Open Access Article
Open Access ArticleZein/gelatin/nanohydroxyapatite nanofibrous scaffolds are biocompatible and promote osteogenic differentiation of human periodontal ligament stem cells†
Qianmin
Ou‡
a,
Yingling
Miao‡
 b,
Fanqiao
Yang
c,
Xuefeng
Lin
b,
Fanqiao
Yang
c,
Xuefeng
Lin
 a,
Li-Ming
Zhang
*b and
Yan
Wang
a,
Li-Ming
Zhang
*b and
Yan
Wang
 *a
*a
aGuanghua School of Stomatology, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Stomatology, Guangzhou 510080, China. E-mail: wang93@mail.sysu.edu.cn; Tel: +86 2087330592
bSchool of Materials Science and Engineering, Sun Yat-sen University, Guangzhou 510275, China. E-mail: ceszhlm@mail.sysu.edu.cn; Tel: +86 2084112354
cShunde hospital of Southern Medical University, Southern Medical University, Shunde, 528300, China
First published on 27th February 2019
Abstract
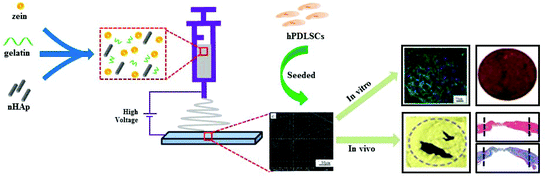
In bone tissue engineering, it is important for biomaterials to promote the osteogenic differentiation of stem cells to achieve tissue regeneration. Therefore, it is critical to develop biomaterials with excellent cytocompatibility and osteoinductive ability. In our previous study, we found a zein/gelatin electrospinning scaffold with good biocompatibility, but low osteoinductive ability for human periodontal ligament stem cells (hPDLSCs). Therefore, herein, we fabricated novel zein/gelatin/nanohydroxyapatite (zein/gelatin/nHAp) nanofibrous membranes to overcome the drawbacks of the zein/gelatin scaffold. The results showed that the surface wettability of the zein/gelatin/nHAp nanofiber membranes was increased. Moreover, the inclusion of nHAp facilitated the attachment, proliferation, and osteogenic differentiation of hPDLSCs. Overall, the zein/gelatin/nHAp nanofiber membranes showed good biocompatibility and osteoinductive activity for hPDLSCs in vitro and in vivo; this suggested potential applications of these membranes in bone tissue engineering.
Introduction
Periodontitis is a chronic inflammatory disease that invades the gums and the supporting periodontal tissues, leading to an irreversible loss of attachment and alveolar bone resorption. It is difficult to restore the destroyed tissue in terms of structure and function once it is damaged. Conventional therapies currently applied to treat periodontal diseases have shown limited potential for complete periodontal regeneration.1 The regeneration of a functional tooth-supporting attachment apparatus, especially osseous tissue, is of significant importance.2,3 Human periodontal ligament stem cells (hPDLSCs) have characteristics similar to those of mesenchymal stem cells (MSCs) and are a type of stem cell discovered by Seo.4 hPDLSCs have become a hotspot in periodontal tissue engineering because of their ease of access, low immunogenicity, multi-directional differentiation ability, and cementum-periodontal ligament complex formation.5–8 However, only a few studies have been reported on biomaterials with osteoinductive abilities for hPDLSCs. Therefore, it is urgent to develop biomaterials that facilitate the cytocompatibility and osteogenic differentiation of hPDLSCs.The extracellular matrix (ECM) is a complex interconnected structure composed of mucopolysaccharides and fibrin that provides mechanical strength and structural support for cells.9 Electrospinning is a popular technique for generating fibres ranging from the nanometre to the micrometre scale to mimic the characteristics of the ECM to some extent.9–11 Thus, electrospun nanofibers have been extensively applied in tissue engineering, wound repair, and drug delivery.12,13 Zein and gelatin are natural polymers with excellent biocompatibility and biodegradability, which have been extensively investigated.14–16 In our previous study, zein/gelatin membranes were fabricated by electrospinning, and their compatibility with hPDLSCs was evaluated.17 The electrospun zein/gelatin membranes enhanced the attachment and growth of the hPDLSCs. However, hPDLSCs cultured on the electrospun zein/gelatin membranes showed weak alkaline phosphatase (ALP) activity. Therefore, it is necessary to incorporate a component with osteoinductive activity into the zein/gelatin nanofibres.
Hydroxyapatite (HAp) [Ca10(PO4)6(OH)2] is the main inorganic substitute of alveolar bone with high mechanical strength, good biocompatibility, and osteoinductive ability.9,18,19 Natural crystalline hydroxyapatite in the needle form is about 15–200 nm in length, 10–80 nm in width, and 2–7 nm in thickness.20 Therefore, compared to apatite, nano hydroxyapatite (nHAp) is more similar to natural bone in terms of crystallinity and morphology. The nHAp component is believed to promote the formation and deposition of calcium phosphate in the human body. It can enhance the intrinsic strength and biological activity of other biological materials and thus reduce their negative effects.21–23 Thus, nHAp is considered to be the most promising material in bone regeneration.
In this study, we fabricated zein/gelatin/nHAp nanofiber membranes by electrospinning. The cytocompatibility of the zein/gelatin/nHAp membranes was evaluated by in vitro cell attachment and proliferation assays. The effects of the zein/gelatin/nHAp membranes on the osteogenic differentiation of hPDLSCs were investigated by in vitro and in vivo experiments (Scheme 1). The results of this study may provide useful information for scaffold selection in periodontal tissue regeneration.
Materials and methods
Materials
Alpha-minimum essential medium was purchased from Gibco (Grand Island, NY, USA). The osteogenic medium consisted of a standard culture medium supplemented with 100 μM L-ascorbic-2-phosphate, 20 μM dexamethasone, and 2 M β-glycerophosphate purchased from Sigma-Aldrich. Adipogenic medium was purchased from Cyagen Biosciences Inc. (Sunnyvale, CA, USA). The CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) kit was obtained from Promega Corporation. Zein (Z3625), gelatin from porcine skin (gel strength = 300 g Bloom, Type A), and collagenase type I were purchased from Sigma. 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP, 99%) was purchased from Energy Chemical. Nano hydroxyapatite (nHAp, ≥97%, <100 nm particle size) was purchased from Aladdin.Preparation of the nanofibrous membranes
| Sample | Zein/gelatin (g/g) | nHAP (g) | HFIP (mL) | Zein ratio (w/w) | Gelatin ratio (w/w) | nHAp ratio (w/w) |
|---|---|---|---|---|---|---|
| Zein/gelatin | 0.50/0.17 | 0 | 5.0 | 74.63% | 25.37% | 0 |
| Zein/gelatin/nHAp-1 | 0.50/0.17 | 0.05 | 5.0 | 69.44% | 23.61% | 6.94% |
| Zein/gelatin/nHAp-2 | 0.50/0.17 | 0.10 | 5.0 | 64.94% | 22.08% | 12.98% |
| Zein/gelatin/nHAp-3 | 0.50/0.17 | 0.17 | 5.0 | 59.52% | 20.24% | 20.24% |
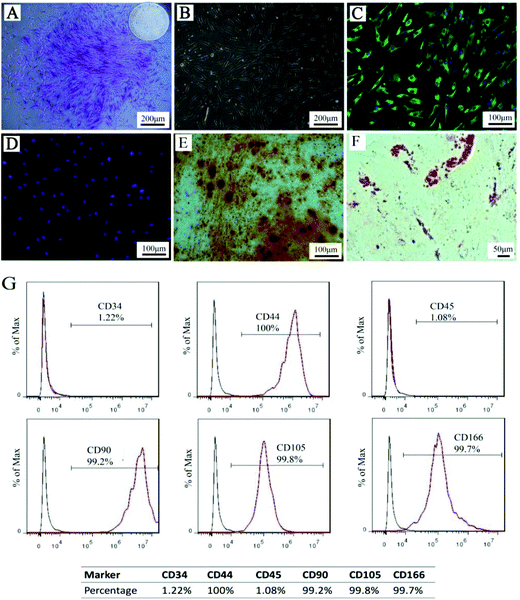
hPDLSC identification
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Abcam) for 1 hour followed by incubation with secondary antibodies (1
200, Abcam) for 1 hour followed by incubation with secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, Abcam) for 45 min in the dark. After this, Hoechst 33342 (1
300, Abcam) for 45 min in the dark. After this, Hoechst 33342 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; 1 mg mL−1) was applied for nuclear staining. The results were observed, and images were obtained using fluorescence microscopy.
000; 1 mg mL−1) was applied for nuclear staining. The results were observed, and images were obtained using fluorescence microscopy.
Biocompatibility and osteogenesis in vitro and in vivo
| Gene target | Sequence | Predicted size (bp) |
|---|---|---|
| ALP | Forward: 5′-GTGAACCGCAACTGGTACTC-3′ | 81 |
| Reverse: 5′-GAGCTGCGTAGCGATGTCC-3′ | ||
| OCN | Forward: 5′-CACTCCTCGCCCTATTGGC-3′ | 112 |
| Reverse: 5′-CCCTCCTGCTTGGACACAAAG-3′ | ||
| RUNX2 | Forward: 5′-TGGTTACTGTCATGGCGGGTA-3′ | 101 |
| Reverse: 5′-TCTCAGATCGTTGAACCTTGCTA-3′ | ||
| GAPDH | Forward: 5′-AGCCACATCGCTCAGACAC-3′ | 67 |
| Reverse: 5′-GCCCAATACGACCAAATCC-3′ |
Statistical analysis
Statistical analyses were performed using the SPSS 20.0 software (SPSS, Chicago, IL, USA). Values are expressed as the mean ± SD. One-way-analysis of variance was used for comparison among the groups. P < 0.05 was considered as the statistical significance level, and P < 0.01 was considered as the higher significance level.Results
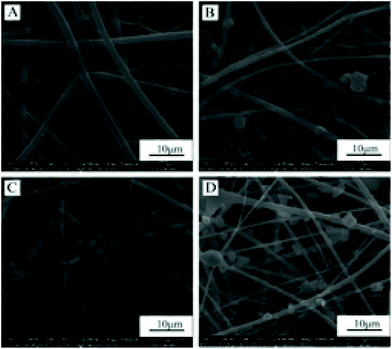
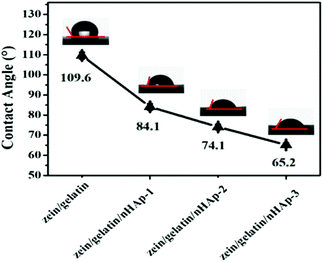
Characterisation of the zein/gelatin/nHAp scaffolds
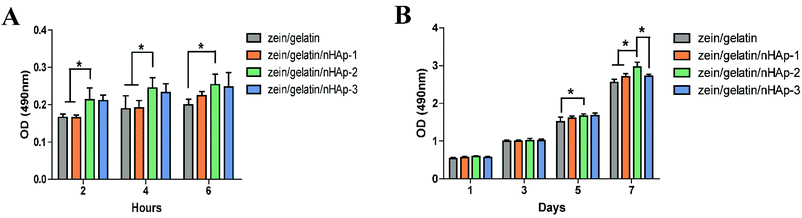
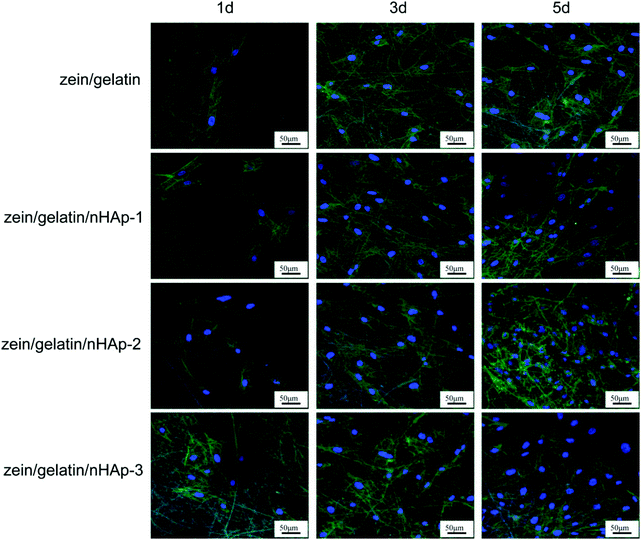
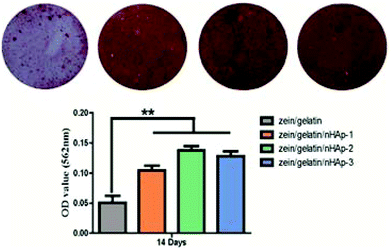
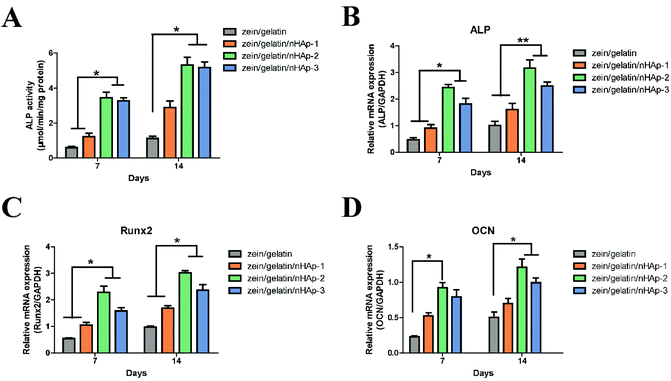
Biocompatibility and osteogenesis in vitro
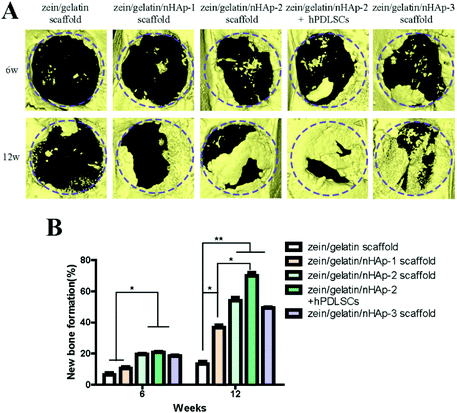
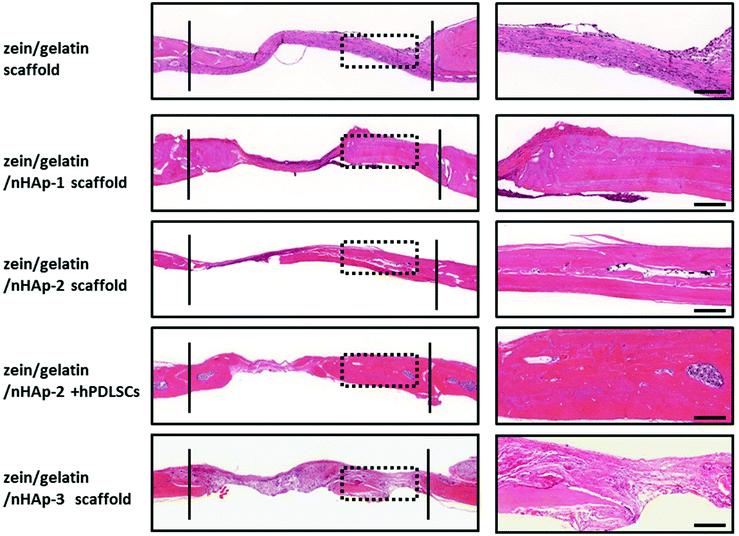 | ||
| Fig. 9 HE staining for the bone formation at 12 weeks. Between the two solid lines is the newly formed bone. The images on the right are the high magnification images of the dotted box (bar: 500 m). | ||
Discussion
Bioactive scaffolds are an indispensable element of bone tissue regeneration. Ideally, a biomaterial scaffold should best mimic the natural ECM in terms of compositional and structural features.21,24 In this study, the electrospinning technique was applied to produce three-dimensional zein/gelatin/nHAp nanofibrous membranes with a porous interconnected nanostructure. The water contact angle of the membranes had a direct correlation with the mass ratio of nHAp, and the surface wettability was improved after the nHAp content was increased because of the hydrophilic nature of nHAp.25 In a similar finding, it was reported the contact angle of poly (L-lactide) nanofibres was reduced by nHAp inclusion.26The biocompatibility of biomaterials is one of the prerequisites for medical therapy and is closely related to the biological behaviour of cells when they are in contact with the biomaterials. The first interaction is cell attachment, followed by the natural process of cell spreading. Thus, the quality of the initial cell adhesion will affect the proliferation capacity as well as the directional differentiation of cells.27–29 The attachment of the cells on the scaffolds increases the survival time and viability of the cells; the zein/gelatin/nHAp membranes in this study enable better cell adhesion in the early stages and better cell proliferation when compared with the zein/gelatin membranes because of multiple factors. First, the gelatin and nHAp components enhanced the surface wettability of the membrane, providing a hydrophilic microenvironment to promote cell affinity. Second, gelatin with short RGD oligopeptide sequences provided various integrin binding sites for cells.30 Moreover, nHAp could adsorb adhesion proteins, such as fibronectin and vitronectin, on its surface; this led to a better binding of integrins to nHAp.31 Third, the rough surface of zein/gelatin/nHAp also favoured cell attachment.32 Therefore, it takes a very short time for the hPDLSCs to adapt to the zein/gelatin/nHAp membranes.
Pore size is connected with the attachment, proliferation and infiltration of cells in tissue scaffolds.33–35 The optimal pore size approximates the diameter of cells.33 A small pore size limits the infiltration and ingrowth of cells into the nanofibers, whereas a large pore size prevents cell attachment due to an insufficient surface area.36,37 Nowadays, there are many novel techniques for optimizing the pore size to improve the effectiveness of biomaterials such as the sacrificial fibres and the 3D gradient pore scaffolds.36,38 The sacrificial fibres support infiltration, colonization and maturation of MC3T3-E1 pre-osteoblasts.39 The 3D gradient pore scaffolds are beneficial for cell infiltration depth, which are promising biomaterials for tissue engineering with gradient pore sizes.36 Therefore, we will focus on optimizing the pore size of the zein/gelatin/nHAp membranes in future research.
The osteoinductive bioactivity of a scaffold is essential for bone defect construction. In the zein/gelatin/nHAp nanofiber membranes, osteogenic biomarker genes (including ALP, OCN, and Runx2) were highly expressed. The results indicated that the addition of nHAp could effectively promote the differentiation of hPDLSCs towards the osteoblastic lineage. Calcium ions released from the partial dissolution of nHAp can promote osteogenic differentiation and bone mineralisation.40 Moreover, nHAp could “cross-link” the fibres by mechanical interlocking or forming calcium ion bridges, thus having an osteoinductive function.41 ALP is a widely recognised marker of early osteoblastic differentiation associated with undifferentiated pluripotent stem cells.42,43 Runx2 is the key transcription factor that initiates and regulates early osteogenesis and late mineralisation of bone.44,45 The high expression of ALP and Runx2 at 7 and 14 days illustrated that the zein/gelatin/nHAp membranes had positive effects on the differentiation of hPDLSCs into osteoblasts at the early stage. OCN, a non-collagenous protein exclusively synthesised by osteoblasts in the process of bone matrix mineralisation, is an important marker reflecting osteogenic activity in the late stage of maturation.45,46 In addition, OCN is considered effective in controlling the nucleation of Hap crystals.47
During the process of stem cell differentiation into osteoblasts, osteoblast precursor cells could bind to nHAp via surface adhesion proteins.31 Rat bone marrow stem cells cultured on silk fibroin fibres with 20 wt% HAp showed the best osteogenic differentiation ability.48 However, polycaprolactone/HAp scaffolds with 15 wt% HAp content exhibited higher ALP activity.49 In our study, the zein/gelatin/nHAp scaffolds with 13% nHAp facilitated robust osteogenesis. We speculate that the different results may be influenced by the cell phenotype, nHAp content, and constituents of the biomaterials and are worthy of further investigation.
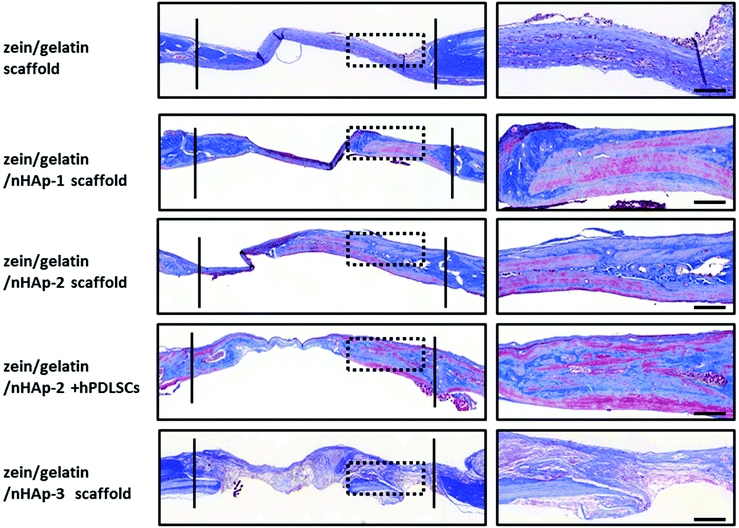
To assess the ability of zein/gelatin/nHAp as scaffolds for hPDLSCs to promote bone repair in vivo, a rat cranial defect model was established. Micro-CT, HE staining and Masson staining showed new bone formation in the zein/gelatin/nHAp scaffolds and the composites of hPDLSCs and zein/gelatin/nHAp. These findings illustrated that both zein/gelatin/nHAp and hPDLSCs contributed to bone regeneration in vivo. Zein/gelatin/nHAp facilitated osteogenesis in vivo likely owing to the topography and components that mimic the ECM as well as the osteoinductive function of nHAp. The hPDLSCs may directly differentiate into osteocytes by secreting diverse soluble molecules to modulate the microenvironment because they have similar characteristics as MSCs.50,51 Thus, further investigation is necessary to clarify the mechanism of zein/gelatin/nHAp in affecting the hPDLSC osteogenesis.
Conclusions
This study demonstrated that the electrospun zein/gelatin/nHAp nanofibres could facilitate the attachment, proliferation, and osteogenic differentiation of hPDLSCs. Moreover, the electrospun zein/gelatin/nHAp nanofibers had good biocompatibility and osteogenic activity in vivo. Hence, the electrospun zein/gelatin/nHAp membranes are a good candidate for applications in human bone tissue engineering.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support received from the Special Funds for Public Welfare Research and Capacity Building of Guangdong Province in China (No. 2014A010105025), the Natural Science Foundation of China (No. 81371793, 81530069) and the Guangdong Innovative Research Team program (No. 2009010058).References
- R. Pokrowiecki, K. Palka and A. Mielczarek, Nanomedicine, 2018, 13, 639–667 CrossRef CAS PubMed.
- J. Han, D. Menicanin, S. Gronthos and P. M. Bartold, Aust. Dent. J., 2014, 59, 117–130 CrossRef PubMed.
- F. Chen, H. Sun, H. Lu and Q. Yu, Biomaterials, 2012, 33, 6320–6344 CrossRef CAS PubMed.
- B. M. Seo, M. Miura, S. Gronthos, P. M. Bartold, S. Batouli, J. Brahim, M. Young, P. G. Robey, C. Y. Wang and S. Shi, Lancet, 2004, 364, 149–155 CrossRef CAS.
- W. Zhu and M. Liang, Stem Cells Int., 2015, 2015, 1–11 Search PubMed.
- M. Lei, K. Li, B. Li, L. N. Gao, F. M. Chen and Y. Jin, Biomaterials, 2014, 35, 6332–6343 CrossRef CAS PubMed.
- C. Zhang, B. Yan, Z. Cui, S. Cui, T. Zhang, X. Wang, D. Liu, R. Yang, N. Jiang, Y. Zhou and Y. Liu, Sci. Rep., 2017, 7, 10519 CrossRef PubMed.
- F. M. Chen, L. N. Gao, B. M. Tian, X. Y. Zhang, Y. J. Zhang, G. Y. Dong, H. Lu, Q. Chu, J. Xu, Y. Yu, R. X. Wu, Y. Yin, S. Shi and Y. Jin, Stem Cell Res. Ther., 2016, 7, 33 CrossRef PubMed.
- J. Zhang, H. Liu, J. Ding, J. Wu, X. Zhuang, X. Chen, J. Wang, J. Yin and Z. Li, ACS Biomater. Sci. Eng., 2016, 2, 1471–1482 CrossRef CAS.
- C. Zhijiang, Z. Qin, S. Xianyou and L. Yuanpei, Mater. Sci. Eng., C, 2017, 71, 797–806 CrossRef PubMed.
- P. Pal, P. Dadhich, P. K. Srivas, B. Das, D. Maulik and S. Dhara, Biomater. Sci., 2017, 5, 1786–1799 RSC.
- A. Deng, Y. Yang, S. Du and S. Yang, Biomater. Sci., 2018, 6, 2197–2208 RSC.
- R. Pugliese, M. Maleki, R. N. Zuckermann and F. Gelain, Biomater. Sci., 2018, 7, 76–91 RSC.
- J. Lin, J. Ding, Y. Dai, X. Wang, J. Wei and Y. Chen, Mater. Sci. Eng., C, 2017, 81, 321–326 CrossRef CAS PubMed.
- S. Babitha, M. Annamalai, M. M. Dykas, S. Saha, K. Poddar, J. R. Venugopal, S. Ramakrishna, T. Venkatesan and P. S. Korrapati, J. Tissue Eng. Regener. Med., 2018, 12, 991–1001 CrossRef CAS PubMed.
- C. Sanhueza, F. Acevedo, S. Rocha, P. Villegas, M. Seeger and R. Navia, Int. J. Biol. Macromol., 2018, 124, 102–110 CrossRef PubMed.
- F. Yang, Y. Miao, Y. Wang, L. Zhang and X. Lin, Materials, 2017, 10, 1168 CrossRef PubMed.
- A. Tampieri, A. Ruffini, A. Ballardini, M. Montesi, S. Panseri, F. Salamanna, M. Fini and S. Sprio, Biomater. Sci., 2018, 7, 307–321 RSC.
- B. Li, P. Gao, H. Zhang, Z. Guo, Y. Zheng and Y. Han, Biomater. Sci., 2018, 6, 3202–3218 RSC.
- A. El-Fiqi, J. O. Buitrago, S. H. Yang and H. W. Kim, Acta Biomater., 2017, 60, 38–49 CrossRef CAS PubMed.
- G. Dalmonico, P. F. Franczak, N. J. Levandowski, N. Camargo, A. L. Dallabrida, C. B. Da, O. G. Gil, O. Cambra-Moo, M. A. Rodriguez and M. Canillas, Biomater. Sci., 2017, 5, 1315–1325 RSC.
- M. Ribeiro, M. P. Ferraz, F. J. Monteiro, M. H. Fernandes, M. M. Beppu, D. Mantione and H. Sardon, Nanomedicine, 2017, 13, 231–239 CrossRef CAS PubMed.
- X. Wang, X. Wu, H. Xing, G. Zhang, Q. Shi, L. E, N. Liu, T. Yang, D. Wang, F. Qi, L. Wang and H. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 11380–11391 CrossRef CAS PubMed.
- S. Wang, F. Hu, J. Li, S. Zhang, M. Shen, M. Huang and X. Shi, Nanomedicine, 2018, 14, 2505–2520 CrossRef CAS PubMed.
- H. Samadian, M. Salehi, S. Farzamfar, A. Vaez, A. Ehterami, H. Sahrapeyma, A. Goodarzi and S. Ghorbani, Artif. Cells, Nanomed., Biotechnol., 2018, 1–11 Search PubMed.
- S. Fu, P. Ni, B. Wang, B. Chu, J. Peng, L. Zheng, X. Zhao, F. Luo, Y. Wei and Z. Qian, Biomaterials, 2012, 33, 8363–8371 CrossRef CAS PubMed.
- M. I. Hassan and N. Sultana, 3 Biotech., 2017, 7, 249 CrossRef PubMed.
- J. E. Song, N. Tripathy, D. H. Lee, J. H. Park and G. Khang, ACS Appl. Mater. Interfaces, 2018, 10, 32955–32964 CrossRef CAS PubMed.
- J. Wu, K. Zhang, X. Yu, J. Ding, L. Cui and J. Yin, Biomater. Sci., 2017, 5, 2251–2267 RSC.
- A. R. El-Ghannam, P. Ducheyne, M. Risbud, C. S. Adams, I. M. Shapiro, D. Castner, S. Golledge and R. J. Composto, J. Biomed. Mater. Res., Part A, 2004, 68, 615–627 CrossRef CAS PubMed.
- H. Liu, H. Peng, Y. Wu, C. Zhang, Y. Cai, G. Xu, Q. Li, X. Chen, J. Ji, Y. Zhang and H. W. OuYang, Biomaterials, 2013, 34, 4404–4417 CrossRef CAS PubMed.
- H. J. Wang, M. Q. Li, W. Liu, G. D. Yao, M. Y. Xia, T. Hayashi, H. Fujisaki, S. Hattori, S. Tashiro, S. Onodera and T. Ikejima, Connect. Tissue Res., 2016, 57, 262–269 CrossRef CAS PubMed.
- A. Paim, I. C. Tessaro, N. Cardozo and P. Pranke, J. Biol. Phys., 2018, 44, 245–271 CrossRef CAS PubMed.
- J. Zhang, T. Zheng, E. Alarcin, B. Byambaa, X. Guan, J. Ding, Y. S. Zhang and Z. Li, Small, 2017, 13, 1949 Search PubMed.
- Z. Z. Zhang, D. Jiang, J. X. Ding, S. J. Wang, L. Zhang, J. Y. Zhang, Y. S. Qi, X. S. Chen and J. K. Yu, Acta Biomater., 2016, 43, 314–326 CrossRef CAS PubMed.
- L. Huang, J. Huang, H. Shao, X. Hu, C. Cao, S. Fan, L. Song and Y. Zhang, Mater. Sci. Eng., C, 2019, 94, 179–189 CrossRef CAS PubMed.
- J. Li, W. Xu, D. Li, T. Liu, Y. S. Zhang, J. Ding and X. Chen, ACS Nano, 2018, 12, 6685–6699 CrossRef CAS PubMed.
- J. Wu and Y. Hong, Bioact. Mater., 2016, 1, 56–64 CrossRef PubMed.
- B. M. Whited, J. R. Whitney, M. C. Hofmann, Y. Xu and M. N. Rylander, Biomaterials, 2011, 32, 2294–2304 CrossRef CAS PubMed.
- X. Wu, L. Miao, Y. Yao, W. Wu, Y. Liu, X. Chen and W. Sun, Int. J. Nanomed., 2014, 9, 4135–4143 CrossRef CAS PubMed.
- C. Hellmich and F. J. Ulm, J. Biomech., 2002, 35, 1199–1212 CrossRef PubMed.
- P. M. Favi, R. S. Benson, N. R. Neilsen, R. L. Hammonds, C. C. Bates, C. P. Stephens and M. S. Dhar, Mater. Sci. Eng., C, 2013, 33, 1935–1944 CrossRef CAS PubMed.
- J. Tan, D. Wang, H. Cao, Y. Qiao, H. Zhu and X. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 15724 Search PubMed.
- T. Wang, X. Yang, X. Qi and C. Jiang, J. Transl. Med., 2015, 13, 152 CrossRef PubMed.
- S. K. Boda, G. Thrivikraman, B. Panigrahy, D. D. Sarma and B. Basu, ACS Appl. Mater. Interfaces, 2017, 9, 19389–19408 CrossRef CAS PubMed.
- X. Chen, S. Bai, B. Li, H. Liu, G. Wu, S. Liu and Y. Zhao, Int. J. Nanomed., 2016, 11, 4707–4718 CrossRef CAS PubMed.
- M. Sila-Asna, A. Bunyaratvej, S. Maeda, H. Kitaguchi and N. Bunyaratavej, Kobe J. Med. Sci., 2007, 53, 25–35 CAS.
- B. Niu, B. Li, Y. Gu, X. Shen, Y. Liu and L. Chen, J. Biomater. Sci., Polym. Ed., 2017, 28, 257–270 CrossRef CAS PubMed.
- S. Eosoly, N. E. Vrana, S. Lohfeld, M. Hindie and L. Looney, Mater. Sci. Eng., C, 2012, 32, 2250–2257 CrossRef CAS.
- Y. Fu, S. Liu, S. J. Cui, X. X. Kou, X. D. Wang, X. M. Liu, Y. Sun, G. N. Wang, Y. Liu and Y. H. Zhou, ACS Appl. Mater. Interfaces, 2016, 8, 15958–15966 CrossRef CAS PubMed.
- L. Li, W. Liu, H. Wang, Q. Yang, L. Zhang, F. Jin and Y. Jin, Cell Death Dis., 2018, 9, 480 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8bm01653d |
| ‡ The first two authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2019 |