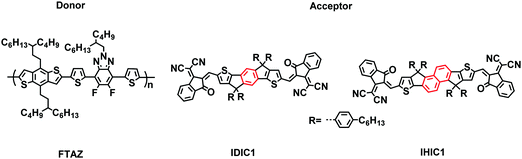
Enhancing the performance of a fused-ring electron acceptor via extending benzene to naphthalene†
Jingshuai
Zhu
ab,
Yang
Wu
c,
Jeromy
Rech
 d,
Jiayu
Wang
b,
Kuan
Liu
b,
Tengfei
Li
b,
Yuze
Lin
*ae,
Wei
Ma
c,
Wei
You
d,
Jiayu
Wang
b,
Kuan
Liu
b,
Tengfei
Li
b,
Yuze
Lin
*ae,
Wei
Ma
c,
Wei
You
 d and
Xiaowei
Zhan
d and
Xiaowei
Zhan
 *b
*b
aDepartment of Chemistry, Capital Normal University, Beijing 100048, China. E-mail: linyz@iccas.ac.cn
bDepartment of Materials Science and Engineering, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, College of Engineering, Peking University, Beijing 100871, China. E-mail: xwzhan@pku.edu.cn
cState Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049, China
dDepartment of Chemistry, University of North Carolina at Chapel Hill, North Carolina 27599, USA
eDepartment of Applied Physical Sciences, University of North Carolina at Chapel Hill, North Carolina 27599, USA
First published on 29th November 2017
Abstract
We compared an indacenodithiophene(IDT)-based fused-ring electron acceptor IDIC1 with its counterpart IHIC1 in which the central benzene unit is replaced by a naphthalene unit, and investigated the effects of the benzene/naphthalene core on the optical and electronic properties as well as on the performance of organic solar cells (OSCs). Compared with benzene-cored IDIC1, naphthalene-cored IHIC1 shows a larger π-conjugation with stronger intermolecular π–π stacking. Relative to benzene-cored IDIC1, naphthalene-cored IHIC1 shows a higher lowest unoccupied molecular orbital energy level (IHIC1: −3.75 eV, IDIC1: −3.81 eV) and a higher electron mobility (IHIC1: 3.0 × 10−4 cm2 V−1 s−1, IDIC1: 1.5 × 10−4 cm2 V−1 s−1). When paired with the polymer donor FTAZ that has matched energy levels and a complementary absorption spectrum, IHIC1-based OSCs show higher values of open-circuit voltage, short-circuit current density, fill factor and power conversion efficiency relative to those of the IDIC1-based control devices. These results demonstrate that extending benzene in IDT to naphthalene is a promising approach to upshift energy levels, enhance electron mobility, and finally achieve higher efficiency in nonfullerene acceptor-based OSCs.
Introduction
Solution-processed bulk heterojunction (BHJ) organic solar cells (OSCs) are a cost-effective alternative for utilizing solar energy, and possess some advantages, such as light weight, low cost and flexibility.1–6 Fullerene derivatives, such as PC61BM and PC71BM, are widely used electron acceptors in OSCs since they have high electron affinity, isotropic charge transport and high electron mobility.7–9 However, the deficiencies of fullerene acceptor materials, such as difficult chemical modification, limited energy level variability, weak absorption in the visible region, and morphology instability in a BHJ, hinder the further development of OSCs.10,11 Therefore, it is necessary to develop nonfullerene acceptors. Compared with fullerene acceptors, the chemical structure and the electronic and optical properties of nonfullerene acceptors are readily tuned.12–15Recently, we reported the original fused-ring electron acceptors (FREAs) with an acceptor–donor–acceptor structure based on indacenodithiophene (IDT) or indacenodithienothiophene (IDTT) end-capped with 1,1-dicyanomethylene-3-indanone, exemplified by the star molecule ITIC.16 The rigid extended fused-ring structure of IDT and IDTT prevents rotational disorder, reduces reorganization energy, and exhibits suitable lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels, broad and strong absorption, and relatively high electron mobility.17–19 OSCs based on these FREAs exhibit high power conversion efficiencies (PCEs) with good device stability.20–32
The naphthalene ring shows larger π-conjugation than the benzene ring, and integration of naphthalene into a rigid and coplanar backbone may facilitate π-electron delocalization, reduce energetic disorder and induce strong intermolecular interactions for efficient charge transport.33–37 A few naphthalene-based nonfullerene acceptors were reported and exhibited promising performance in OSCs with PCEs of 8–9%.38,39 However, there is no work to compare naphthalene- and benzene-cored nonfullerene acceptors and probe how naphthalene and benzene differ in affecting the performance of nonfullerene acceptors.
In this work, we synthesized a FREA based on the naphthalene ring as a core (IHIC1, Chart 1), which was published when we prepared this paper,38 compared with its counterpart based on the benzene core (IDIC1, Chart 1) reported in our previous work, and investigated how naphthalene and benzene affect the FREA performance. In comparison with benzene-cored IDIC1, naphthalene-cored IHIC1 exhibits upshifted HOMO and LUMO energy levels and enhanced electron mobility. Furthermore, as-cast OSCs based on blends of IHIC1 and a wide-bandgap polymer donor FTAZ40 (Chart 1) exhibit a PCE of 9.21%, higher than that of IDIC1-based OSCs (7.13%).
Results and discussion
Optical and electronic properties
The HOMO and LUMO energies of the IDIC1 film are estimated to be −5.51 eV and −3.81 eV from the onset oxidation and reduction potentials (Fig. S1a, ESI†), respectively. IHIC1 shows higher energy levels (HOMO = −5.47 eV; LUMO = −3.75 eV) relative to IDIC1 (Fig. 1a). The higher LUMO energy level of IHIC1 is beneficial to high open-circuit voltage (VOC) in OSCs. In solution, IDIC1 and IHIC1 exhibit maximum absorption peaks at 656 nm and 651 nm, respectively (Fig. S1b, ESI†). In the thin film, IDIC1 and IHIC1 show maximum absorption peaks at 686 nm and 674 nm, respectively. Relative to IDIC1, IHIC1 exhibits slightly blue-shifted absorption. The optical bandgaps of IDIC1 and IHIC1 films are 1.67 eV and 1.69 eV, estimated from the absorption edge at 743 nm and 732 nm, respectively (Fig. 1b). The electron mobilities of IDIC1 and IHIC1 films, measured using the space-charge-limited current (SCLC) method, are 1.5 × 10−4 and 3.0 × 10−4 cm2 V−1 s−1, respectively (Fig. S2, ESI†).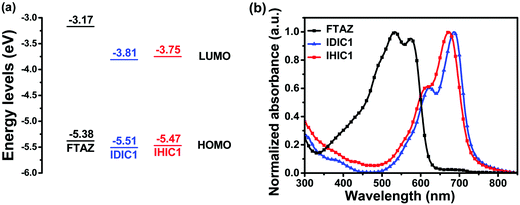 | ||
| Fig. 1 (a) Energy levels of FTAZ, IDIC1 and IHIC1 calculated from cyclic voltammetry. (b) UV-vis absorption spectra of FTAZ, IDIC1 and IHIC1 in a thin film. | ||
Photovoltaic properties
In our previous work, we reported a wide-bandgap polymer donor FTAZ.40 FTAZ exhibits strong absorption at 400–620 nm, which complements those of IDIC1 and IHIC1 at 500–750 nm. Energy levels of FTAZ (HOMO = −5.38 eV; LUMO = −3.17 eV) match with those of IDIC1 and IHIC1. Thus, we used FTAZ as a donor and IDIC1 or IHIC1 as an acceptor to fabricate OSCs with a structure of indium tin oxide (ITO)/ZnO/FTAZ:IDIC1(or IHIC1)/MoO3/Ag. The optimized donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor (D/A) weight ratio is 1
acceptor (D/A) weight ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (Table S1, ESI†). Table 1 summarizes VOC, short-circuit current density (JSC), fill factor (FF) and PCE of the optimized devices. OSCs based on the as-cast FTAZ
1.5 (Table S1, ESI†). Table 1 summarizes VOC, short-circuit current density (JSC), fill factor (FF) and PCE of the optimized devices. OSCs based on the as-cast FTAZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IHIC1 (1
IHIC1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) film give a VOC of 0.950 V, a JSC of 14.3 mA cm−2, a FF of 67.9% and a PCE of 9.21%, while OSCs based on the as-cast FTAZ
1.5, w/w) film give a VOC of 0.950 V, a JSC of 14.3 mA cm−2, a FF of 67.9% and a PCE of 9.21%, while OSCs based on the as-cast FTAZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDIC1 (1
IDIC1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) film give a VOC of 0.896 V, a JSC of 13.6 mA cm−2, a FF of 58.5% and a PCE of 7.13% (Fig. 2a). Compared with the FTAZ:IDIC1 system, FTAZ:IHIC1-based devices show a higher VOC, a higher JSC and a higher FF. The higher VOC is related to the higher LUMO level of IHIC1.
1.5, w/w) film give a VOC of 0.896 V, a JSC of 13.6 mA cm−2, a FF of 58.5% and a PCE of 7.13% (Fig. 2a). Compared with the FTAZ:IDIC1 system, FTAZ:IHIC1-based devices show a higher VOC, a higher JSC and a higher FF. The higher VOC is related to the higher LUMO level of IHIC1.
| Active layers | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Calculated JSC (mA cm−2) |
|---|---|---|---|---|---|
| FTAZ:IDIC1 | 0.894 ± 0.005 (0.896) | 13.5 ± 0.1 (13.6) | 58.1 ± 1.2 (58.5) | 7.05 ± 0.17 (7.13) | 13.2 |
| FTAZ:IHIC1 | 0.947 ± 0.005 (0.950) | 14.2 ± 0.2 (14.3) | 66.4 ± 0.6 (67.9) | 8.91 ± 0.16 (9.21) | 13.8 |
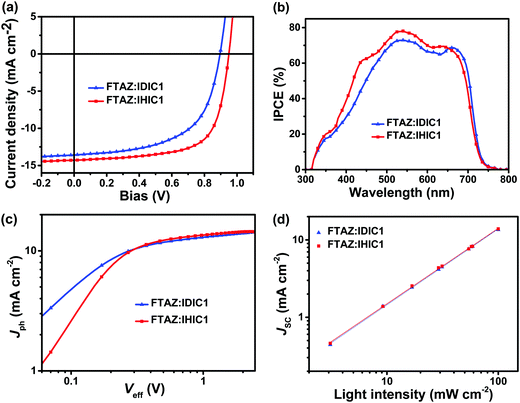 | ||
| Fig. 2 (a) J–V curves, (b) IPCE spectra, (c) Jphversus Veff characteristics, and (d) J versus light intensity of devices with the structure ITO/ZnO/active layer/MoO3/Ag. | ||
The incident photon to converted current efficiency (IPCE) spectra of the optimized devices are shown in Fig. 2b. The OSCs based on FTAZ:IDIC1 and FTAZ:IHIC1 show a broad photoresponse from 300 to 750 nm. The IPCE of FTAZ:IHIC1 based devices is higher than that of FTAZ:IDIC1 based devices at 300–650 nm. The IPCE of FTAZ:IDIC1 based devices red shifts relative to that of FTAZ:IHIC1 based devices at 650–750 nm, due to red-shifted absorption of IDIC. The maximum IPCEs of FTAZ:IDIC1 and FTAZ:IHIC1 are 72.92% and 76.83%, respectively. The JSC values of FTAZ:IDIC1 and FTAZ:IHIC1-based devices calculated from the integration of the IPCE spectra with the AM 1.5G reference spectrum are 13.2 and 13.8 mA cm−2, respectively, which is in good agreement with JSC measured from J–V (the error is <5%).
To probe the exciton/charge dynamics, we measured the photocurrent density (Jph) versus the effective voltage (Veff) to study the charge generation, dissociation and extraction properties. In Fig. 2c, at a high applied voltage (Veff > 2 V), Jph reaches saturation, implying that almost all excitons are dissociated and photo-generated charge carriers are completely collected by the electrodes. The charge dissociation probability can be calculated from JSC/Jsat.41 The Jph/Jsat ratios for the devices of FTAZ:IDIC1 and FTAZ:IHIC1 is calculated to be 95.8% and 96.7% under the short circuit conditions, respectively, indicating efficient charge dissociation and collection.
To gain insights into charge recombination in the active layer, light intensity dependent photocurrent measurement was carried out for the optimized device. JSC follows a power-law dependence on incident light intensity (Plight): JSC ∝ Pαlight (Fig. 2d).42 For extreme conditions, α is equal to 0.75 when space charge buildup reaches a fundamental limit; α is equal to 1 when no space charge exists. The α values of FTAZ:IDIC1 and FTAZ:IHIC1 are 0.983 and 0.978, respectively, suggesting negligible bimolecular charge recombination under the short circuit conditions.
The SCLC method was employed to measure the hole mobility and electron mobility of FTAZ:IDIC1 and FTAZ:IHIC1 blended films (Fig. S3, ESI†). The as-cast blended film of FTAZ:IDIC1 exhibits a hole mobility (μh) of 1.3 × 10−4 cm2 V−1 s−1 and an electron mobility (μe) of 2.0 × 10−5 cm2 V−1 s−1 with a μh/μe ratio of 6.5, while the as-cast blended film of FTAZ:IHIC1 exhibits a μh of 1.5 × 10−4 cm2 V−1 s−1 and a μe of 2.6 × 10−5 cm2 V−1 s−1 with a μh/μe ratio of 5.8. The higher and more balanced charge mobilities of the FTAZ:IHIC1 blend are responsible for the higher JSC and the higher FF of the device.
Film morphology
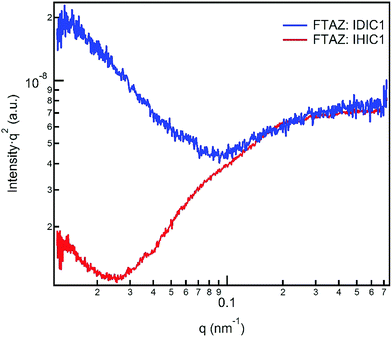
The morphology of the blended films of FTAZ:IDIC1 and FTAZ:IHIC1 is studied by atomic force microscopy (AFM), transmission electron microscopy (TEM), grazing-incidence wide-angle X-ray scattering (GIWAXS) and Resonant soft X-ray scattering (R-SoXS). We used AFM to characterize the surface morphology of active layers (Fig. S4, ESI†). Both FTAZ:IDIC1 and FTAZ:IHIC1 films have a smooth and uniform surface. The root-mean-square roughness values of FTAZ:IDIC1 and FTAZ:IHIC1 films are 0.64 nm and 0.95 nm, respectively. In the TEM images (Fig. S5, ESI†), the blended film of FTAZ:IDIC1 shows low contrast, while the FTAZ:IHIC1 blended film shows visible contrast, which is consistent with the AFM images, suggesting the stronger crystallinity of the FTAZ:IHIC1 film. The GIWAXS measurement was used to probe the molecular packing of FTAZ:IDIC1 and FTAZ:IHIC1 blended films (Fig. 3).43 The peak at q ≈ 0.34 Å−1 is generated from the lamellar packing of FTAZ. The π–π stacking peaks of FTAZ, IDIC1 and IHIC1 are located at q ≈ 1.67, 1.82 and 1.78 Å−1, respectively. The scattering profiles of the corresponding neat films are shown in Fig. S6 (ESI†). IHIC1 shows stronger π–π stacking than IDIC1 in the as-cast films. The coherence length of π–π stacking for IDIC1 and IHIC1 in blended films is calculated to be 1.9 and 2.2 nm, respectively. The enhanced π–π stacking of IHIC1 is beneficial to the charge transport, leading to higher electron mobility in neat and blended films. The phase separation of FTAZ:IDIC1 and FTAZ:IHIC1 blended films was investigated by R-SoXS (Fig. 4).44 The photon energy of 285.2 eV was selected to provide enhanced material contrasts. Both blended films exhibit a scattering peak at ∼0.25 nm−1, corresponding to the characterized length scale of ∼25 nm. These length scales are close to the diffusion length of exciton, thus are favorable for charge separation. Furthermore, the total scattering intensity (TSI, calculated by integrating the scattering profiles) can be used to describe the relative domain purity of the blended films. The higher the TSI, the purer the domains. The relative domain purity of FTAZ:IDIC1 and FTAZ:IHIC1 blend films is the same (88%).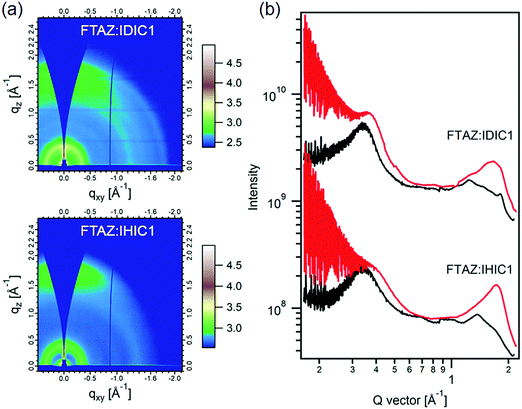 | ||
| Fig. 3 (a) 2D GIWAXS patterns and (b) scattering profiles of in-plane and out-of-plane for blended films. | ||
Conclusions
In summary, we compared benzene-cored (IDIC1) and naphthalene-cored (IHIC1) FREAs and investigated the effects of the benzene/naphthalene unit on the optical and electronic properties as well as on the performance of OSCs. Compared with benzene-cored IDIC1, naphthalene-cored IHIC1 shows a larger π-conjugation with stronger intermolecular π–π stacking. Thus, IHIC1 has upshifted energy levels and higher electron mobility. When paired with the polymer donor FTAZ that has matched energy levels and a complementary absorption spectrum, the IHIC1-based OSCs show higher values of VOC, JSC and FF. The as-cast OSCs based on FTAZ:IHIC1 without any additional treatment yield PCEs of up to 9.21%, much higher than that of the control devices based on FTAZ:IDIC1 (7.13%). The higher VOC in FTAZ:IHIC1-based devices is attributed to the higher LUMO level of IHIC1 in comparison with IDIC1. The FTAZ:IHIC1-based devices show better and balanced charge transport, contributing to the higher JSC and higher FF. These results demonstrate that extending benzene in the electron-donating fused-ring IDT unit to naphthalene is a promising approach to upshift energy levels, enhance electron mobility, and finally achieve higher efficiency in FREA-based OSCs.Experimental section
Materials
Unless stated otherwise, all chemical reagents and solvents used were obtained commercially and were used without further purification. IDIC1,13 IHIC138 and FTAZ40 were synthesized according to the literature procedure.Measurements
Solution (chloroform) and thin film (on quartz substrate) UV-vis absorption spectra were recorded using a JΛSCO V-570 spectrophotometer. Electrochemical measurements were carried out under nitrogen in a solution of tetra-n-butylammonium hexafluorophosphate ([nBu4N]+[PF6]−) (0.1 M) in CH3CN employing a computer-controlled CHI660C electrochemical workstation, a glassy carbon working electrode coated with IDIC1 and IHIC1 films, an Ag/AgCl reference electrode, and a platinum-wire auxiliary electrode. The potentials were referenced to a ferrocenium/ferrocene (FeCp2+/0) couple using ferrocene as an internal standard. The transmission electron microscopy (TEM) characterization was carried out on a JEM-2100 transmission electron microscope operated at 200 kV. The samples for the TEM measurements were prepared as follows: the active layer films were spin-casted on ITO/poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) substrates, and the substrates with the active layers were submerged in deionized water to make the active layers float at the air–water interface. Then, the floated films were picked up on unsupported 200 mesh copper grids for the TEM measurements. The nanoscale morphology of the blends was observed using a Multimode 8 scanning probe microscope (Bruker) in the tapping mode.GIWAXS measurements were performed at beamline 7.3.3 at the Advanced Light Source (ALS). Samples were prepared on Si substrates using identical blend solutions as used in OSC devices. The 10 keV X-ray beam was incident at a grazing angle of 0.11–0.15°, which maximized the scattering intensity from the samples. The scattered X-rays were detected using a Dectris Pilatus 2 M photon counting detector. R-SoXS transmission measurements were performed at beamline 11.0.1.2 at the ALS. Samples for R-SoXS measurements were prepared on a PEDOT:PSS modified Si substrate under the same conditions as used for the OSC device fabrication, and then transferred by floating in water to a 1.5 mm × 1.5 mm, 100 nm thick Si3N4 membrane supported by a 5 mm × 5 mm, 200 mm thick Si frame (Norcada Inc.). Two dimensional scattering patterns were collected on an in-vacuum CCD camera (Princeton Instrument PI-MTE). The beam size at the sample is 100 μm × 200 μm. The composition variation (or relative domain purity) over the length scales probed can be extracted by integrating scattering profiles to yield the total scattering intensity.
Fabrication and characterization of OSCs
OSCs were fabricated with the structure of ITO/ZnO/FTAZ:acceptor/MoO3/Ag. The patterned ITO glass (sheet resistance = 10 Ω □−1) was pre-cleaned in an ultrasonic bath with acetone and isopropanol. The ZnO electron transport layer was prepared onto the ITO glass through spin coating at 4000 rpm from a ZnO precursor solution, then the ZnO substrates were immediately baked in air at 200 °C for 30 min. A CHCl3 solution (totally 12.5 mg mL−1) of the FTAZ:acceptor was spin-coated (3000 rpm) on the ZnO layer to form a photoactive layer (ca. 100 nm). The thickness of the photoactive layer was measured using a Bruker Dektak-XT. The MoO3 layer (ca. 5 nm) and Ag (ca. 80 nm) were successively evaporated onto the surface of the photoactive layer under vacuum (ca. 10−5 Pa). The active area of the device was ca. 4 mm2. An XES-70S1 (SAN-EI Electric Co., Ltd) solar simulator (AAA grade, 70 × 70 mm2 photobeam size) coupled with AM 1.5G solar spectrum filters was used as the light source, and the optical power at the sample was 100 mW cm−2. A 2 × 2 cm2 monocrystalline silicon reference cell (SRC-1000-TC-QZ) was purchased from VLSI Standards Inc. The current–voltage (J–V) measurement of the devices was conducted using a computer-controlled Agilent B2912A Precision Source/Measure Unit (Agilent Technologies). The IPCE spectrum was measured using a Solar Cell Spectral Response Measurement System QE-R3011 (Enlitech Co., Ltd). The light intensity at each wavelength was calibrated using a standard single crystal Si photovoltaic cell.Mobility measurements
Hole-only or electron-only diodes were fabricated using the architectures: ITO/PEDOT:PSS/FTAZ:acceptor/Au for holes and Al/FTAZ:acceptor/Al or Al/acceptor/Al for electrons. A chloroform solution (totally 12.5 mg mL−1) of the acceptor or the FTAZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor (1
acceptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) was spin-coated (3000 rpm). Mobilities were extracted by fitting the current density–voltage curves using space charge limited current (SCLC).45 The J–V curves of the devices were plotted as ln[Jd3/V2] versus [V/d]0.5 using equation J = 9ε0εrμh(μe)V2/8d3 for holes and electrons, where J is the current density, d is the film thickness of the active layer (ca. 100 nm), μh is the hole mobility, μe is the electron mobility, εr is the relative dielectric constant of the transport medium, and ε0 is the permittivity of free space (8.85 × 10−12 F m−1). V = Vappl − Vbi, Vappl is the applied voltage and Vbi is the offset voltage.
1.5, w/w) was spin-coated (3000 rpm). Mobilities were extracted by fitting the current density–voltage curves using space charge limited current (SCLC).45 The J–V curves of the devices were plotted as ln[Jd3/V2] versus [V/d]0.5 using equation J = 9ε0εrμh(μe)V2/8d3 for holes and electrons, where J is the current density, d is the film thickness of the active layer (ca. 100 nm), μh is the hole mobility, μe is the electron mobility, εr is the relative dielectric constant of the transport medium, and ε0 is the permittivity of free space (8.85 × 10−12 F m−1). V = Vappl − Vbi, Vappl is the applied voltage and Vbi is the offset voltage.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
X. Z. thanks NSFC (21734001). Y. L. thanks NSFC (21504058) and Scientific Research Project of Beijing Educational Committee (KM201610028006). W. M. thanks NSFC (21504066, 21534003) and the Ministry of Science and Technology of China (2016YFA0200700). W. Y. thanks NSF (DMR-1507249 and CBET-1639429). X-ray data were acquired at beamlines 7.3.3 and 11.0.1.2 at the Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.Notes and references
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666 CrossRef CAS PubMed.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2016, 49, 175 CrossRef CAS PubMed.
- F. C. Krebs, N. Espinosa, M. Hösel, R. R. Søndergaard and M. Jørgensen, Adv. Mater., 2014, 26, 29 CrossRef CAS PubMed.
- A. J. Heeger, Adv. Mater., 2014, 26, 10 CrossRef CAS PubMed.
- L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed.
- J. Wang, K. Liu, L. Ma and X. Zhan, Chem. Rev., 2016, 116, 14675 CrossRef CAS PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- C. Z. Li, J. Huang, H. Ju, Y. Zang, J. Zhang, J. Zhu, H. Chen and A. K. Jen, Adv. Mater., 2016, 28, 7269 CrossRef CAS PubMed.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470 RSC.
- Y. Lin and X. Zhan, Adv. Energy Mater., 2015, 5, 1501063 CrossRef.
- S. Holliday, R. S. Ashraf, A. Wadsworth, D. Baran, S. A. Yousaf, C. B. Nielsen, C. Tan, S. D. Dimitrov, Z. Shang, N. Gasparini, M. Alamoudi, F. Laquai, C. J. Brabec, A. Salleo, J. R. Durrant and I. McCulloch, Nat. Commun., 2016, 7, 11585 CrossRef CAS PubMed.
- Y. Lin, T. Li, F. Zhao, L. Han, Z. Wang, Y. Wu, Q. He, J. Wang, L. Huo, Y. Sun, C. Wang, W. Ma and X. Zhan, Adv. Energy Mater., 2016, 6, 1600854 CrossRef.
- Y. Wu, H. Bai, Z. Wang, P. Cheng, S. Zhu, Y. Wang, W. Ma and X. Zhan, Energy Environ. Sci., 2015, 8, 3215 CAS.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C.-J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- F. Liu, Z. Zhou, C. Zhang, T. Vergote, H. Fan, F. Liu and X. Zhu, J. Am. Chem. Soc., 2016, 138, 15523 CrossRef CAS PubMed.
- W. Wang, C. Yan, T. K. Lau, J. Wang, K. Liu, Y. Fan, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1701308 CrossRef PubMed.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed.
- J. Zhu, Z. Ke, Q. Zhang, J. Wang, S. Dai, Y. Wu, Y. Xu, Y. Lin, W. Ma, W. You and X. Zhan, Adv. Mater., 2017 DOI:10.1002/adma.201704713.
- S. Dai, F. Zhao, Q. Zhang, T. K. Lau, T. Li, K. Liu, Q. Ling, C. Wang, X. Lu, W. You and X. Zhan, J. Am. Chem. Soc., 2017, 139, 1336 CrossRef CAS PubMed.
- P. Cheng, M. Zhang, T. K. Lau, Y. Wu, B. Jia, J. Wang, C. Yan, M. Qin, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1605216 CrossRef PubMed.
- Y. Lin, F. Zhao, Y. Wu, K. Chen, Y. Xia, G. Li, S. K. Prasad, J. Zhu, L. Huo, H. Bin, Z. G. Zhang, X. Guo, M. Zhang, Y. Sun, F. Gao, Z. Wei, W. Ma, C. Wang, J. Hodgkiss, Z. Bo, O. Inganas, Y. Li and X. Zhan, Adv. Mater., 2017, 29, 1604155 CrossRef PubMed.
- Y. Cui, H. Yao, B. Gao, Y. Qin, S. Zhang, B. Yang, C. He, B. Xu and J. Hou, J. Am. Chem. Soc., 2017, 139, 7302 CrossRef CAS PubMed.
- H. Bin, L. Gao, Z. G. Zhang, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang, M. Xiao and Y. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Rohr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2017, 16, 363 CrossRef CAS PubMed.
- S. Chen, Y. Liu, L. Zhang, P. C. Y. Chow, Z. Wang, G. Zhang, W. Ma and H. Yan, J. Am. Chem. Soc., 2017, 139, 6298 CrossRef CAS PubMed.
- J. Wang, W. Wang, X. Wang, Y. Wu, Q. Zhang, C. Yan, W. Ma, W. You and X. Zhan, Adv. Mater., 2017, 29, 1702125 CrossRef PubMed.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148 CrossRef CAS PubMed.
- Y. Yang, Z. G. Zhang, H. Bin, S. Chen, L. Gao, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 15011 CrossRef CAS PubMed.
- T. Li, Z. Ke, L. Yang, J. Wang, C. Yan, W. Ma and X. Zhan, Adv. Mater., 2017 DOI:10.1002/adma.201705969.
- Y. Ma, Q. Zheng, L. Wang, D. Cai, C. Tang, M. Wang, Z. Yin and S.-C. Chen, J. Mater. Chem. A, 2014, 2, 13905 CAS.
- Y. Ma, Q. Zheng, Z. Yin, D. Cai, S.-C. Chen and C. Tang, Macromolecules, 2013, 46, 4813 CrossRef CAS.
- I. Osaka, S. Shinamura, T. Abe and K. Takimiya, J. Mater. Chem. C, 2013, 1, 1297 RSC.
- I. Osaka and K. Takimiya, Adv. Mater., 2017, 29, 1605218 CrossRef PubMed.
- I. Osaka, T. Abe, S. Shinamura and K. Takimiya, J. Am. Chem. Soc., 2011, 133, 6852 CrossRef CAS PubMed.
- Y. Yi, H. Feng, M. Chang, H. Zhang, X. Wan, C. Li and Y. Chen, J. Mater. Chem. A, 2017, 5, 17204 CAS.
- Y. Ma, M. Zhang, Y. Yan, J. Xin, T. Wang, W. Ma, C. Tang and Q. Zheng, Chem. Mater., 2017, 29, 7942 CrossRef CAS.
- S. C. Price, A. C. Stuart, L. Yang, H. Zhou and W. You, J. Am. Chem. Soc., 2011, 133, 4625 CrossRef CAS PubMed.
- V. D. Mihailetchi, L. J. Koster, J. C. Hummelen and P. W. Blom, Phys. Rev. Lett., 2004, 93, 216601 CrossRef CAS PubMed.
- I. Riedel, J. Parisi, V. Dyakonov, L. Lutsen, D. Vanderzande and J. C. Hummelen, Adv. Funct. Mater., 2004, 14, 38 CrossRef CAS.
- A. Hexemer, W. Bras, J. Glossinger, E. Schaible, E. Gann, R. Kirian, A. MacDowell, M. Church, B. Rude and H. Padmore, J. Phys.: Conf. Ser., 2010, 247, 012007 CrossRef.
- E. Gann, A. T. Young, B. A. Collins, H. Yan, J. Nasiatka, H. A. Padmore, H. Ade, A. Hexemer and C. Wang, Rev. Sci. Instrum., 2012, 83, 045110 CrossRef CAS PubMed.
- G. G. Malliaras, J. R. Salem, P. J. Brock and C. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 13411 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: CV curves, absorption spectra, SCLC measurements, AFM images, TEM images, GIWAXS, OSC data. See DOI: 10.1039/c7tc04520d |
| This journal is © The Royal Society of Chemistry 2018 |