–4440 RSC.
- S. Askin, J. K. Cockcroft, L. S. Price, A. D. Gonçalves, M. Zhao, D. A. Tocher, G. R. Williams, S. Gaisford and D. Q. M. Craig, Cryst. Growth Des., 2019, 19, 2751–2757 CrossRef CAS.
- P. Damman, L. Paternostre, J. Moulin and M. Dosiere, Macromol. Symp., 1999, 138, 57–71 CrossRef CAS.
- L. Paternostre, P. Damman and M. Dosie, Macromolecules, 1999, 32, 153–161 CrossRef CAS.
- J. J. Point and C. Coutelier, J. Polym. Sci., Polym. Phys. Ed., 1985, 23, 231–239 Search PubMed.
- R. Iwamoto, Y. Saito, H. Ishihara and H. Tadokoro, J. Polym. Sci., Part A-1: Polym. Chem., 1968, 6, 1509–1525 CAS.
- A. A. Blumberg, S. S. Pollack and C. A. J. Hoeve, J. Polym. Sci., Part A-1: Polym. Chem., 1964, 2, 2499–2502 Search PubMed.
- E. Delaite, J. J. Point, P. Damman and M. Dosiere, Macromolecules, 1992, 25, 4768–4778 CrossRef CAS.
- R. M. Myasnikova, E. F. Titova and E. S. Obolonkova, Polymer, 1980, 21, 403–407 CrossRef CAS.
- J. J. Point and P. Damman, Macromolecules, 1992, 25, 1184–1188 CrossRef CAS.
- L. Paternostre, P. Damman and M. Dosiere, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 1197–1208 CrossRef CAS.
- J. J. Point and P. Damman, Macromolecules, 1991, 24, 2019–2023 CrossRef CAS.
- L. A. Belfiore and E. Ueda, Polymer, 1992, 33, 38 CrossRef.
- F. E. Bailey and H. G. France, J. Polym. Sci., 1961, 49, 397–406 CrossRef CAS.
- Z. Zhong, C. Guo, L. Chen, J. Xu and Y. Huang, Chem. Commun., 2014, 50, 6375–6378 RSC.
- F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2007, 2613–2626 RSC.
- A. R. Ploszajski, M. Billing, A. S. Nathanson, M. Vickers and S. M. Bennington, Int. J. Hydrogen Energy, 2018, 43, 5645–5656 CrossRef CAS.
- V. R. Hathwar, A. K. Paul, S. Natarajan and T. N. Row, J. Phys. Chem. A, 2011, 115, 12818–12825 CrossRef CAS PubMed.
- M. Z. Visi, C. B. Knobler, J. J. Owen, M. I. Khan and D. M. Schubert, Cryst. Growth Des., 2006, 6, 538–545 CrossRef CAS.
- D. A. Kose, M. A. Beckett and N. Colak, Hacettepe J. Biol. Chem., 2012, 40, 219–225 Search PubMed.
- M. A. Beckett, P. N. Horton, M. B. Hursthouse, D. A. Knox and J. L. Timmis, Dalton Trans., 2010, 39, 3944–3951 RSC.
- M. A. Beckett, P. N. Horton, M. B. Hursthouse and J. L. Timmis, RSC Adv., 2013, 3, 15185–15191 RSC.
- M. Wiebcke, C. C. Freyhardt, J. Felsche and G. Engelhardt, Z. Naturforsch., 1993, 48, 978–985 CAS.
- P. Li, C. H. Fan and J. F. Ge, Z. Kristallogr. – New Cryst. Struct., 2016, 231, 537–539 CAS.
- M. A. Beckett, P. N. Horton, M. B. Hursthouse and J. L. Timmis, Polyhedron, 2014, 77, 96–102 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2014, 71, 9–18 CrossRef PubMed.
- A. Sachse and J. Garcia-Martinez, Chem. Mater., 2017, 29, 3827–3853 CrossRef CAS.
- C. Duan, F. Li, H. Zhang, J. Li, X. Wang and H. Xi, RSC Adv., 2017, 7, 52245–52251 RSC.
- P. Eaton, N. Vasanthan, I. D. Shin and A. E. Tonelli, Macromolecules, 1996, 29, 2531–2536 CrossRef CAS.
- C. C. Rusa, C. Luca and A. E. Tonelli, Macromolecules, 2001, 34, 1318–1322 CrossRef CAS.
- H. Yu, H. Wu, S. Pan, Y. Wang, Z. Yang and X. Su, Inorg. Chem., 2013, 52, 5359–5365 CrossRef CAS PubMed.
- C. Bai, S. Han, S. Pan, B. Zhang, Y. Yang, L. Li and Z. Yang, RSC Adv., 2015, 5, 12416–12422 RSC.
- U. B. Demirci, S. Bernard, R. Chiriac, F. Toche and P. Miele, J. Power Sources, 2011, 196, 279–286 CrossRef CAS.
- O. T. Summerscales and J. C. Gordon, Dalton Trans., 2013, 42, 10075–10084 RSC.
- L. Z. Ouyang, H. Zhong, Z. M. Li, Z. J. Cao, H. Wang, J. W. Liu, X. K. Zhu and M. Zhu, J. Power Sources, 2014, 269, 768–772 CrossRef CAS.
- Z. H. Liu, J. J. Zhang and W. J. Zhang, Inorg. Chim. Acta, 2006, 359, 519–524 CrossRef CAS.
- J. Di, X. Xu, C. Xia, H. Zeng, Y. Cheng, D. Li, D. Zhou, F. Wu, J. Cheng and J. Xu, J. Alloys Compd., 2012, 536, 20–25 CrossRef CAS.
- W. R. Cook and H. Jaffe, Acta Crystallogr., 1957, 10, 705–707 CrossRef CAS.
- A. Akella and D. A. Keszler, Mater. Res. Bull., 1995, 30, 105–111 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental detail, crystallographic tables, and additional supporting figures are supplied. CCDC 1903934. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc02720c |
| This journal is © The Royal Society of Chemistry 2019 |