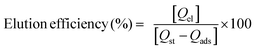Fe3O4-based composite magnetic nanoparticles for volatile compound sorption in the gas phase: determination of selenium(IV) †
Irina
Timofeeva
 *,
Mariya
Alikina
,
Anna
Vlasova
,
Mikhail
Osmolowsky
,
Mikhail
Voznesenskiy
,
Olga
Volina
,
Leonid
Moskvin
,
Olga
Osmolovskaya
and
Andrey
Bulatov
*,
Mariya
Alikina
,
Anna
Vlasova
,
Mikhail
Osmolowsky
,
Mikhail
Voznesenskiy
,
Olga
Volina
,
Leonid
Moskvin
,
Olga
Osmolovskaya
and
Andrey
Bulatov
Department of Analytical Chemistry, Institute of Chemistry, Saint-Petersburg University, St Petersburg State University, SPbSU, SPbU, 7/9 Universitetskaya nab., St Petersburg, 199034 Russia. E-mail: i.i.timofeeva@spbu.ru
First published on 15th November 2018
Abstract
In this study, Fe3O4-based composite magnetic nanoparticles were found to separate volatile compounds directly in the gas phase for the first time. The phenomenon of H2Se sorption on the magnetic nanoparticles was studied in detail and applied for separation and preconcentration. The developed approach was applied for the determination of selenium in dietary supplement samples after microwave digestion by ETA-AAS as a proof-of-concept example.
Recently, magnetic nanoparticles (MNPs) have been widely used for separation and preconcentration of diverse compounds from various matrixes (biological samples,1 foods,2 environmental samples3etc.). As is known, magnetic materials as sorbents have several advantages over conventional sorbents for sorption in the aqueous phase. The separation and preconcentration processes can be performed directly in aqueous solution containing MNPs, and after sorption MNPs can easily be collected and separated from the liquid phase using an external magnetic field.4 Due to the large surface-area-to-volume ratios of nanometer (nm)-sized sorbents, MNPs are particularly useful for extracting and enriching a large volume of diverse compounds without tedious centrifugation. The compounds absorbed on MNPs can easily be eluted by a suitable eluent for subsequent determination.
It should be pointed out that sorption procedures based on MNPs were only implemented for the separation of compounds from liquid phase sample matrixes.5–7
In this work, a novel phenomenon – gas sorption on magnetic Fe3O4-based nanoparticles held in a gas phase by an external magnetic field was established and coupled with hydride generation. To the best of our knowledge, this discovered phenomenon has not been presented in the literature.
To demonstrate the efficiency of the suggested approach, the proposed strategy was applied to determine Se(IV) as a proof-of-concept analyte in dietary supplement samples after microwave digestion using electrothermal atomization-atomic absorption spectroscopy (ETA-AAS). Selenium is an essential micronutrient for humans and animals,8 and it is widely used as a supplement ingredient.9 It acts as an active center of selenoenzymes and selenoproteins and plays an important role in energy metabolism and gene expression in organisms.10 Currently, dietary supplements containing selenium are widely consumed over the world for health-related reasons. Even though, most of these supplements are beneficial for human health, they can also have side effects due to an excess of one of the supplement ingredients including selenium. It is then important to ensure their efficacy and safety.11
The most frequently used magnetic MNPs are the materials based on Fe3O4.12 Modification of the Fe3O4 MNP surface can improve the selectivity of the sorption procedure. Fe3O4@AlOOH was chosen because it is known as an effective sorbent for ion separation.13 In this research, Fe3O4 and Fe3O4@AlOOH MNPs with different shell parameters (thickness and crystallinity) were synthesized and investigated as sorbents for H2Se separation in the gas phase obtained by analyte hydride generation based on Se(IV) reduction in the presence of acid and NaBH4. H2Se formation is widely used for analyte separation and preconcentration for its subsequent sensitive determination by various instrumental methods.14–16
Synthesis of Fe3O4-based MNPs was performed as follows.17 Firstly, 10.5 g of FeSO4·7H2O, 15 mL of 3.7 mol L−1 FeCl3 (Sigma-Aldrich, USA) in the molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 360 mL of H2O were mixed in a round flask using an overhead stirrer under an argon flow. Then, 198 mL of NH4OH solution (3 mmol L−1) was dropwise added, and the reaction mixture was kept for 15 min. The resulting product was separated by centrifugation (Sigma 2-16P, 9000g, 5 min) and washed with distilled water six times followed by freeze-drying for 3 h.
2 and 360 mL of H2O were mixed in a round flask using an overhead stirrer under an argon flow. Then, 198 mL of NH4OH solution (3 mmol L−1) was dropwise added, and the reaction mixture was kept for 15 min. The resulting product was separated by centrifugation (Sigma 2-16P, 9000g, 5 min) and washed with distilled water six times followed by freeze-drying for 3 h.
To obtain composite Fe3O4@AlOOH nanoparticles 0.1 g of Fe3O4 MNPs was placed into Al(NO3)3 solution (75 mL, 0.1–4.3 g L−1). Then, the pH value was adjusted to 4 by adding NaOH solution (6 mol L−1). After that, the white-black suspension obtained was transferred into a 200 mL Teflon lined autoclave (SPBU, Russia), sealed and heated at 180 °C for 2 h. After cooling, the resulting product was separated by centrifugation (Sigma 2-16P, 8000g, 3 min) and washed with distilled water and ethanol four times followed by freeze-drying for 3 h.
All studied MNPs were stable during the storage under ambient conditions.
A microcolumn (5 mg of MNPs in a pipette tip (100 μL volume)) equipped with an external magnet was hermetically connected with a mixing chamber through a PTFE tube (Fig. 1). At the beginning 5 mL of 0.5 mg L−1 Se(IV) solution (in 2 mol L−1 HNO3) was placed into the mixing chamber. Then, 3 mL of 3% (w/v) NaBH4 (in 0.1 mol L−1 NaOH) was delivered into the mixing chamber using a peristaltic pump over 6 min. Formation of H2Se and H2 was observed. H2Se was collected on MNPs when the gas mixture was delivered through the microcolumn with MNPs held by an external magnetic field. Then, the microcolumn was disconnected and MNPs were transferred to a PTFE vial (5 mL). For analyte elution 1 mL of H2O2 (30%) was added to MNPs, and a sonication using an ultrasonic homogenizer (Bandelin Sonoplus HD2070, MS 72, Germany, power – 30%) for 10 min at 30% amplitude was carried out. After that, the vial was placed on the magnet (permanent neodymium iron boron magnet) to precipitate magnetic particles and the clear supernatant was analyzed by ETA-AAS (a Shimadzu Model AA-7000 atomic absorption spectrometer). MNPs were used once, since the particle structure was irreparably changed after sonication used for analyte elution.
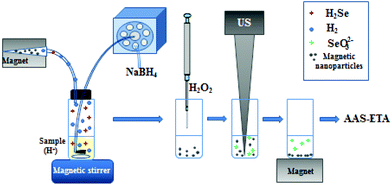 | ||
| Fig. 1 Schematic representation of H2Se generation and sorption on the MNPs followed by selenium determination. | ||
MNPs synthesized were characterized by XRD (ESI Fig. 1†), TEM (ESI Fig. 2 and 3†), SSA estimation, FTIR (ESI Fig. 4†) and VSM (ESI Fig. 5†). The data obtained were in good agreement with each other and indicated the presence of AlOOH shells with different parameters on the magnetic core. The observed decrease of a shell thickness (ESI Table 1†) with the increase of AlOOH amount up to 33 mol% (Fe3O4@33AlOOH) may indicate a gradual process of its crystallization on the Fe3O4 surface because a more crystalline structure has a higher packing density than the amorphous one. A further increase of AlOOH amount led to the appearance of more intensive peaks from OH groups in the 3400–3500 cm−1 region (ESI Fig. 4†), which indicated the presence of a hydroxide amorphous phase.
To be effectively held in the microcolumn MNPs should have good magnetic properties. The magnetic characteristics of Fe3O4-based composite magnetic nanoparticles can be decreased because of the presence of a non-magnetic shell. According to the data obtained (ESI Table 1†) the decrease in the value of the saturation magnetization (MS) for the Fe3O4@AlOOH MNPs was not critical (not more than 15%) in comparison with Fe3O4 MNPs.
The evaluation of magnetic interaction between the nanoparticles of bare and Fe3O4@AlOOH types in the gas and aqueous phases was also performed. We supposed that since the gas sorption and elution processes were carried out in the external magnetic field, they probably could be determined to a considerable degree by the magnetic interactions between the MNPs. For the gas phase the energy values (E) of interaction between MNPs under the external magnet field were calculated according to a handbook.18 MNPs with the lowest energy should be more effectively held in the external magnetic field. For the aqueous phase the velocity values (V) of nanoparticle movement under the external magnet field were also calculated according to a handbook.19 The V values determine the efficiency of MNP collection during the elution process. As expected both the parameters (ESI Table 1†) depended on the shell thickness and will be discussed below.
Seven types of MNPs (Fe3O4, Fe3O4@5AlOOH, Fe3O4@10AlOOH, Fe3O4@20AlOOH, Fe3O4@33AlOOH, Fe3O4@50AlOOH and Fe3O4@60AlOOH) were investigated for H2Se sorption. To choose the optimal sorbent the efficiency of sorption and elution was studied. The sorption efficiency was calculated using the following equation:
The elution efficiency was calculated using the following equation:
After H2Se sorption and its elution the concentration of Se was determined in the eluate and absorption solution by ETA-AAS.
The obtained experimental and calculated results (Fig. 2) indicated that the maximum sorption efficiency and the minimum interaction energy correspondingly were observed for bare Fe3O4. Both these values showed the same tendencies depending on the shell thickness for the Fe3O4-based nanoparticles under study. Note that the maximum difference between the experimental and calculated results and comparable to bare Fe3O4 sorption efficiency were observed for Fe3O4@10AlOOH MNPs with high SSA and shell thickness values. Thus, for composite MNPs not only magnetic interaction but also the surface composition influences the sorption properties.
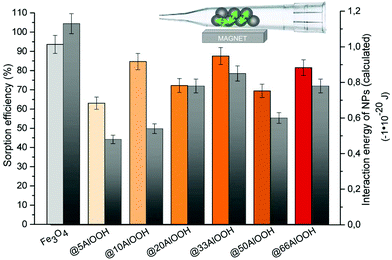 | ||
| Fig. 2 Sorption efficiency (left) and interaction energy values (right) in the gas phase under an external magnetic field for the MNPs synthesized (5 mg of MNPs). | ||
Moreover, we can assume that the efficiency of sorbent and analyte binding in the gas phase was determined by two processes: magnetic interactions between MNPs and the direct adsorption process. In the case of Fe3O4-based MNPs, they were evenly distributed under the magnetic field on the inner surface of the microcolumn and formed a layer that interacted with the gas phase. The higher interaction energy of Fe3O4 MNPs led to the formation of more elongated needles, which significantly increased the surface area available for gas molecule interaction.
To understand the sorption mechanisms of H2Se on the Fe3O4 MNP surface, an adsorption isotherm was plotted. Two widely used models based on Langmuir and Freundlich isotherm equations were applied. The linearization results indicated that in our case the adsorption process was polymolecular, the Freundlich model represented good agreement with the data obtained (adjusted R-square value for linear least squares fitting was equal to 0.998 (ESI Fig. 7†)). Thus, it can be concluded that H2Se adsorption on Fe3O4 MNPs was characterized by the sorbent surface heterogeneity because the Freundlich isotherm model represents heterogeneity of solid surfaces.20 The n value (0.947) obtained from the Freundlich model was smaller than 1. Thus, a nonlinear sorption process takes place.
The flow rate of NaBH4 solution injected in a sample solution influenced the flow rate of the gas phase generated. The flow rate of NaBH4 solution (3%) in the range of 0.2–1 mL min−1 was varied, and the gas flow rate was measured using a gas flow meter. It was found that the NaBH4 solution flow rate of 0.5 mL min−1 provided optimal conditions for gas phase generation (gas flow rate 10 mL min−1) and H2Se sorption (sorption efficiency 95 ± 5%). At a higher reagent flow rate, excessive hydrogen generation was observed, which caused solution spraying and transferring of droplets of the aqueous phase into the microcolumn. In this case, the MNPs were wetted and H2Se sorption was not observed.
As can be seen from Fig. 3 the optimal elution efficiency and the maximum velocity as expected were observed for bare Fe3O4. Similar to that discussed above the experimental and calculated data demonstrated the same behavior. But in the case of a high AlOOH amount the elution efficiency values became lower.
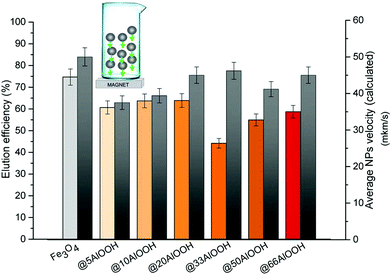 | ||
| Fig. 3 Elution efficiency (left) and average velocity in the aqueous phase under an external magnetic field (right) for the MNPs synthesized (5 mg of MNPs). | ||
Thus, Fe3O4 MNPs provided maximum sorption of H2Se and its satisfactory elution, therefore Fe3O4 MNPs were chosen for future experiments. The amount of MNPs is an important factor affecting the sorption efficiency and reproducibility. The effect of MNP (bare Fe3O4) amount was investigated in the range from 2.5 to 10 mg. The optimal sorbent amount was determined as 5 mg (ESI Fig. 6†). At a lower sorbent amount, it was probably not enough to completely adsorb H2Se from the gas phase. At a higher sorbent amount its surface area was less because the MNPs were tightly packed into the microcolumn resulting in a lower sorption efficiency. In addition, the low elution efficiency values at higher sorbent masses could be due to the shift of Se adsorption/desorption equilibrium in the aqueous phase during the sorption process.
In order to find the optimal conditions for H2Se formation, the concentrations of NaBH4 and HNO3 were varied from 1 to 5% and from 1 to 5 mol L−1, respectively. It was established, that the highest analytical signal was achieved using 3% NaBH4 and 2 mol L−1 HNO3.
Taking into account the need to convert H2Se to a water-soluble form for its elution from MNPs as well as the features of pyrolytic graphite tubes and the properties of MNPs, oxidants such as KMnO4 and H2O2 were studied as components of elution solution. Initially, a solution of 10−5 mol L−1 KMnO4 was investigated. This concentration of KMnO4 was chosen to eliminate background interferences taking place during the ETA-AAS analysis and to exclude its negative effect on the pyrolytic graphite tube. The results obtained showed a low elution efficiency (30%). Thus, another oxidant – H2O2 – was studied in the concentration range of 5–30%. It was established that the maximum absorbance peak area was observed by using 30% H2O2, thus, this eluent was chosen for further experiments. The elution efficiency was 2.5 times higher compared to the one with KMnO4 solution.
It is well known that sonication is widely used to activate the MNP surface.21,22 Ultrasonic irradiation can improve the mass transfer between two immiscible phases and reduce the equilibrium time, and thus can be helpful for the elution process. It was established that the use of ultrasonic irradiation enhanced analytical signals at two different times. To choose the optimal sonication time, the elution process was implemented at 1, 5, 10, and 15 min of sonication (325 W, 35 kHz). The maximal absorbance peak area was observed at 10 min of sonication and no improvement was found at 15 min. Thus, 10 min of sonication was chosen for analyte elution from MNPs.
The effect of potentially interfering volatile compounds (AsH3 and H2S) on H2Se sorption and selenium determination was also investigated. It was done by addition of known concentrations of KAsO2 and Na2S to 50 μg L−1 selenium(IV) solution. The tolerable concentration of each taken foreign compound is considered to be less than 5% of relative error in the signal. It was shown that AsH3 and H2S generated did not interfere with H2Se sorption and selenium determination up to the highest studied concentration of As(III) and S2− (1 mg L−1).
The developed procedure was applied for the determination of Se(IV) in three types of dietary supplements. The dietary supplements were complex matrices, which included selenoxanthen (sample no. 1), sodium selenite (samples no. 2, 3) as biologically active substances, and different other components (cellulose, dextrose, starch), anti-caking agents (magnesium stearate, silicon dioxide, talc), some vitamins ((2R)-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-6-chromanol (vitamin E) and L-ascorbic acid (vitamin C)), and salts/oxides of zinc, iron, and calcium. To destroy complex sample matrices and obtain selenium in the form of Se(IV) all samples were digested in a single run using a laboratory microwave digestor MDS-10 Master (Sineo, China).23 0.15 ± 0.5 g of powdered samples were placed in individual microwave reactors. The aliquots were treated with 6 mL concentrated HNO3 and 1 mL H2O2. The temperature program is given in ESI Table 2.† The employed microwave power was up to 600 W.
Generally, the obtained results corresponded to the contents indicated on each package (Table 1). Accuracy and reliability of the resulting information were further studied by the add/found method. The procedure developed provides selenium separation from digested samples with recovery from 87 to 102%.
| Sample no. | Labeled value, μg | Added, μg | Found, μg | Recovery, % |
|---|---|---|---|---|
| 1 (selenoxanthen) | 50 | 0 | 45 ± 8 | — |
| 50 | 90 ± 12 | 90 | ||
| 100 | 147 ± 21 | 102 | ||
| 2 (sodium selenite) | 50 | 0 | 54 ± 7 | — |
| 50 | 101 ± 10 | 94 | ||
| 100 | 146 ± 17 | 92 | ||
| 3 (sodium selenite) | 40–60 | 0 | 52 ± 8 | — |
| 50 | 99 ± 11 | 94 | ||
| 100 | 139 ± 19 | 87 |
Moreover, the certified reference material SRM 7340-96 (Se(IV) solution, Ecohim, Russia) was used to validate the developed method. In this case the t-test was applied in order to establish the statistical significance of the results. The results showed no differences, which were found at a confidence level of 95%, between the certified value of 500 ± 5 μg Se(IV) L−1 and the obtained value of 500 ± 5 μg Se(IV) L−1 (n = 3). Thus, this result was in agreement with the certified value (texp < tcr), where t critical and expected values were equal to 2.78 and 1.34, respectively.
The calibration plot was constructed using Se(IV) solutions under developed procedure conditions. The linear calibration range of 1–20 μg L−1 selenium was obtained with a correlation coefficient of 0.998. The LOD, calculated from a blank test, based on 3σ, was found to be 0.3 μg L−1. The LOQ, equal to 1 μg L−1 was calculated that produced a peak with 10 times the signal-to-noise ratio. The obtained relative standard deviation values for 1 and 20 μg L−1 of Se(IV) solutions were in the range from 4 to 8% (within-day precision) and from 6 to 9% (day-to-day precision), respectively.
In conclusion, Fe3O4-based MNPs were investigated for volatile H2Se sorption directly in the gas phase for the first time. In this study sorption of H2Se on Fe3O4-based MNPs was coupled with hydride generation and applied for selenium(IV) determination in dietary supplement samples by ETA-AAS. The proposed approach has the following advantages. First, Fe3O4-based MNPs can be easily held by an external magnetic field in the gas phase resulting in analyte sorption in the gas phase flow. Second, compared with conventional sorbents Fe3O4-based MNPs allowed the implementation of analyte elution without centrifugation and filtration. Thirdly, the Fe3O4-based magnetic nanoparticles can be easily prepared, and MNPs are inexpensive and readily available. The application of this method for other volatile compounds is under investigation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The combined method was developed and supported by the Russian Scientific Foundation (project no. 16-13-10117). Irina Timofeeva gratefully acknowledges the Russian Foundation for Basic Research (project no. 16-33-60126 mol_a_dk) for the financial support in the on-line sample preparation investigations. Mikhail Osmolowsky acknowledges the Russian Scientific Foundation (project no. 18-03-01066) for the financial support in sorbent synthesis and characterization. Scientific research was performed using the equipment of the Research Park of St Petersburg State University (Centre for X-ray Diffraction Studies, Chemical Analysis and Materials Research Centre, Centre for Innovative Technologies of Composite Nanomaterials).Notes and references
- B. Hashemi, P. Zohrabi and M. Shamsipur, Talanta, 2018, 187, 337–347 CrossRef CAS PubMed.
- J. González-Sálamo, B. Socas-Rodríguez, J. Hernández-Borges and M. A. Rodríguez-Delgado, TrAC, Trends Anal. Chem., 2016, 85, 203–220 CrossRef.
- J. Li, Y. Wang, K. Li, Y. Cao, S. Wu and L. Wu, TrAC, Trends Anal. Chem., 2015, 72, 141–152 CrossRef CAS.
- J. Meng, J. Bu, C. Deng and X. Zhang, J. Chromatogr. A, 2011, 1218, 1585–1591 CrossRef CAS PubMed.
- C. Vakh, M. Alaboud, S. Lebedinets, D. Korolev, V. Postnov, L. Moskvin, O. Osmolovskaya and A. Bulatov, Anal. Chim. Acta, 2018, 1001, 59–69 CrossRef CAS PubMed.
- F. Maya, C. P. Cabello, J. M. Estela, V. Cerdà and G. T. Palomino, Anal. Chem., 2015, 87, 7545–7549 CrossRef CAS PubMed.
- Q. Gao, H. B. Zheng, D. Luo, J. Ding and Y. Q. Feng, Anal. Chim. Acta, 2012, 720, 57–62 CrossRef CAS PubMed.
- T. Ferri and De L. L. Ticconi, Anal. Lett., 2001, 34, 975–988 CrossRef CAS.
- A. Rocco, E. Donati, E. Touloupakis and Z. Aturki, TrAC, Trends Anal. Chem., 2018, 103, 156–183 CrossRef CAS.
- F. Zhou, W. Yang, M. Wang, Y. Miao, Z. Cui, Z. Li and D. Liang, Food Chem., 2018, 265, 182–188 CrossRef CAS PubMed.
- A. Rocco, E. Donati, E. Touloupakis and Z. Aturki, TrAC, Trends Anal. Chem., 2018, 103, 156–183 CrossRef CAS.
- H. Hu, L. Yang, Z. Lin, X. Xiang, X. Jiang and L. Hou, Int. J. Biol. Macromol., 2018, 114, 256–262 CrossRef CAS PubMed.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef.
- A. Shishov, M. Wieczorek, P. Kościelniak, D. Dudek-Adamska, A. Telk, L. Moskvin and A. Bulatov, Talanta, 2018, 181, 359–365 CrossRef CAS PubMed.
- X. Tang, Z. Xu and J. Wang, Spectrochim. Acta, Part B, 2005, 60, 1580–1585 CrossRef.
- N. Zhang, G. Sun and H. Ma, Miner. Eng., 2007, 20, 1397–1400 CrossRef CAS.
- A. A. Hernández-Hernández, G. A. Álvarez-Romero, E. Contreras Lopez, K. AguilarArteaga and A. Castañeda-Ovando, Food Anal. Methods, 2017, 10, 2974–2993 CrossRef.
- A. Stone, The Theory of Intermolecular Forces, Oxford University Pres, 2nd edn, 2016 Search PubMed.
- L. D. Landau and E. M. Lifshitz, Fluid Mechanics: Volume 6 (Course of Theoretical Physics), Butterworth-Heinemann, 2nd edn, 1987 Search PubMed.
- L. Yin, S. Song, X. Wang, F. Niu, R. Ma, S. Yu, T. Wen, Y. Chen, T. Hayat, A. Alsaedi and X. Wang, Environ. Pollut., 2018, 238, 725–738 CrossRef CAS PubMed.
- N. Jaafarzadeh, A. Takdastan, S. Jorfi, F. Ghanbari, M. Ahmadi and G. J. Barzegar, Mol. Liq., 2018, 256, 462–470 CrossRef CAS.
- A. Keramat and R. Zare-Dorabei, Ultrason. Sonochem., 2017, 38, 421–429 CrossRef CAS PubMed.
- Q.-X. Zhao, Y.-W. Chen, N. Belzile and M. Wang, Anal. Chim. Acta, 2010, 665, 123–128 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8an01894d |
| This journal is © The Royal Society of Chemistry 2019 |