Bioinspired radical cyclization of tryptamines: synthesis of peroxypyrroloindolenines as potential anti-cancer agents†
Yan
Li
,
Longjie
Li
 ,
Xunbo
Lu
,
Yulong
Bai
,
Yufan
Wang
,
Yuzhou
Wu
,
Xunbo
Lu
,
Yulong
Bai
,
Yufan
Wang
,
Yuzhou
Wu
 * and
Fangrui
Zhong
* and
Fangrui
Zhong
 *
*
Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), 1037 Luoyu road, Wuhan 430074, China. E-mail: wuyuzhou@hust.edu.cn; chemzfr@hust.edu.cn; Fax: +86-27-8754 3632
First published on 24th November 2018
Abstract
Inspired by the heme iron-catalyzed radical insertion of dioxygen to the tryptophan indole ring, herein we utilize alkylperoxy radical species as a coupling partner to trigger a peroxycyclization of readily accessible tryptophan derivatives and enable the first synthesis of peroxypyrroloindolenines. A preliminary biological evaluation revealed promising anti-cancer activities (IC50 = 22.00 μM for compound 2a), and revealed that both the indolenine core and the peroxy functionality are responsible for the antiproliferation effect against Hela cell lines.
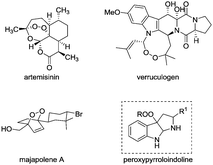
The peroxy functionality has been regarded as an important structural motif linked to the antitumor, antibacterial, and other biological activities of many pharmaceutical compounds, such as artemisinin, verruculogen, and majapolene A (Fig. 1).1,2 Notably, the cleavage of the relatively weak O–O bond is widely recognized to be responsible for the mechanism of action of drugs,3 thus spurring great synthetic interest in effectively constructing organic peroxides.4 Since the pioneering studies by Kharasch et al. on cobalt-catalyzed peroxidation with hydroperoxide,5 many state-of-the-art peroxidation methodologies have been disclosed by using transition metal catalysis6 or organocatalysis.7 Despite these significant advances, the structural diversity of the resulting peroxides is still limited. Particularly, it remains a standing challenge to selectively install a peroxy unit into complex molecular frameworks.8
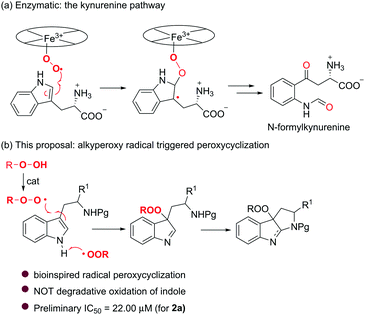
In the course of our studies toward the efficient synthesis of N-heterocyclic compounds,9 we were impressed by the molecular architecture of pyrroloindoles because of their frequent occurrence in many natural alkaloids.10 With their wide range of biological activities, together with the aforementioned pharmacophoric significance of a peroxy unit, it is quite interesting to hybridize these two entities and obtain a novel family of peroxypyrroloindoles (Fig. 1). Of note, the C3a substitution pattern of pyrroloindoles appears to be essential for their distinct biological profiles, thus having stimulated considerable synthetic efforts to functionalize this site with various groups (e.g., alkyl, aryl, halogen, azido, hydroxy, and amino).11 However, to our knowledge, the preparation of the respective peroxylated counterpart remains elusive. The only known synthesis was achieved through photooxygenation of tryptophan to give a hydroperoxypyrroloindoline adduct.12 Unfortunately, the highly labile nature of this peroxide substantially retards practical synthesis and biological evaluation. Inspired by the heme dioxygenase-catalyzed insertion of oxygen to the indole rings13 (i.e., kynurenine pathway, Scheme 1a), we envisaged that an alkylperoxy radical might be capable of abstracting the hydrogen of indole NH14 and undergoing an electrophilic addition to the C3a of indole. A subsequent intramolecular cyclization would render the assembly of interesting peroxypyrroloindoles (Scheme 1b). In this communication, we report the Bu4NI (TBAI) catalyzed synthesis of unexpected peroxypyrroloindolenine derivatives from readily accessible tryptamine derivatives by the use of t-BuOOH (TBHP) (Scheme 1b). A preliminary biological evaluation showed that such an unprecedented class of peroxides exhibit promising cytotoxic activities against Hela cell lines.
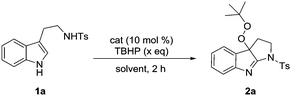
Recent advances in the TBAI/TBHP catalytic system shed light on the utilization of the t-BuOO˙ and t-BuO˙ species as radical coupling partners for organic transformations.15 We were encouraged to apply them in the radical cyclization reaction of tryptamines. The initial attempt was commenced with a model reaction of N-tosyl tryptamine 1a and TBHP (2.0 eq.) in the presence of 10 mol% of TBAI. Unexpectedly, the major product isolated in CH3CN under reflux (20% yield) was identified to be peroxypyrroloindolenine 2a. Given its potential interesting properties,16 it became our synthetic target for subsequent optimization (Table 1). It was evident that TBHP served a dual role as an oxidant and a reactant. In line with this, appropriately excess TBHP (4.0 eq.) was found to be helpful in improving the yield of product 2a (entries 2–4). A control experiment demonstrated the indispensability of TBAI, as without it, no reaction took place (entry 5). I2 and NaI were proven to be less effective than TABI (entries 6 and 7). Subsequent optimization of the solvent revealed 1,4-dioxane as the best medium (Table S1, ESI†), and 62% yield was recorded (entry 8). While thermoactivation was a requisite for this transformation, performing the reaction at 90 °C provided a superior result (70%, entry 10). Further improvement of the yield was achieved by increasing the loading of TBAI (20 mol%). Under the optimal conditions, pyrroloindolenine 2a was isolated in 78% yield (entry 12).
| Entry | Catalyst | x | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 1a (0.2 mmol), a catalyst (10 mol%), and TBHP in a solvent (4.0 ml) for 2 hours. b Isolated yield. c 20 mol% of TBAI was used. | |||||
| 1 | TBAI | 2 | CH3CN | Reflux | 20 |
| 2 | TBAI | 3 | CH3CN | Reflux | 27 |
| 3 | TBAI | 4 | CH3CN | Reflux | 35 |
| 4 | TBAI | 5 | CH3CN | Reflux | 26 |
| 5 | — | CH3CN | Reflux | NR | |
| 6 | I2 | 4 | CH3CN | Reflux | 22 |
| 7 | NaI | 4 | CH3CN | Reflux | 20 |
| 8 | TBAI | 4 | 1,4-Dioxane | Reflux | 62 |
| 9 | TBAI | 4 | 1,4-Dioxane | rt | NR |
| 10 | TBAI | 4 | 1,4-Dioxane | 90 | 70 |
| 11 | TBAI | 4 | 1,4-Dioxane | 70 | 64 |
| 12c | TBAI | 4 | 1,4-Dioxane | 90 | 78 |
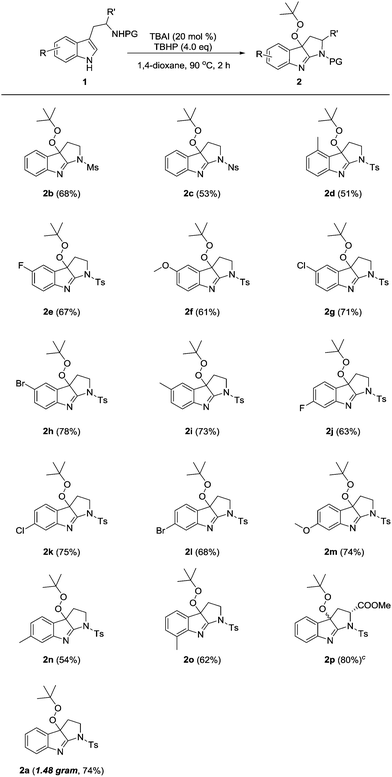
The generality of this peroxycyclization system was next evaluated (Table 2). N-Mesyl and N-nosyl tryptamines were smoothly transformed into the desired products 2b–c in synthetically useful yields. Of special note is that product 2d was delivered in a somewhat lower yield (51%), likely due to the steric congestion between the 4-methyl and peroxy groups. A series of other diversely substituted tryptamines bearing either electron-withdrawing or electron-donating groups on the indole ring were all readily processed, thus affording fused indolenines 2e–o in 54–78% yields. The employment of tryptophan as a substrate resulted in the formation of compound 2p as a mixture of diastereoisomers (d.r. = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Notably, all these reactions were implemented with aqueous TBHP under aerobic conditions, without concern for their sensitivities to moisture and oxygen. Moreover, a gram-scale preparation of product 2a was successfully carried out in a consistently good efficiency (1.48 gram, 74% yield).
1). Notably, all these reactions were implemented with aqueous TBHP under aerobic conditions, without concern for their sensitivities to moisture and oxygen. Moreover, a gram-scale preparation of product 2a was successfully carried out in a consistently good efficiency (1.48 gram, 74% yield).
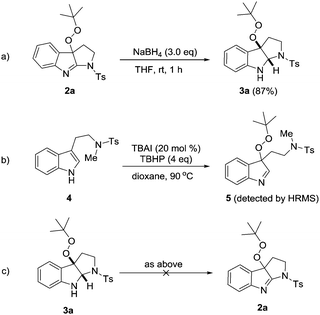
Peroxypyrroloindolenine 2a could be selectively reduced by NaBH4 in THF to give fused indoline 3a in 87% yield with the peroxy group untouched (Scheme 2a). Some additional experiments were performed to probe the reaction mechanism. First, when N-methylated substrate 4 was subjected to the peroxidation reaction conditions (Scheme 2b), although we failed to isolate intermediate 5 probably due to its instability, its existence was validated by the HRMS analysis of the reaction mixture (see the ESI†). These results suggest that the peroxidation takes place before the intramolecular C–N bond formation. Surprisingly, treatment of pyrroloindoline 3a with TBAI/TBHP did not give rise to any trace of compound 2a (Scheme 2c), thus firmly excluding the event of a second oxidation that converts the former as an intermediate to the latter. Moreover, the introduction of TEMPO (4.0 eq.) to the model reaction mixture led to a significant inhibition of the cyclization (36% conversion of 1a at 2 hours).
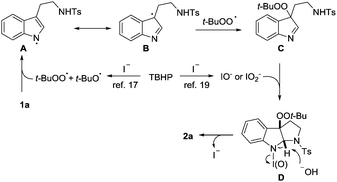
In view of the above observations, it seems safe to conclude that a radical process is involved in the catalysis. A reaction mechanism is tentatively proposed (Scheme 3). Exposure of TBHP to TBAI generates reactive radicals, t-BuOO˙ and t-BuO˙,17 which might abstract hydrogen of the indole NH to give radical A. Due to the persistent radical effect, this species likely undergoes radical coupling with t-BuOO˙ at the C3a site,18 which is analogous to the heme-catalyzed dioxygenation of tryptophan. Despite failing to isolate it, the formation of adduct C is somewhat supported by the intermediacy of compound 5. Subsequently, an (hypo)iodite mediated oxidative coupling19 of C is likely in operation to give intermediate D, which was detected by mass spectroscopy (see the ESI†). Finally, a base facilitated elimination of D would take place to regenerate iodide and release product 2a.
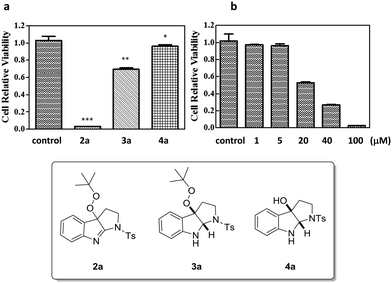
Given that many organic peroxides and pyrroloindoline alkaloids possess anti-cancer properties, compounds 2a and 3a and the respective C3a-hydroxylated pyrroloindoline 4a were taken for a preliminary cytotoxicity study against Hela cell lines. Among these, indole derivatives 2a and 3a displayed a significant antiproliferation effect at a concentration of 100 μM, sharply contrasting the negligible effect from compound 4a obviating the peroxy group (Fig. 2a). Interestingly, it was evident that the unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the indolenine structure was also essential for the anti-cancer activity, as 2a showed a tendency for a much enhanced antiproliferation profile compared to 3a. Closer investigations of the anti-cancer activity of 2a were then carried out at different concentrations for 48 h (Fig. 2b). The IC50 was determined to be 22.00 μM. A comprehensive SAR study of a large collection of analogues of 2a for cytotoxic activity against multiple human cancer cell lines is currently underway.
N bond of the indolenine structure was also essential for the anti-cancer activity, as 2a showed a tendency for a much enhanced antiproliferation profile compared to 3a. Closer investigations of the anti-cancer activity of 2a were then carried out at different concentrations for 48 h (Fig. 2b). The IC50 was determined to be 22.00 μM. A comprehensive SAR study of a large collection of analogues of 2a for cytotoxic activity against multiple human cancer cell lines is currently underway.
In summary, we have disclosed a bioinspired, iodide-catalyzed oxidative radical cyclization of readily accessible tryptamines that renders the first synthesis of peroxypyrroloindolenines. The significant cytotoxicity of parent compound 2a against Hela cell lines underscores the significance of both the indolenine core and the peroxy functionality for the anti-cancer activity. This serves as a promising starting point for a further comprehensive SAR study and might shed light on the design of novel anti-cancer agents. As such, we believe that the iodide catalysis protocol described herein will find useful applications in the preparation of valuable peroxylated compounds with considerable molecular complexity.
We thank the National Natural Science Foundation of China (21602067) for financial support. We are also grateful to the Analytical and Testing Centre of HUST, Analytical and Testing Centre of School of Chemistry and Chemical Engineering (HUST) for access to their facilities.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D. A. Casteel, Nat. Prod. Rep., 1999, 16, 55–73 RSC; (b) M. Jung, H. Kim, K. Lee and M. J. Park, Mini-Rev. Med. Chem., 2003, 3, 159–165 CrossRef CAS; (c) Z. Rappoport, Chem. Peroxides, Wiley, New York, 2006 CrossRef.
- (a) K. L. Erickson, J. A. Beutler, G. N. Gray, J. H. I. Cardellina and M. R. Boyd, J. Nat. Prod., 1995, 58, 1848–1860 CrossRef CAS; (b) V. M. Dembitsky, T. A. Gloriozova and V. W. Poroikov, Mini-Rev. Med. Chem., 2007, 7, 571–589 CrossRef CAS; (c) R. D. Slack, A. M. Jacobine and G. H. Posner, MedChemComm, 2012, 3, 281–297 RSC.
- (a) S. R. Meshnick, Int. J. Parasitol., 2002, 32, 1655–1660 CrossRef CAS; (b) D. Chaturvedi, A. Goswami, P. P. Saikia, N. C. Barua and P. G. Rao, Chem. Soc. Rev., 2010, 39, 435–454 RSC.
- For an elegant review, see: H. Gandhi, K. O’Reilly, M. K. Gupta, C. Horgan, E. M. O’Leary and T. P. O’Sullivan, RSC Adv., 2017, 7, 19506–19556 RSC.
- (a) M. S. Kharasch, P. Pauson and W. Nudenberg, J. Org. Chem., 1953, 18, 322 CrossRef CAS; (b) M. S. Kharasch and A. Fono, J. Org. Chem., 1958, 23, 324 Search PubMed; (c) M. S. Kharasch and A. Fono, J. Org. Chem., 1959, 24, 72 CrossRef CAS.
- For selected examples, see: (a) W. Liu, Y. Li, K. Liu and Z. Li, J. Am. Chem. Soc., 2011, 133, 10756–10759 CrossRef CAS; (b) S. W. Krabbe, D. T. Do and J. S. Johnson, Org. Lett., 2012, 14, 5932–5935 CrossRef CAS; (c) A. Banerjee, S. K. Santra, N. Khatun, W. Ali and B. K. Patel, Chem. Commun., 2015, 51, 15422–15425 RSC; (d) X. Sun, X. Li, S. Song, Y. Zhu, Y.-F. Liang and N. Jiao, J. Am. Chem. Soc., 2015, 137, 6059–6066 CrossRef CAS PubMed; (e) V. R. Sabbasani, H. Lee, Y.-Z. Xia and D. Lee, Angew. Chem., Int. Ed., 2016, 55, 1151–1155 CrossRef CAS PubMed; (f) J.-K. Cheng and T.-P. Loh, J. Am. Chem. Soc., 2015, 137, 42–45 CrossRef CAS PubMed; (g) Y. Lan, X.-H. Chang, P. Fan, C.-C. Shan, B.-Z. Liu, T.-P. Loh and Y.-H. Xu, ACS Catal., 2017, 7, 7120–7125 CrossRef CAS; (h) Y. Lan, C. Yang, Y.-H. Xu and T.-P. Loh, Org. Chem. Front., 2017, 4, 1411–1415 RSC; (i) Y. Chen, Y. Chen, S. Lu and Z. Li, Org. Chem. Front., 2018, 5, 972–976 RSC.
- (a) K. Žmitek, M. Zupan, S. Stavber and J. Iskra, J. Org. Chem., 2007, 72, 6534–6540 CrossRef; (b) X.-J. Lu, Y. Liu, B.-F. Sun, B. Cindric and L. Deng, J. Am. Chem. Soc., 2008, 130, 8134–8135 CrossRef CAS; (c) A. Russo and A. Lattanzi, Adv. Synth. Catal., 2008, 350, 1991–1995 CrossRef CAS; (d) W.-H. Zheng, L. Wojtas and J. C. A. Antilla, Angew. Chem., Int. Ed., 2010, 49, 6589–6591 CrossRef CAS; (e) B. Schweitzer-Chaput, J. Demaerel, H. Engler and M. Klussmann, Angew. Chem., Int. Ed., 2014, 53, 8737–8740 CrossRef CAS; (f) T. J. Fisher and A. E. Mattson, Org. Lett., 2014, 16, 5316–5319 CrossRef CAS; (g) H. Yu and J. Shen, Org. Lett., 2014, 16, 3204–3207 CrossRef CAS; (h) L. Hu, X.-J. Lu and L. Deng, J. Am. Chem. Soc., 2015, 137, 8400–8403 CrossRef CAS.
- (a) Y. Yang, M. Farhana, C. Willy, T. Ma, Z. Jiang and C. H. Tan, Org. Lett., 2012, 14, 4762–4765 CrossRef CAS; (b) X. Wang, Y. Pan, K.-W. Huang and Z. Lai, Org. Lett., 2015, 17, 5630–5633 CrossRef CAS; (c) S. Nakamura and S. Takahashi, Org. Lett., 2015, 17, 2590–2593 CrossRef CAS; (d) M. Kischkewitz, C.-G. Daniliuc and A. Studer, Org. Lett., 2016, 18, 1206–1209 CrossRef CAS PubMed.
- (a) Y. Ni, H. Zuo, Y. Li, Y. Wu and F. Zhong, Org. Lett., 2018, 20, 4350–4353 CrossRef CAS; (b) Y. Ni, Q. Yu, Q. Liu, H. Zuo, H.-B. Yu, W.-J. Wei, R.-Z. Liao and F. Zhong, Org. Lett., 2018, 20, 1404–1408 CrossRef CAS; (c) X. Lu, Y. Shi and F. Zhong, Green Chem., 2018, 20, 113–117 RSC.
- (a) D. Crich and A. Banerjee, Acc. Chem. Res., 2007, 40, 151–161 CrossRef CAS; (b) P. Ruiz-Sanchis, S. A. Savina, F. Albericio and M. Álvarez, Chem. – Eur. J., 2011, 17, 1388–1408 CrossRef CAS.
- For selected examples, see: (a) D. Tu, L. Ma, X. Tong, X. Deng and C. Xia, Org. Lett., 2012, 14, 4830–4833 CrossRef CAS; (b) X. Deng, K. Liang, X. Tong, M. Ding, D. Li and C. Xia, Org. Lett., 2014, 16, 3276–3279 CrossRef CAS; (c) H. Yin, T. Wang and N. Jiao, Org. Lett., 2014, 16, 2302–2305 CrossRef CAS; (d) W. Yang, L. Huang, Y. Yu, D. Pflästerer, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2014, 20, 3927–3931 CrossRef CAS; (e) P. Zhang, W. Sun, G. Li, L. Hong and R. Wang, Chem. Commun., 2015, 51, 12293–12296 RSC; (f) C. Liu, Q. Yin, L.-X. Dai and S.-L. You, Chem. Commun., 2015, 51, 5971–5974 RSC; (g) A. A. Adhikari and J. D. Chisholm, Org. Lett., 2016, 18, 4100–4103 CrossRef CAS; (h) C. Liu, J.-C. Yi, Z.-B. Zheng, Y. Tang, L.-X. Dai and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 751–754 CrossRef CAS PubMed; (i) C. Ma, T. Zhang, J.-Y. Zhou, G.-J. Mei and F. Shi, Chem. Commun., 2017, 53, 12124–12127 RSC; (j) J. Xu and R. Tong, Green Chem., 2017, 19, 2952–2956 RSC; (k) T. Horibe, S. Ohmura and K. Ishihara, Org. Lett., 2017, 19, 5525–5528 CrossRef CAS; (l) J.-C. Yi, C. Liu, L.-X. Dai and S.-L. You, Chem. – Asian J., 2017, 12, 2975–2979 CrossRef CAS; (m) W. Xie, G. Jiang, H. Liu, J. Hu, X. Pan, H. Zhang, X. Wan, Y. Lai and D. Ma, J. Am. Chem. Soc., 2018, 140, 3394–3402 CrossRef.
- (a) M. Nakagawa, S. Kato, S. Kataoka, S. Kodato, H. Watanabe, H. Okajima, T. Hino and B. Witkop, Chem. Pharm. Bull., 1981, 29, 1013–1026 CrossRef CAS; (b) M. Nakagawa, Y. Yokoyama, S. Kato and T. Hino, Tetrahedron, 1985, 41, 2125–2132 CrossRef CAS.
- (a) J. Basran, I. Efimov, N. Chauhan, S. J. Thackray, J. L. Krupa, G. Eaton, G. A. Griffith, C. G. Mowat, S. Handa and E. L. Raven, J. Am. Chem. Soc., 2011, 133, 16251–16257 CrossRef CAS; (b) I. Shin, B. R. Ambler, D. Wherritt, W. P. Griffith, A. C. Maldonado, R. A. Altman and A. Liu, J. Am. Chem. Soc., 2018, 140, 4372–4379 CrossRef CAS.
- (a) S. E. Walden and R. A. Wheeler, J. Chem. Soc., Perkin Trans. 2, 1996, 2663–2672 RSC; (b) A. Piatkivskyi, S. Osburn, K. Jaderberg, J. Grzetic, J. D. Steill, J. Oomens, J. Zhao, J. K.-C. Lau, U. H. Verkerk and A. C. Hopkinson, J. Am. Soc. Mass Spectrom., 2013, 24, 513–523 CrossRef CAS.
- For selected reviews, see: (a) M. Uyanik and K. Ishihara, ChemCatChem, 2012, 4, 177–185 CrossRef CAS; (b) X.-F. Wu, J.-L. Gong and X. Qi, Org. Biomol. Chem., 2014, 12, 5807–5817 RSC; (c) D. Liu and A. Lei, Chem. – Asian J., 2015, 10, 806–823 CrossRef CAS; (d) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328–3435 CrossRef CAS.
- The pyrroloindolenine is present in naturally occurring Flustramine C, for relevant studies see: (a) T. Lindel, L. Bräuchle, G. Golz and P. Böhrer, Org. Lett., 2007, 9, 283–286 CrossRef CAS; (b) T. Kawasaki, M. Shinada, M. Ohzono, A. Ogawa, R. Terashima and M. Sakamoto, J. Org. Chem., 2008, 73, 5959–5964 CrossRef CAS.
- (a) H. Yu and J. Shen, Org. Lett., 2014, 16, 3204–3207 CrossRef CAS; (b) M. Kischkewitz, C.-G. Daniliuc and A. Studer, Org. Lett., 2016, 18, 1206–1209 CrossRef CAS.
- A. Studer, Chem. – Eur. J., 2001, 7, 1159–1164 CrossRef CAS.
- (a) M. Uyanik, H. Okamoto, T. Yasui and K. Ishihara, Science, 2010, 328, 1376–1379 CrossRef; (b) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. J. Nachtsheim, Org. Lett., 2011, 13, 3754–3757 CrossRef CAS; (c) M. Uyanik, H. Hayashi and K. Ishihara, Science, 2014, 345, 291–294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc08866g |
| This journal is © The Royal Society of Chemistry 2019 |