Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functionalisation of isoindolinones via a calcium catalysed Hosomi–Sakurai allylation†
Ashley J.
Basson
and
Mark G.
McLaughlin
 *
*
Faculty of Science & Engineering, Department of Natural Sciences, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, UK. E-mail: m.mclaughlin@mmu.ac.uk
First published on 24th June 2019
Abstract
A rapid and functionally tolerant calcium catalysed Hosomi–Sakurai reaction has been realised. Employing 1 mol% calcium, allylated isoindolinones can be synthesised in high yields and the reaction is shown to be tolerant to a range of medicinally relevant functional groups including heterocycles. The synthetic utility of the reaction has been shown, and a plausible reaction mechanism is provided.
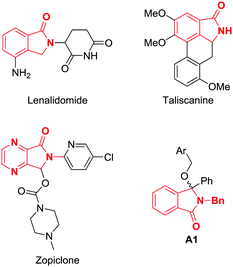
Isoindolinones are a common structural motif found in both drugs and natural products alike (Fig. 1).1 For example, lenalidomide is used in the treatment of cancer,2 taliscanine has been shown to have potential as treatment for neurological disorders,3 and zopiclone is an approved treatment for insomnia. Furthermore, investigational compounds such as A1 act as inhibitors of the MDM2-p53 protein–protein interaction.4
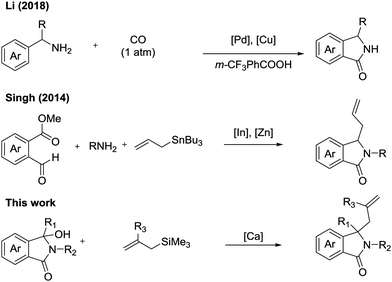
Due to their pronounced biological activities against a range of disease targets, their synthesis has attracted the attention of numerous synthetic chemists (Fig. 2).5 Of particular interest is the development of catalytic transformations to produce these important scaffolds. Copper,6 palladium,7 rhodium,8 platinum9 and other Lewis acids10 have found use in the synthesis of a wide range of isoindolinone scaffolds. Although these methods are useful, many of them rely on the ready access to functionalised building blocks, as well as typically producing mono-functionalised isoindolinones. Furthermore, although elegant, many of the procedures suffer from functional group intolerances, particularly functional groups with relevance in medicinal chemistry.11
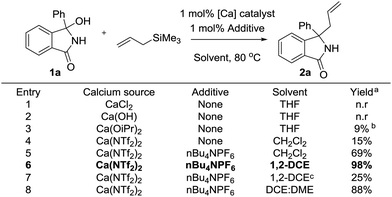
During the course of a medicinal chemistry program focused on the development of inhibitors against a metabolic disease target, we required access to a range of substituted isoindolinones bearing a functional group handle for further exploitation. Due to our groups’ burgeoning interest in the use of calcium as a sustainable catalyst in synthesis,12 we decided to explore the use of 3-hydroxyisoindolinones as easily prepared precursors to N-acyliminium ions. We reasoned that through catalytic dehydration, these reactive intermediates could be produced and subsequently trapped by an allyl nucleophile.13 We started our investigation on model substrate 1a, employing a range of differing calcium catalysts and additive to effect the desired transformation (Fig. 3). We surveyed a range of calcium salts to determine the most appropriate source for the catalytic reaction, and saw no reaction with either CaCl2 or Ca(OH)2. Interestingly we saw a stoichiometric reaction when Ca(OiPr)2 was used, with yield increasing as loading increased.
Gratifyingly, when Ca(NTf2)2 was employed as a catalyst,14 we isolated 15% of the desired product after 1 hour.10c Addition of nBu4NPF6 further improved the yield,15 as did changing the solvent to 1,2-DCE. Further attempts at improving the reaction were not successful, including performing the reaction in a binary mix of solvents. To ensure the reaction required a calcium salt, we attempted the reaction using HNTf2, however no reaction was observed.
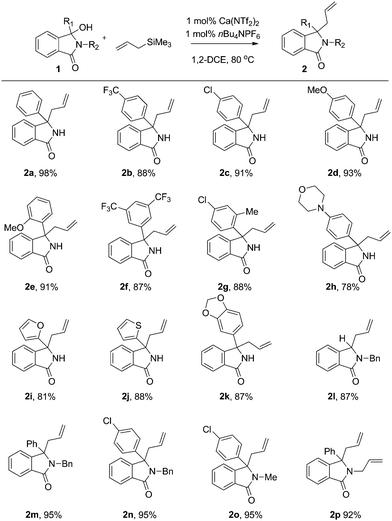
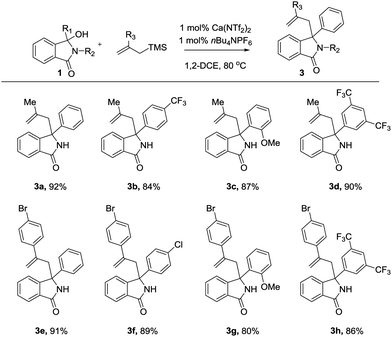
With these optimised conditions in hand, we probed the substrate scope of the reaction (Fig. 4). As observed, both electron donating (2a,d) and electron withdrawing (2b,c) groups were well tolerated, each providing the allylated product in excellent yields and high efficiency.
We next moved our attention onto compounds exhibiting a range of substitution patterns, with ortho (2e), meta (2f) and ortho/para (2g) substituents all tolerated well. As compounds of this class have been shown to exhibit a range of biological activities,16 we next turned our attention to heterocyclic moieties. As shown, the catalyst system is unaffected by traditionally difficult functional groups, with morpholine (2h), furan (2i), and thiophene (2j) all working well. Furthermore, acetal (2k) was also tolerated, affording the allylated product in high yield. All the examples shown thus far contained a free NH embedded within the lactam core. We therefore decided to investigate the effect of nitrogen substitution has on the reaction. We focused our attention on either easily removable groups (-Me, -Bn) or N-allyl group. As shown, these examples also worked well, efficiently providing the desired allylated produce in high yields.
Our focus then turned to the use of branched allyl silanes, as these will produce compounds with increased levels of diversity (R3 = Me or 4-BrPh). The reactions once again proceeded smoothly, affording the disubstituted γ-lactams good yield (Fig. 5 and 6).
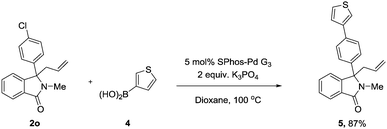
With the substrate scope completed, we next decided to investigate the synthetic applicability of our synthesised compounds. Employing Buchwald's system for Suzuki cross couplings of aryl chlorides (2o),17 and using commercial boronic acid 4, 5 was obtained in excellent yield (Scheme 1).
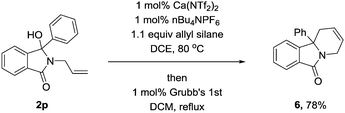
Indolizidines are privileged scaffolds within medicinal chemistry and new routes towards these important scaffolds remains a priority.18 We reasoned that due to the high level of structural diversity achieved via this methodology,19 we could produce these important scaffolds easily and in high yields. Using 2p as a model, we initially performed a ring closing metathesis with Grubb's 1st generation catalyst.20 This proceeded well and in expected high yield (Scheme 2). To further increase the utility, we performed the reaction in a one-pot fashion. Once again this produced the desired compound (6) in nearly identical yield to that observed with the isolated.
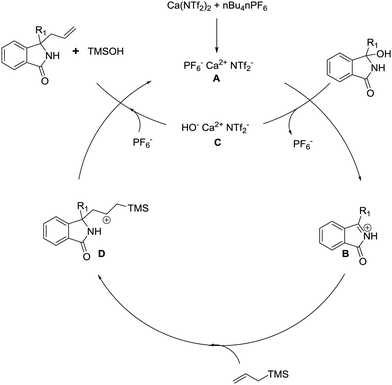
Finally, as the Hosomi–Sakurai reaction is traditionally non-catalytic,21 we wanted to perform preliminary studies to elucidate the potential mechanism of the reaction. The reaction proceeded slowly in the absence of nBu4NPF6, suggesting the crucial role the weakly coordinating PF6− ligand has. Furthermore, analysis of the crude reaction mixture by 1H NMR showed the presence of TMS-OH. Taken together, as well as inferring from previous studies,22 a plausible reaction mechanism is present below.
The active catalyst A is produced which reacts with the Lewis basic hydroxyl group. The resultant N-acyliminium ion B is produced, along with a postulated calcium alkoxy species C. The N-acyliminium ion is then attacked by the allyl silane affording the stabilised cation D. We then reason that due to the nucleophilic nature of the ligands in calcium complex C, a facile elimination with concomitant reintroduction of PF6− occurs, providing the desired compound, releasing TMSOH and regenerating catalyst A.
In summary, we have developed a facile and high yielding calcium catalysed Hosomi–Sakurai reaction. The reaction proceeds well, and is unencumbered by both steric and electronic factors. Furthermore, we have shown that both linear and branched silanes can react, providing much needed diversity of structure. Synthetic applicability has been demonstrated, and a plausible reaction mechanism proposed.
This work was supported by a Research Accelerator Grant from Manchester Metropolitan University. MML thanks MMU for startup funding. AJB thanks the Royal Society of Chemistry for an Undergraduate Research Bursary. We thank Liban Van Haren and Anthony Kromodimedjo for synthetic assistance.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Speck and T. Magauer, Beilstein J. Org. Chem., 2013, 9, 2048–2078 CrossRef PubMed.
- X. Armoiry, G. Aulagner and T. Facon, J. Clin. Pharm. Ther., 2008, 33, 219–226 CrossRef CAS PubMed.
- H. A. Priestap, Phytochemistry, 1985, 24, 849–852 CrossRef CAS.
- I. R. Hardcastle, S. U. Ahmed, H. Atkins, G. Farnie, B. T. Golding, R. J. Griffin, S. Guyenne, C. Hutton, P. Källblad, S. J. Kemp, M. S. Kitching, D. R. Newell, S. Norbedo, J. S. Northen, R. J. Reid, K. Saravanan, H. M. G. Willems and J. Lunec, J. Med. Chem., 2006, 49, 6209–6221 CrossRef CAS PubMed.
- (a) M.-D. Chen, X. Zhou, M.-Z. He, Y.-P. Ruan and P.-Q. Huang, Tetrahedron, 2004, 60, 1651–1657 CrossRef CAS; (b) D. L. Comins, S. Schilling and Y. Zhang, Org. Lett., 2005, 7, 95–98 CrossRef CAS PubMed; (c) T. R. Belliotti, W. A. Brink, S. R. Kesten, J. R. Rubin, D. J. Wustrow, K. T. Zoski, S. Z. Whetzel, A. E. Corbin, T. A. Pugsley, T. G. Heffner and L. D. Wise, Bioorg. Med. Chem. Lett., 1998, 8, 1499–1502 CrossRef CAS PubMed; (d) Y. Tian, J. Sun, K. Zhang, G. Li and F. Xu, Synthesis, 2018, 2255–2265 CAS; (e) D. Brahmchari, A. K. Verma and S. Mehta, J. Org. Chem., 2018, 83, 3339–3347 CrossRef CAS PubMed; (f) C. Alonso, M. González, F. Palacios and G. Rubiales, J. Org. Chem., 2017, 82, 6379–6387 CrossRef CAS PubMed; (g) J. Maury, G. Force, B. Darses and D. Lebœuf, Adv. Synth. Catal., 2018, 360, 2752–2756 CrossRef CAS.
- (a) R. B. Bedford, J. G. Bowen and C. Méndez-Gálvez, J. Org. Chem., 2017, 82, 1719–1725 CrossRef CAS PubMed; (b) H. S. P. Rao and A. V. B. Rao, J. Org. Chem., 2015, 80, 1506–1516 CrossRef CAS PubMed; (c) S. R. Chemler, Beilstein J. Org. Chem., 2015, 11, 2252–2253 CrossRef CAS PubMed.
- (a) C. Zhang, Y. Ding, Y. Gao, S. Li and G. Li, Org. Lett., 2018, 20, 2595–2598 CrossRef CAS PubMed; (b) W. Chen, L. Jin, Y. Zhu, X. Cao, L. Zheng and W. Mo, Synlett, 2013, 1856–1860 CrossRef CAS.
- Z.-Q. Wang, C.-G. Feng, M.-H. Xu and G.-Q. Lin, J. Am. Chem. Soc., 2007, 129, 5336–5337 CrossRef CAS PubMed.
- L. Shi, L. Hu, J. Wang, X. Cao and H. Gu, Org. Lett., 2012, 14, 1876–1879 CrossRef CAS PubMed.
- (a) S. Dhanasekaran, V. Bisai, R. A. Unhale, A. Suneja and V. K. Singh, Org. Lett., 2014, 16, 6068–6071 CrossRef CAS PubMed; (b) S. Dhanasekaran, A. Suneja, V. Bisai and V. K. Singh, Org. Lett., 2016, 18, 634–637 CrossRef CAS PubMed; (c) C. Qi, V. Gandon and D. Lebœuf, Adv. Synth. Catal., 2017, 359, 2671–2675 CrossRef CAS.
- D. D. Carolina, J. B. Eliezer and A. M. F. Carlos, Mini-Rev. Med. Chem., 2007, 7, 1108–1119 CrossRef.
- H. J. Kiely-Collins, I. Sechi, P. E. Brennan and M. G. McLaughlin, Chem. Commun., 2018, 54, 654–657 RSC.
- (a) C. Masusai, D. Soorukram, C. Kuhakarn, V. Reutrakul and M. Pohmakotr, Eur. J. Org. Chem., 2018, 160–169 CrossRef CAS; (b) T. Bootwicha, D. Panichakul, C. Kuhakarn, S. Prabpai, P. Kongsaeree, P. Tuchinda, V. Reutrakul and M. Pohmakotr, J. Org. Chem., 2009, 74, 3798–3805 CrossRef CAS PubMed; (c) R. D. Dura, I. Modolo and L. A. Paquette, Heterocycles, 2007, 74, 145 CrossRef CAS; (d) I. Osante, E. Lete and N. Sotomayor, Tetrahedron Lett., 2004, 45, 1253–1256 CrossRef CAS.
- D. Lebœuf, M. Presset, B. Michelet, C. Bour, S. Bezzenine-Lafollée and V. Gandon, Chem. – Eur. J., 2015, 21, 11001–11005 CrossRef PubMed.
- (a) M. Niggemann and M. J. Meels, Angew. Chem., Int. Ed., 2010, 49, 3684–3687 CrossRef CAS PubMed; (b) J. M. Begouin and M. Niggemann, Chem. – Eur. J., 2013, 19, 8030–8041 CrossRef CAS PubMed; (c) J.-M. Begouin, F. Capitta, X. Wu and M. Niggemann, Org. Lett., 2013, 15, 1370–1373 CrossRef CAS PubMed; (d) L. Fu and M. Niggemann, Chem. – Eur. J., 2015, 21, 6367–6370 CrossRef CAS PubMed; (e) S. Gao, T. Stopka and M. Niggemann, Org. Lett., 2015, 17, 5080–5083 CrossRef CAS PubMed; (f) V. J. Meyer, C. Ascheberg and M. Niggemann, Chem. – Eur. J., 2015, 21, 6371–6374 CrossRef CAS PubMed; (g) T. Stopka and M. Niggemann, Org. Lett., 2015, 17, 1437–1440 CrossRef CAS PubMed; (h) V. J. Meyer and M. Niggemann, Eur. J. Org. Chem., 2011, 3671–3674 CrossRef CAS.
- J. Caruano, G. G. Muccioli and R. Robiette, Org. Biomol. Chem., 2016, 14, 10134–10156 RSC.
- M. A. Düfert, K. L. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 12877–12885 CrossRef PubMed.
- J. P. Michael, Nat. Prod. Rep., 2007, 24, 191–222 RSC.
- A. K. Maity and S. Roy, J. Org. Chem., 2012, 77, 2935–2941 CrossRef CAS PubMed.
- (a) P. Schwab, M. B. France, J. W. Ziller and R. H. Grubbs, Angew. Chem., Int. Ed. Engl., 1995, 34, 2039–2041 CrossRef CAS; (b) P. Schwab, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1996, 118, 100–110 CrossRef CAS.
- J. J. Lade, S. D. Pardeshi, K. S. Vadagaonkar, K. Murugan and A. C. Chaskar, RSC Adv., 2017, 7, 8011–8033 RSC.
- J. Davies and D. Leonori, Chem. Commun., 2014, 50, 15171–15174 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental conditions and copies of spectra are available. See DOI: 10.1039/c9cc02450f |
| This journal is © The Royal Society of Chemistry 2019 |