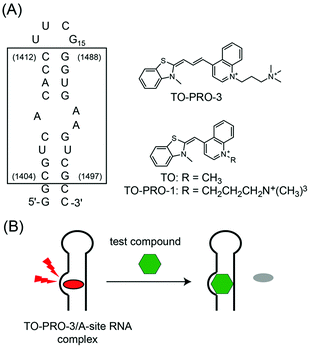
Trimethine cyanine dyes as deep-red fluorescent indicators with high selectivity to the internal loop of the bacterial A-site RNA†
Yusuke
Sato
 ,
Sayaka
Yajima
,
Akifumi
Taguchi
,
Kyosuke
Baba
,
Mayu
Nakagomi
,
Yuri
Aiba
and
Seiichi
Nishizawa
,
Sayaka
Yajima
,
Akifumi
Taguchi
,
Kyosuke
Baba
,
Mayu
Nakagomi
,
Yuri
Aiba
and
Seiichi
Nishizawa
 *
*
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan. E-mail: nishi@m.tohoku.ac.jp; Fax: +81-22-795-6552; Tel: +81-22-795-6549
First published on 30th January 2019
Abstract
We report that TO-PRO-3, a thiazole orange analogue with a trimethine bridge, functions as a deep-red fluorescent indicator for the internal loop structure of the bacterial (Escherichia coli) ribosomal decoding region of the aminoacyl-tRNA site (A-site), which enables the assessment of A-site binding capability of various test compounds including blue and even-green-emitting compounds.
With increasing knowledge about the diverse roles of RNAs within the cells, targeting RNA structures with small ligands has received considerable attention in drug discovery.1 Aminoacyl-tRNA site (A-site) of the 16S RNA decoding region in the bacterial ribosome represents one of the most validated RNA targets.1,2 Aminoglycosides are well-characterized ligands that can strongly and selectively bind to the asymmetric internal loop formed by three adenines in the bacterial A-site (Fig. 1A).3 This causes mistranslation or premature termination of protein synthesis,4 which ultimately results in the bacterial cell death.5 Although aminoglycosides are widely used as antibiotics in the treatment of the bacterial infections, they possess several undesirable properties for clinical use, such as the emergence of resistant bacterial strains and off-target effects.6 In order to circumvent these concerns with aminoglycosides, it is of great importance to develop new A-site-binding ligands whose structures are distinct from the aminoglycosides.7,8
In this context, the fluorescence indicator displacement (FID) assay has been useful for the screening of RNA-binding ligands because neither covalent labeling nor immobilization of RNAs and test compounds on the solid support is needed.9,10 This is based on the model in which a fluorescent indicator bound to target RNA is displaced by a test compound in a competitive manner (Fig. 1B). The binding of test compounds can be evaluated by monitoring the fluorescence change of the indicator in the displacement event.
Aminoglycosides tagged with a fluorophore (pyrene or fluorescein)9a,b and TO-PRO-1 (Fig. 1)9c were employed as the indicators in the FID assay targeting the bacterial A-site RNA. However, the emission wavelengths of these indicators are in the blue or green region (pyrene-tagged aminoglycoside: λem = 380 nm,9a fluorescein-tagged aminoglycoside: λem = 517 nm,9b and TO-PRO-1: λem = 533 nm9c), which would suffer from the “compound optical interference” for high-throughput screening (HTS) using chemical libraries.11 Chemical libraries often contain innate fluorescent compounds in these spectral regions,12 by which these compounds cannot be correctly evaluated in the FID assays due to the emission overlap between the compounds and the indicators. In fact, the assays using the previous indicators evaluated only non-fluorescent or blue-emitting test compounds.9 Furthermore, such interference from test compounds can produce false results (either positive or negative) as it is reproducible and concentration-dependent.11 Based on the success in overcoming the compound optical interference for targeting proteins and enzymes,13 we have sought to develop a deep-red fluorescent indicator for the bacterial A-site because a negligible number of test compounds in the chemical library exhibit the emission in this spectral region.12 In addition, we consider that high binding selectivity of the indicators to the internal loop in the A-site RNA is highly desirable. These indicators enable the elimination of non-specific binders, for example, test compounds binding to double-stranded region in the bacterial A-site,10a leading to the efficient identification of the internal loop-binding antibiotics. There are, however, no reports of such deep-red indicators capable of selectively binding to the specific RNA secondary structure to date.
In this work, we found that TO-PRO-3 (Fig. 1A), a thiazole orange (TO; Fig. 1A) analogue with a trimethine bridge, functioned as a deep-red probe with high selectivity for the internal loop structure in the bacterial A-site RNA. Like TO and TO-PRO-1, TO-PRO-3 is a commercially available dye for light-up sensing of DNA structures such as DNA duplexes14 and G-quadruplexes,15 but it has as yet to be examined for the binding to the biological relevant RNAs. The comparison with a series of cyanine dyes including TO and TO-PRO-1 revealed that the trimethine-bridged structure was a key structural element for high selectivity of TO-PRO-3 to the internal loop. Moreover, by virtue of its deep-red emission property with high binding selectivity to the internal loop, TO-PRO-3 facilitated the assessment of various test compounds including aminoglycosides, blue- and even green-emitting compounds in a FID assay (Fig. 1B).
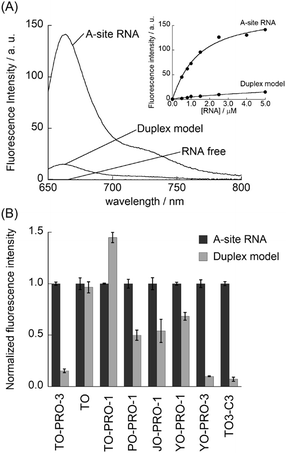
Fig. 2A shows the fluorescence spectra of TO-PRO-3 (0.50 μM) in the absence and presence of the 27-mer model RNA containing the bacterial (Escherichia coli) A-site (5.0 μM; A-site RNA), measured at 20 °C in a 10 mM phosphate buffer solution (pH 7.0) containing 50 mM NaCl and 1.0 mM EDTA. TO-PRO-3 exhibits negligible fluorescence in the absence of A-site RNA (ϕfree < 0.01) due to non-radiative energy loss by the free rotation of the benzothiazole and quinoline rings.14a The addition of A-site RNA causes a remarkable increase in the fluorescence intensity of TO-PRO-3 in the deep-red spectral region (λem = 663 nm), which can be attributed to the restricted rotation of TO-PRO-3 upon interaction with A-site RNA.14,15 The quantum yield for TO-PRO-3 bound to A-site RNA (ϕbound) reached 0.41. It highlights the significant light-up property of TO-PRO-3 for A-site RNA. This response was much more pronounced compared to that for the control RNA having no internal loops (duplex model: 5′-GGC GUC ACA CCU UCG GGU GUG UCG CC-3′). These results suggest that TO-PRO-3 is a promising candidate as the deep-red probe with high selectivity to the internal loop structure in A-site RNA.
Fluorescence titration experiments were performed in order to determine the dissociation constant (Kd) of TO-PRO-3 to A-site RNA (Fig. 2A, inset). The resulting titration curve was well fitted with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model,16 which suggests one TO-PRO-3 molecule binds to the internal loop structure in A-site RNA. Kd value was thus obtained as 1.1 ± 0.12 μM (N = 3), which is almost comparable to a non-aminoglycoside A-site binder, ATMND-C2-NH2 developed by our group (Kd = 0.44 μM; I = 0.06 M, pH 7.0, 5 °C)7 and several aminoglycosides (ribostamycin: Kd = 8.3 μM, paromomycin: Kd = 0.48 μM; I = 0.10 M, pH 7.5, 25 °C).17 On the other hand, the deletion of the internal loop (duplex model) remarkably reduced TO-PRO-3 binding (Fig. 2A, inset). This clearly shows the high selectivity of TO-PRO-3 binding to the internal loop structure of the A-site RNA. Interestingly, such an internal loop selectivity of TO-PRO-3 was not obtained for the corresponding DNA construct (A-site DNA). This arises from non-specific binding of TO-PRO-3 for DNAs, as was clearly seen from large response for DNA duplex model having no internal loops (Fig. S1, ESI†). Hence, a low level of non-specific binding toward RNAs is important for TO-PRO-3 to show high selectivity to the internal loop in the A-site RNA.
1 binding model,16 which suggests one TO-PRO-3 molecule binds to the internal loop structure in A-site RNA. Kd value was thus obtained as 1.1 ± 0.12 μM (N = 3), which is almost comparable to a non-aminoglycoside A-site binder, ATMND-C2-NH2 developed by our group (Kd = 0.44 μM; I = 0.06 M, pH 7.0, 5 °C)7 and several aminoglycosides (ribostamycin: Kd = 8.3 μM, paromomycin: Kd = 0.48 μM; I = 0.10 M, pH 7.5, 25 °C).17 On the other hand, the deletion of the internal loop (duplex model) remarkably reduced TO-PRO-3 binding (Fig. 2A, inset). This clearly shows the high selectivity of TO-PRO-3 binding to the internal loop structure of the A-site RNA. Interestingly, such an internal loop selectivity of TO-PRO-3 was not obtained for the corresponding DNA construct (A-site DNA). This arises from non-specific binding of TO-PRO-3 for DNAs, as was clearly seen from large response for DNA duplex model having no internal loops (Fig. S1, ESI†). Hence, a low level of non-specific binding toward RNAs is important for TO-PRO-3 to show high selectivity to the internal loop in the A-site RNA.
The selectivity of TO-PRO-3 for the internal loop in A-site RNA was compared with that of TO-PRO-1 that was used as the bacterial A-site-targeting fluorescent indicator (Fig. 2B).9c We also examined a TO molecule that lacks the cationic moiety because it was used for the screening for other kinds of RNAs.18 We found that both dyes displayed the remarkable light-up response for not only A-site RNA but also duplex model (Fig. S2, ESI†). Although A-site RNA selectivity was observed at low RNA concentration, it disappeared for 10 equiv. amount of RNA. Evidently, TO-PRO-3's selectivity for the internal loop is superior to those of TO and TO-PRO-1. Further examination of TO analogues with a monomethine bridge (PO-PRO-1, JO-PRO-1, YO-PRO-1) and a trimethine bridge (YO-PRO-3 and TO3-C3) revealed the key structural elements for TO-PRO-3 binding to the internal loop in A-site RNA (Fig. 2B). In contrast to TO and TO-PRO-1, these monomethine cyanines exhibited the selective response for the internal loop (Fig. S3, ESI†); however the selectivity was much lower relative to TO-PRO-3. In sharp contrast, both trimethine cyanines showed high selectivity to A-site RNA over duplex model RNA (Fig. 2B and Fig. S4, ESI†), where the selectivity is almost comparable to that of TO-PRO-3 (Fig. 2B). Therefore, it is highly likely that trimethine-bridged structure is crucial for highly selective binding to the internal loop structure in A-site RNA. While further structural studies such as NMR or X-ray analysis are needed, we reason that this can be attributed to the suitable molecular size of trimethine cyanine dyes to be accommodated into the internal loop structure. YO-PRO-3 has the strong binding affinity (Kd = 1.1 ± 0.4 μM (N = 3); Fig. S4, ESI†) as well as the remarkable light-up property for A-site RNA (ϕbound = 0.49). Although its emission wavelength is blue-shifted by 37 nm compared to TO-PRO-3 (λem = 626 nm), YO-PRO-3 would be a useful fluorescent probe for A-site RNA as well. It was seen that TO3-C3 having no cationic trimethylammonium moieties showed weaker binding to A-site RNA than TO-PRO-3 (Kd = 6.6 ± 1.1 μM (N = 3); Fig. S4, ESI†). Thus, the cationic moiety in TO-PRO-3 favorably contributes to the binding to A-site RNA through electrostatic interaction. This is supported by the examination of the apparent charge of TO-PRO-3 bound to the A-site RNA (Fig. S5, ESI†).
Finally, TO-PRO-3 was applied as an indicator in a FID assay for the bacterial A-site. In this assay, the fluorescence of TO-PRO-3 bound to the internal loop in A-site RNA would decrease when displacement with test compound occurs in a competitive manner (Fig. 1B). Here, we chose three kinds of ribosome-targeting antibiotics (neomycin, amikacin and chloramphenicol) as test compounds (Fig. S6, ESI†). Neomycin and amikacin can selectively bind to the internal loop structure in the A-site17,19 whereas chloramphenicol targets other sites in the ribosome to exert its antibiotic activity.20 Moreover, we examined fluorescent A-site binding ligands, namely blue-emissive ATMND-C2-NH2 (λem = 406 nm: Fig. S6, ESI†)7 and green-emissive JO-PRO-1 (λem = 546 nm; cf. Fig. S3B, ESI†). Fig. 3A shows the fluorescence spectra of TO-PRO-3/A-site RNA complex (0.50 μM) in the absence and presence of 10 μM test compounds. In the absence of test compounds, strong emission from TO-PRO-3/A-site RNA complex was observed. Hardly any response was observed for chloramphenicol because of its inability of A-site binding. In contrast, the addition of neomycin caused the remarkable decrease in the fluorescence intensity of TO-PRO-3. Fluorescence decrease was saturated at a neomycin concentration of 5.0 μM (Fig. 3B and Fig. S7A, ESI†), where all TO-PRO-3 molecules were displaced by neomycin (100% displacement; Fig. S8, ESI†). It clearly shows that neomycin efficiently competes with TO-PRO-3 in binding to the internal loop of the bacterial A-site RNA. Decrease in TO-PRO-3 fluorescence was also observed for amikacin (Fig. S7B, ESI†), but the response is much more moderate compared to neomycin (Fig. 3B). This can be attributed to inefficient displacement of TO-PRO-3 due to weaker binding of amikacin to the internal loop relative to neomycin (amikacin: Kd = 8.1 μM in I = 0.15 M, pH 7.0, 21 °C; neomycin: Kd = 0.033 μM; I = 0.10 M, pH 7.5, 25 °C).17,19 Thus, the present FID assay using TO-PRO-3 can be useful for assessing the binding capability of test compounds to the internal loop.9 Significantly, the deep-red emission property of TO-PRO-3 indicator enabled the evaluation of the binding of blue-emissive ATMND-C2-NH2 and green emissive JO-PRO-1 (Fig. 3B). Decrease of TO-PRO-3 fluorescence was clearly observed upon addition of ATMND-C2-NH2 and JO-PRO-1 in a concentration dependent manner without suffering from the emission of these test compounds (Fig. S7C and D, ESI†). It is noteworthy that even in the presence of 100 equiv. of these compounds (50 μM), the emission profile of TO-PRO-3 is negligibly affected. Of special note is that JO-PRO-1 became fluorescent by binding to the A-site RNA (cf. Fig. S3B, ESI†), by which the previous indicators9 would fail to assess its binding to A-site RNA in FID assay. The response observed here indicates both ATMND-C2-NH2 and JO-PRO-1 competitively displace TO-PRO-3 bound to the internal loop. It should be noted that the displacement does not reach 100% for ATMND-C2-NH2 although it seems to be saturated at a 30 μM concentration. Considering high selectivity of TO-PRO-3 for the internal loop in the A-site RNA, this would be rationalized by non-specific binding, such as binding to the double-stranded region in A-site RNA, in the case of high concentration of ATMND-C2-NH2, due to its relatively moderate selectivity to the internal loop.7 Such a non-competitive binding between ATMND-C2-NH2 and TO-PRO-3 would yield less change in TO-PRO-3 fluorescence, as discussed in the literature.9c Note that the FID assay using TO-PRO-3 indicator is compatible with 96-well microplate format (Fig. S9, ESI†). This indicates the great potential for the application to HTS screening using chemical libraries.
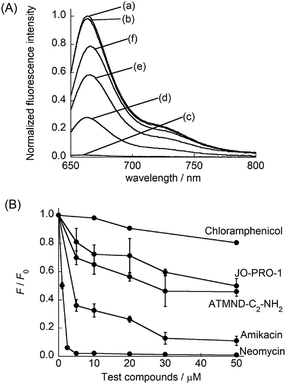 | ||
| Fig. 3 (A) Fluorescence spectra of TO-PRO-3 (0.50 μM)/A-site RNA (0.50 μM) complex in the (a) absence and presence of 10 μM test compounds ((b) chloramphenicol, (c) neomycin, (d) amikacin, (e) ATMND-C2-NH2 and (f) JO-PRO-1). (B) FID results obtained by using TO-PRO-3 as the indicator for test compounds. [TO-PRO-3], [A-site RNA] = 0.50 μM. [test compound] = 0–50 μM. F and F0 denote the fluorescence intensity at 663 nm of the TO-PRO3/A-site RNA complex in the presence and absence of test compounds, respectively. Except for chloramphenicol (N = 1), error bars are the standard deviations (N = 3). Solution conditions are the same as given in Fig. 2A. Temperature, 20 °C. Excitation, 632.5 nm. | ||
In summary, we described TO-PRO-3 functioned as a deep-red emissive indicator with a high binding selectivity to the internal loop of the bacterial A-site RNA. To the best of our knowledge, this is the first report on a deep-red fluorescent indicator capable of selective binding to the RNA secondary structure for the screening of RNA-binding ligands in FID assay. This property would not only expand the assessable test compounds but also reduce the risk of false results, which greatly improves the applicability to HTS screening using chemical libraries. It thus represents a powerful tool in bringing new A-site targeting ligands to light with a view toward the development of novel non-aminoglycoside antibiotics. Besides the bacterial A-site RNA, TO-PRO-3 was found to exhibit light-up response upon strongly binding to human cytoplasmic and mitochondrial A-site RNAs (Kd/μM: cytoplasmic A-site, 1.4; mitochondrial A-site, 3.2; Fig. S10, ESI†). TO-PRO-3 indicator is thus applicable to FID assay for these relevant A-site RNAs. This can enable us to estimate the selectivity of test compounds for the bacterial A-site RNA over these human A-site RNAs, which should be of great importance for designing A-site-targeting antibiotics without suffering from toxicities.21 The obtained results would also contribute to the rational design of bacterial A-site-targeting antibiotics themselves. In addition, we expect that trimethine cyanine dyes would also be useful for the design of this class of indicators targeting other RNA structures. In fact, the preliminary experiments revealed that TO-PRO-3 could function as a deep-red indicator with the binding selectivity to the internal loop region in the influenza A virus RNA promoter22 (data not shown). We also expect that these indicators can find the applications in the development of nucleic acid-based sensors.23 We are now undertaking further studies in these directions.
This work was supported by Grant-in-Aid for Scientific Research (B) (No. 16H04159) and Challenging Exploratory Research (No. 17K19133) from Japan Society for the Promotion of Science (JSPS).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. R. Thomas and P. J. Hergenrother, Chem. Rev., 2008, 108, 1171–1224 CrossRef CAS PubMed; (b) B. S. Morgan, J. E. Forte and A. E. Hargrove, Nucleic Acids Res., 2018, 46, 8025–8037 CrossRef PubMed.
- (a) A. P. Carter, W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly and V. Ramakrishnan, Nature, 2000, 407, 340–348 CrossRef PubMed; (b) Q. Vicens and E. Westhof, Biopolymers, 2003, 70, 42–57 CrossRef CAS PubMed.
- (a) D. Moazed and H. F. Noller, Nature, 1987, 327, 389–394 CrossRef CAS PubMed; (b) P. Purohit and S. Stern, Nature, 1994, 370, 659–662 CrossRef CAS PubMed.
- S. Magnet and J. S. Blanchard, Chem. Rev., 2005, 105, 477–498 CrossRef CAS PubMed.
- (a) G. F. Busscher, F. P. Rutjes and F. L. van Delft, Chem. Rev., 2005, 105, 775–791 CrossRef CAS PubMed; (b) H. F. Noller, Annu. Rev. Biochem., 1991, 60, 191–227 CrossRef CAS PubMed.
- J. L. Houghton, K. D. Green, W. Chen and S. Garneau-Tsodikova, ChemBioChem, 2010, 11, 880–902 CrossRef CAS PubMed.
- Y. Sato, M. Rokugawa, S. Ito, S. Yajima, H. Sugawara, N. Teramae and S. Nishizawa, Chem. – Eur. J., 2018, 24, 13862–13870 CrossRef CAS PubMed.
- (a) L. Yu, T. K. Oost, J. M. Schkeryantz, J. Yang, D. Janowick and S. W. Fesik, J. Am. Chem. Soc., 2003, 125, 4444–4450 CrossRef CAS PubMed; (b) E. E. Swayze, E. A. Jefferson, K. A. Sannes-Lowery, L. B. Blyn, L. M. Risen, S. Arakawa, S. A. Osgood, S. A. Hofsyadler and R. H. Griffy, J. Med. Chem., 2002, 45, 3816–3819 CrossRef CAS PubMed; (c) S. P. Maddaford, M. Motamed, K. B. Turner, M. S. Choi, J. Ramnauth, S. Rakhit, R. R. Hudgins, D. Fabris and P. E. Johnson, Bioorg. Med. Chem. Lett., 2004, 14, 5987–5990 CrossRef CAS PubMed; (d) N. Foloppe, I.-J. Chen, B. Davis, A. Hold, D. Morley and R. Howes, Bioorg. Med. Chem., 2004, 12, 935–947 CrossRef CAS PubMed.
- (a) K. Hamasaki and R. R. Rando, Anal. Biochem., 1998, 261, 183–190 CrossRef CAS PubMed; (b) D. Watkins, F. A. Norris, S. Kumar and D. P. Arya, Anal. Biochem., 2013, 434, 300–307 CrossRef CAS PubMed; (c) P. N. Asare-Okai and C. S. Chow, Anal. Biochem., 2011, 408, 269–276 CrossRef CAS PubMed.
- (a) S. Umemoto, S. Im, J. Zhang, M. Hagihara, A. Murata, Y. Harada, T. Fukuzumi, T. Wazaki, S. Sasaoka and K. Nakatani, Chem. – Eur. J., 2012, 18, 9999–10008 CrossRef CAS PubMed; (b) L. Qi, J.-R. Wei, X.-J. Lv, Y. Huo and Z.-Q. Zhang, Biosens. Bioelectron., 2016, 86, 287–292 CrossRef CAS PubMed; (c) T. Tran and M. D. Disner, Nat. Commun., 2012, 3, 1125–1133 CrossRef PubMed.
- (a) N. Thorne, D. S. Auld and J. Inglese, Curr. Opin. Chem. Biol., 2010, 14, 315–324 CrossRef CAS PubMed; (b) W. P. Janzen, Chem. Biol., 2014, 21, 1162–1170 CrossRef CAS PubMed.
- (a) A. Simeonov, A. Jadhav, C. J. Thomas, Y. Wang, R. Huang, N. T. Southall, P. Shinn, J. Smith, C. P. Austin, D. S. Auld and J. Inglese, J. Med. Chem., 2008, 51, 2363–2371 CrossRef CAS PubMed; (b) A. Jadhav, R. S. Ferreira, C. Klumpp, B. T. Mott, C. P. Austin, J. Inglese, C. J. Thomas, D. J. Maloney, B. K. Shoichet and A. Simeonov, J. Med. Chem., 2010, 53, 37–51 CrossRef CAS PubMed.
- (a) B. P. Duckworth and C. C. Aldrich, Anal. Biochem., 2010, 403, 13–19 CrossRef CAS PubMed; (b) R. Schneider, A. Gohla, J. R. Simard, D. B. Yadav, Z. Fang, W. A. L. van Otterlo and D. Rauh, J. Am. Chem. Soc., 2013, 135, 8400–8408 CrossRef CAS PubMed; (c) J. Qian, B. P. Holskin, J. Theroff, T. Underiner, S. L. Meyer and T. S. Angeles, Assay Drug Dev. Technol., 2012, 10, 375–381 CrossRef CAS PubMed.
- (a) N. Milanovich, M. Suh, R. Jankowiak, G. J. Small and J. M. Hayes, J. Phys. Chem., 1996, 100, 9181–9186 CrossRef CAS; (b) J. Ghasemi, Sh. Ahmadi, A. I. Ahmad and S. Ghobadi, Appl. Biochem. Biotechnol., 2008, 149, 9–22 CrossRef CAS PubMed.
- E. Largy, F. Hamon and M.-P. Teulade-Fichou, Anal. Bioanal. Chem., 2011, 400, 3419–3427 CrossRef CAS PubMed.
- Y. Sato, M. Kudo, Y. Toriyabe, S. Kuchitsu, C.-X. Wang, S. Nishizawa and N. Teramae, Chem. Commun., 2014, 50, 515–517 RSC.
- M. Kaul, C. M. Barbieri and D. S. Pilch, J. Am. Chem. Soc., 2006, 128, 1261–1271 CrossRef CAS PubMed.
- M. Krishnamurthy, N. T. Schirle and P. A. Beal, Bioorg. Med. Chem., 2008, 16, 8914–8921 CrossRef CAS PubMed.
- M. Dudek, J. Romanowska, T. Witula and J. Trylska, Biochimie, 2014, 102, 188–202 CrossRef CAS PubMed.
- D. N. Wilson, Nat. Rev. Microbiol., 2014, 12, 35–48 CrossRef CAS PubMed.
- Q. Vicens and E. Westhof, ChemBioChem, 2003, 4, 1018–1023 CrossRef CAS PubMed.
- M.-K. Lee, A. Bottini, M. Kim, M. F. Bardaro Jr., Z. Zhang, M. Pellecchia, B.-S. Choi and G. Varani, Chem. Commun., 2014, 50, 368 RSC.
- Y. Hu, F. Lin, T. Wu, Y. Zhou, Q. Li, Y. Shao and Z. Xu, Anal. Chem., 2017, 89, 2181–2185 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, fluorescence response of a series of cyanines, examination of the apparent charge, FID results. See DOI: 10.1039/c9cc00414a |
| This journal is © The Royal Society of Chemistry 2019 |