Open Access Article
Open Access ArticleA mixed molecular salt of lithium and sodium breaks the Hume-Rothery rules for solid solutions†
Monica
Lestari
and
Matteo
Lusi
 *
*
Bernal Institute, University of Limerick, Castletroy, Ireland. E-mail: matteo.lusi@ul.ie
First published on 4th February 2019
Abstract
The first molecular solid solution of lithium and sodium ions is reported. In spite of the different chemical and structural properties of the parent compounds, the two cations form a homogeneous mixed phase with the isoorotate ion. Such observation appears in contrast with the Hume-Rothery principles for solid solutions. Furthermore the mixed salts in the series are thermally stable up to 100 °C and non-hygroscopic, which makes them relevant for their potential use as a lithium drug substance.
Solid solutions have long been studied in chemical and pharmaceutical research.1 Such phases allow the variation of composition and properties in continuum, enabling material design and fine-tuning.2 Unfortunately, molecular solubility in the solid state is more limited than in the liquid state.3 For example, the Hume-Rothery rule prescribes that complete miscibility in metals and inorganic salts requires isovalent atoms whose size (atomic or ionic radius) differs by less than 15%, as well as crystal isomorphism of the pure (parent) substances.4,5 In molecular mixed crystals it has been suggested that molecular isostructurality is also required,6 although exceptions are reported.7 Such strict rules explain why solid solutions are rarer than single component crystals and co-crystals. Furthermore, the difficulty in finding molecular components that satisfy the above conditions is an obstacle to the development of solid solutions into products for real world applications.
Lithium ion is the active pharmaceutical ingredient (API) of choice in the treatment of bipolar disorder and is listed as “essential medicine” by the World Health Organization. Among the many available compounds, lithium citrate and lithium carbonate are the only FDA approved salts for therapeutic purposes. Others, like lithium orotate, are commercially available as supplements. In spite of their high efficacy, these drugs are characterised by a narrow therapeutic window, whereby the difference between the serum concentrations required for effectiveness and toxicity is small. As a consequence, the drug must be administrated through medical centres that monitor lithium levels in the blood.8
Recent publications describe drug–drug ionic cocrystals (ICC) such as piracetam with lithium bromide and lithium chloride9 or lithium salicylate and L-proline10,11 that could guarantee higher lithium absorption whilst reducing toxicity. Such products could enable a simpler administration protocol, increasing patience compliance and reducing the costs of therapy. In most cases, the high hygroscopicity of lithium salts makes them unsuited for commercialization. Therefore, we have recently evaluated the hygroscopicity of solid solutions of lithium coordination polymers.12
The mechanism of action of lithium remains largely unknown.8,13 Nonetheless, it is widely accepted that the metabolism of the drug is closely dependant on that of sodium. A low sodium concentration in the serum reduces the clearance of lithium, which causes intoxication. A drug product that contains an optimal ratio of lithium and sodium could reduce toxicity and a solid solution would have the advantage of allowing precise dosage of the cations in a single phase.
Partial solubility of lithium and sodium has been reported for niobate14,15 and metazirconate16 ceramics. On the contrary, examples of mixed crystals (molecular) of the two cations remains unknown. This work reports the first solid solution of lithium and sodium salts with organic counter ions.
Lithium and sodium are two alkali metals whose van der Waals radii are 1.82 Å and 2.27 Å respectively, i.e. sodium is about 25% larger than lithium.17 The smaller radius makes lithium a much harder acid (35 versus 21 eV), and determines a rather different chemical and structural behaviour. Lithium and sodium appear to form a pair of isomorphous crystals with the monoaqua isoorotate (IOR) complex and dihydrogen citrate anion (H2CIT) (Fig. 1). Hence these systems represent ideal candidates to verify whether molecular solid solutions of lithium and sodium are possible in the series of Lix−1NaxIOR·H2O (1x), and Lix−1NaxH2CIT (2x) (where 0 > x > 1).
The monohydrate salts of lithium and sodium isoorotate crystallise in the P21/n space group and have roughly the same unit cell metrics (CSD18 refcodes VEYVUQ19 and ACUFIO,20 respectively). Crystal isomorphism occurs in spite of the different chemical structure of the two compounds. A behaviour that has been referred to as pseudo-isomorphism.21,22 The lithium ion coordinates with five oxygen atoms forming a distorted trigonal bipyramid while the sodium salt with six oxygen atoms in an octahedral manner (Fig. 2). In both cases, IOR chelates the metal atoms with one carboxylate and one keto oxygen. Two of such complexes from a dimer with the carboxylate oxygen bridging between two metals [{M2(IOR-κO,μO)2}]. Above and below the plane of the dimer, two water molecules link the dimers into a 1D polymer (ladder). Such arrangement ensures the π stacking of the aromatic rings. In NaIOR·H2O the distorted octahedral coordination sphere is completed by a further interaction with the carboxylate oxygen from the IOR below in the same polymeric chain: O–Na distance = 2.47 Å. In LiIOR·H2O such interaction is absent: O–Li distance 3.68 Å. Therefore, the resulting underlying net topology is 2,3,5C2 and 2,2,4C1 for sodium and lithium respectively. The different coordination confers a different conformation to the two compounds. In LiIOR·H2O the angle between water molecules is 118.5°, and the carboxylate group lays on the plane of the aromatic ring dihedral angle 2.4°. In NaIOR·H2O the same angles measures 96.1° and −11.0° respectively; i.e. the carboxylate group is rotated towards the upper dimer (Fig. 2 and Tables S1, S2, ESI†). Ultimately, in the lithium complex, the metal ions lay almost in the plane common to the aromatic rings (about 0.08 Å). In the sodium complex, such distance increases to about 0.45 Å. For these reasons, the default calculation of crystal packing similarity23 as implemented in Mercury24 shows a partial matching (RMS 0.408, 8 out of 15 molecules), but only if molecular and bond counts differences are allowed.
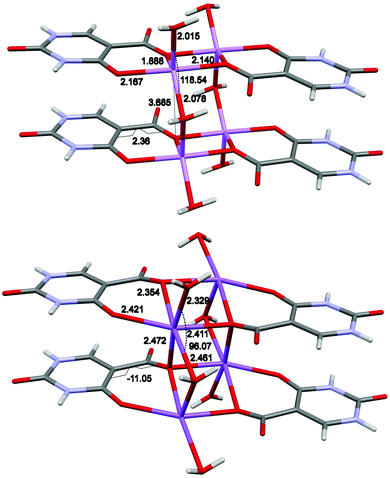 | ||
| Fig. 2 Molecular structure in LiIOR·H2O (top) and NaIOR·H2O (bottom). Selected atomic distances and torsion angle are reported. | ||
The salt can be produced mechanochemically from basic metal salts25 by grinding the carbonate salts with one equivalent of isoorotic acid. PXRD analysis reveals that similar phases form when the synthesis of intermediate Li and Na stoichiometry is attempted (Fig. 3). In the series of powder patterns, peaks shift regularly with composition. A comparison with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 physical mixture of LiIOR·H2O and NaIOR·H2O suggests that the mixed salts are a homogeneous phases, i.e. a solid solution. Rietveld analysis of the mechanochemical product enabled the refinement of the unit cell metric for the series. As expected, unit cell parameters change linearly with composition, following the Vegard's law (ESI†). Recrystallization of the samples with mixed composition affords single crystals for X-ray characterization, which confirmed the homogeneous (solid solution) nature of the mixed salts. The structures with mixed composition show the water molecule disordered over two positions and the thermal ellipsoids of the metals are oblate, as expected from atoms that are disordered over two positions. Then the coordination geometry of the mixed salts is an average of both parent compounds. Notably the metal centres in the mixed salt lay at about 0.34 and 0.42 Å from the plane of the aromatic rings depending on the Li/Na ratio.
1 physical mixture of LiIOR·H2O and NaIOR·H2O suggests that the mixed salts are a homogeneous phases, i.e. a solid solution. Rietveld analysis of the mechanochemical product enabled the refinement of the unit cell metric for the series. As expected, unit cell parameters change linearly with composition, following the Vegard's law (ESI†). Recrystallization of the samples with mixed composition affords single crystals for X-ray characterization, which confirmed the homogeneous (solid solution) nature of the mixed salts. The structures with mixed composition show the water molecule disordered over two positions and the thermal ellipsoids of the metals are oblate, as expected from atoms that are disordered over two positions. Then the coordination geometry of the mixed salts is an average of both parent compounds. Notably the metal centres in the mixed salt lay at about 0.34 and 0.42 Å from the plane of the aromatic rings depending on the Li/Na ratio.
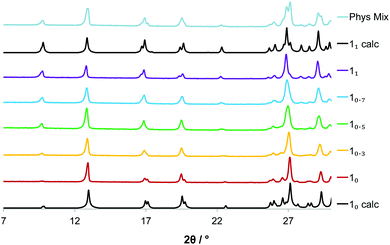 | ||
Fig. 3 PXRD pattern for Lix−1NaxIOR·H2O (1x). The reference patterns are in black. Phys. mix is the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 physical mixture of the parent. 1 physical mixture of the parent. | ||
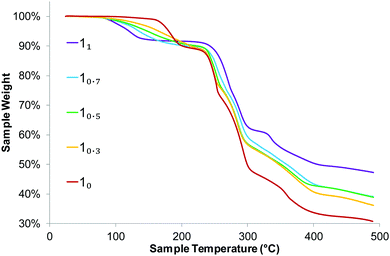
Thermogravimetric analysis shows that the elimination of the coordinated water molecule occurs at lower temperature when the sodium content increases (Fig. 4). This is likely due to both the harder acidity of lithium and the different coordination geometry. In any case, the samples are stable at least until 100 °C. DVS measurement shows that water uptake is higher for the samples with mixed composition than for the pure Li and Na salts, or the physical mixture (ESI†). Nonetheless, it remains always below 1% resulting non-hygroscopic according to the European Pharmacopeia classification.26 Measurements of particle size distribution exclude that such behaviour is a consequence of different particle size, which are rather uniform throughout the series (Fig. 5). It is worth noting that in our previous investigation, solid solutions of lithium coordination polymers with L-proline and 2-methoxybenzoate/benzoate showed partial solubility and that the water uptake for the mixed compositions were higher than that of the end members. On the other hand, solid solutions formed by lithium coordination polymers with succinate/malate showed complete solubility and that the water uptake behaviour appeared to be more regular.12
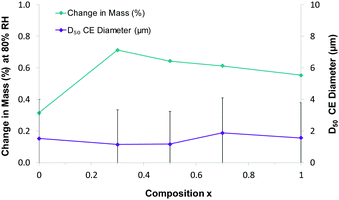 | ||
| Fig. 5 Water sorption (blue) and particle size distribution (purple) as function of composition in Lix−1NaxIOR·H2O (1x). | ||
Lithium and sodium form a pair of isomorphous crystals with the dihydrogen citrate anion. The structures are reported in the P21/a space group (CSD refcode LIHCIT27 and NAHCIT27 respectively) with the metal atoms coordinated to six oxygens in an octahedral manner. A hydrate LiH2CIT·H2O (CSD refcode ZZZREG28), whose atomic coordinates are not determined, and a second form of NaH2CIT (CSD refcode NAHCIT01),29 which crystallizes in the orthorhombic P212121, are also known. In form II, the sodium atom coordinates to seven oxygen atoms.
PXRD analysis shows that co-grinding Li2CO3 or Na2CO3 with one equivalent of citric acid results in the hydrate LiH2CIT and NaH2CIT form I respectively (Fig. 6). Co-grinding of citric acid with both Li and Na in variable ratio affords physical mixtures of the above phases rather than the desired Lix−1NaxH2CIT (2x) solid solution. Thermogravimetric analysis confirmed these conclusions: the first member of the series shows a weight loss about 7% between 100 and 150 °C as expected for pure LiH2CIT monohydrate, whereas in NaH2CIT the weight loss only starts at about 190 °C and coincides with the decomposition of the anion. The samples with mixed composition show an intermediate behaviour (see ESI†). Notably the monoclinic form I of NaH2CIT converts in the thermodynamically stable form II in about two weeks (see ESI†).
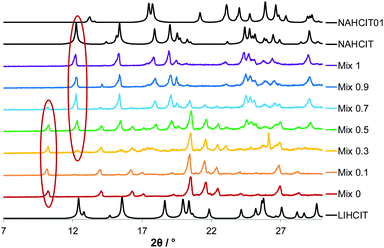 | ||
| Fig. 6 Diffraction patterns for the product of manual grinding of citric acid with LiH2CIT and NaH2CIT in variable ratio. The reference patterns are in black. | ||
In order to verify whether the formation of 2x was possible in the absence of water, the samples were dried in a vacuum oven at 100 °C overnight and then re-ground with dry ethanol. PXRD analysis shows that such treatment produces anhydrous LiH2CIT. However, new peaks appear in all the samples that contain a portion of sodium, which coincide to the orthorhombic polymorph (Fig. 7).
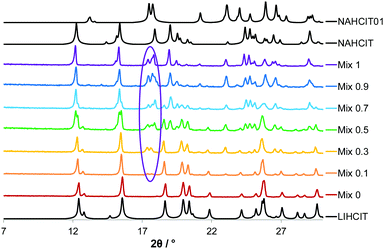 | ||
| Fig. 7 Diffraction patterns for the product of manual grinding of citric acid with LiH2CIT and NaH2CIT in variable ratio in anhydrous conditions. The reference patterns are in black. | ||
A quantification of the mixture composition by Rietveld refinement indicates that the amount of LiH2CIT phase is generally lower than expected from the reactants stoichiometry (Table 1). The difference is always small (4 to 10%) and close to limits of detections of this technique for organic samples with an in-house diffractometer. Therefore, the solubility of lithium and sodium dihydrogen citrate cannot be confirmed.
| Expected (wt%) | Found (wt%) | ||||
|---|---|---|---|---|---|
| Li | Na | LIHCIT | NAHCIT | NAHCIT01 | |
| Mix 0.1 | 89.28 | 10.72 | 86.7(4) | 4.8(9) | 8.4(3) |
| Mix 0.3 | 64.34 | 31.66 | 64.7(3) | — | 35.3(6) |
| Mix 0.5 | 48.05 | 51.95 | 43.7(7) | 44.0(8) | 12.2(5) |
| Mix 0.7 | 28.39 | 71.61 | 19(1) | 62.3(2) | 18.6(9) |
| Mix 0.9 | 9.32 | 90.68 | — | 52.8(3) | 47.2(8) |
This work demonstrates that, despite the large different size and chemical properties, lithium and sodium can homogeneously mix in a single phase when they are combined with the appropriate anion. In the case of isoorotate salt, a virtually complete solubility exists despite the different coordination geometry in the parent compounds. On the other hand, in the isomorphous and isostructural dihydrogen citrate system, the extent of solid-state solubility is, at most, limited. These observations are in open contrast with Hume-Rothery rules.
We speculate that, in the non-isostructural isoorotate system, the high solubility is enabled by the presence of a water molecule that can freely pivot between the lithium and sodium coordination without affecting the overall crystal packing. In contrast, the low solubility it the citrate system could be explained by the existence of more stable solid forms (a hydrate and a polymorph). In a previous work we demonstrated that, solid solution could serve to control polymorphism in a series Co/Zn coordination polymer.30 Here that effect is not observed.
Ultimately, we have presented the first example of molecular solid solution of lithium and sodium salts. This phase shows enough thermal and humidity stability to be employable as lithium drug substances medical therapy. From a pharmaceutical point of view, such mixed salt could help regulating the sodium concentration in the serum and the metabolism of lithium in the treatment of bipolar disorder.
The authors acknowledge the financial support of Science Foundation Ireland that contributed with Grant 15/SIRG/3577.
Conflicts of interest
The authors have no conflicts to declare.Notes and references
- A. H. Goldberg, M. Gibaldi and J. L. Kanig, J. Pharm. Sci., 1965, 54, 1145–1148 CrossRef CAS PubMed.
- M. Lusi, Cryst. Growth Des., 2018, 18, 3704–3712 CrossRef CAS.
- M. Lusi, CrystEngComm, 2018, 20, 7042–7052 RSC.
- W. Hume-Rothery, J. Inst. Met., 1926, 35, 295–361 Search PubMed.
- W. D. Callister and D. G. Rethwisch, Materials Science and Engineering: An Introduction, Wiley, 8th edn, 2009 Search PubMed.
- A. I. Kitaigorodskii, Mixed crystals, Springer-Verlag, Berlin, 1984 Search PubMed.
- E. Schur, E. Nauha, M. Lusi and J. Bernstein, Chem. – Eur. J., 2015, 21, 1735–1742 CrossRef CAS PubMed.
- R. Oruch, M. A. Elderbi, H. A. Khattab, I. F. Pryme and A. Lund, Eur. J. Pharmacol., 2014, 740, 464–473 CrossRef CAS PubMed.
- D. Braga, F. Grepioni, L. Maini, D. Capucci, S. Nanna, J. Wouters, L. Aerts and L. Quéré, Chem. Commun., 2012, 48, 8219 RSC.
- A. J. Smith, S.-H. Kim, N. K. Duggirala, J. Jin, L. Wojtas, J. Ehrhart, B. Giunta, J. Tan, M. J. Zaworotko and R. D. Shytle, Mol. Pharmaceutics, 2013, 10, 4728–4738 CrossRef CAS PubMed.
- N. K. Duggirala, M. L. Perry, Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2016, 52, 640–655 RSC.
- M. Lestari, M. Lusi, A. O'Leary, D. O'Nolan and M. J. Zaworotko, CrystEngComm, 2018, 20, 5940–5944 RSC.
- F. Marmol, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2008, 32, 1761–1771 CrossRef CAS PubMed.
- T. Nitta, J. Am. Ceram. Soc., 1968, 51, 623–630 CrossRef.
- R. C. R. Franco, E. R. Camargo, M. A. L. Nobre, E. R. Leite, E. Longo and J. A. Varela, Ceram. Int., 1999, 25, 455–460 CrossRef CAS.
- H. Pfeiffer, E. Lima and P. Bosch, Chem. Mater., 2006, 18, 2642–2647 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- C. C. Wagner, E. J. Baran, O. E. Piro and E. E. Castellano, J. Inorg. Biochem., 1999, 77, 209–213 CrossRef CAS.
- G. S. Nichol and W. Clegg, Polyhedron, 2006, 25, 1043–1056 CrossRef CAS.
- G. Y. Chao and J. D. McCullough, Acta Crystallogr., 1961, 14, 940–945 CrossRef CAS.
- S. d’Agostino, D. Braga, F. Grepioni and P. Taddei, Cryst. Growth Des., 2014, 14, 821–829 CrossRef.
- J. A. Chisholm and S. Motherwell, J. Appl. Crystallogr., 2005, 38, 228–231 CrossRef.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- C. J. Adams, M. A. Kurawa, M. Lusi and A. G. Orpen, CrystEngComm, 2008, 10, 1790–1795 RSC.
- European Pharmacopoeia, Directorate for the Quality of Medicine & Healthcare of the Council of Europe (EDQM), Strasburg, 8th edn, 2013, p. 695.
- J. P. Glusker, D. Van Der Helm, W. E. Love, M. Dornberg, J. A. Minkin, C. K. Johnson and A. L. Patterson, Acta Crystallogr., 1965, 19, 561–572 CrossRef CAS.
- W. E. Love and A. L. Patterson, Acta Crystallogr., 1960, 13, 426–428 CrossRef CAS.
- A. Rammohan and J. A. Kaduk, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2016, 72, 854–857 CrossRef CAS PubMed.
- C. J. Adams, A. L. Gillon, M. Lusi and A. G. Orpen, CrystEngComm, 2010, 12, 4403 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, supplementary figures and tables. CCDC 1880097, 1880098, 1884568 and 1884569. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc09850f |
| This journal is © The Royal Society of Chemistry 2019 |