pH-Sensitive nanogels based on the electrostatic self-assembly of radionuclide 131I labeled albumin and carboxymethyl cellulose for synergistic combined chemo-radioisotope therapy of cancer†
Kefeng
Liu
a,
Dan
Zheng
a,
Jingyang
Zhao
a,
Yinghua
Tao
a,
Yingsa
Wang
a,
Jing
He
a,
Jiandu
Lei
 *a and
Xingjun
Xi
*b
*a and
Xingjun
Xi
*b
aBeijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, P. R. China. E-mail: ljd2012@bjfu.edu.cn; Tel: +86 10 62337251
bInstitute of Food and Agricultural Standardization, China National Institute of Standardization, Beijing 100191, China. E-mail: xixj@cnis.gov.cn
First published on 15th June 2018
Abstract
Development of biocompatible and biodegradable nanocarriers with multiple functionalities has attracted great interest in recent years. In this study, a hybrid hydrogel nanoparticle (nanogel) platform based on the self-assembly of carboxymethyl cellulose (CMC) and bovine serum albumin (BSA) is presented for the first time. It was facile to realize the efficient co-delivery of radionuclide 131I and chemotherapeutic drugs such as camptothecin (CPT) to achieve the combined chemo-radioisotope therapy of cancer. Notably, a nanogel was prepared by a simple and green electrostatic interaction approach, instead of chemical reaction, showing typical spherical shape with average size about 120 nm, high drug loading capacity, robust stability and low hemolysis. Interestingly, such nanogels exhibited pH-dependent drug release profile, leading to significant reduction of damage to normal tissues. Furthermore, the as-prepared nanogels could effectively promote intracellular uptake, prolong blood circulation time and enhance accumulation in the tumor tissues. As a result, an excellent therapeutic effect was achieved both in vitro and in vivo through combined chemo-radioisotope therapy. Collectively, this study presents the preparation of a novel green nanocarrier by a reliable and simple approach, and offers an effective strategy for the combination of chemotherapy and radiotherapy.
1. Introduction
Cancer remains one of the leading causes of death worldwide in recent years.1 To date, surgery, chemotherapy and radiotherapy are the three widely used strategies for cancer therapy in clinic.2 However, single treatments such as chemotherapy and radiotherapy suffer from some drawbacks. The major drawbacks of chemotherapy are limited efficiency and severe toxic side effects owing to the nonspecific distribution of drugs and poor tissue permeability.3,4 Moreover, high doses of ionizing radiation are often needed for radiotherapy owing to the low radiation absorption efficiency of tumors, leading to unoptimistic therapeutic outcomes and severe damage to normal tissues adjacent to tumors that are exposed to radiation beams.5,6 To overcome these problems, combination therapy is a commonly used strategy in clinical treatments.7–11 However, simply mixing different therapeutic agents to treat cancer may not always achieve the desired therapeutic effects owing to the inconsistent tumor uptake and different pharmacokinetic profiles. In order to achieve better combination therapy effects, many drug delivery systems (DDSs) have been explored and used in preclinical trials.12–16 However, many currently explored DDSs, particularly inorganic DDSs, have poor biocompatibility, which has limited their further in vivo applications. Moreover, various chemical regents and tedious chemical synthesis procedures are often required to develop DDSs with multiple functionalities, which not only lead to low reaction yield, but also cause damage to the environment. Hence, developing biocompatible and eco-friendly DDSs that can intelligently incorporate different therapeutic agents by reliable and simple strategies for synergetic combination therapy of cancer remains a challenge.Recently, hybrid nanogels based on the self-assembly of natural protein and polysaccharide has attracted great attention and were regarded as promising DDSs because of their inherent biocompatibility and biodegradability, renewability, and well-defined structure.17,18 To date, few self-assembly nanogels based on polysaccharides and proteins have been studied.19–22 However, using protein/polysaccharide nanogels for synergetic combined chemo-radioisotope therapy has not been reported according to our knowledge. Furthermore, hybrid nanogels formed by simple electrostatic interaction instead of chemical reaction between renewable carboxymethyl cellulose (CMC) and bovine serum albumin (BSA) have not been reported.
CMC is an anionic ether of natural cellulose. As a naturally abundant and renewable polysaccharide, CMC has been applied in diverse fields, such as petroleum, food, textile and papermaking industries.23–25 Recently, because of some excellent properties of CMC, such as biodegradability, low toxicity and biocompatibility, it has attracted growing interest in biomedical field.26 There are many reports on the use of CMC as a carrier for drug delivery. For example, Ernsting et al. used CMC as a backbone to conjugate docetaxel and demonstrated its anticancer efficiency.27 Uglea et al. used CMC as a carrier for benzocaine delivery.28 Furthermore, BSA, which is the major component of serum proteins, has received extensive attention from researchers owing to its in inherent biodegradability, biocompatibility, abundance, non-toxicity, non-antigenicity, patient-tolerance and high bioavailability, and has been widely used as a diagnostic agent and drug carrier.29–32
In this study, we developed a novel hybrid BSA/CMC nanogel by a simple and green electrostatic approach, instead of a chemical reaction, for co-delivery of radionuclide 131I and chemotherapeutic drug CPT to achieve chemo-radioisotope synergistic therapy (Fig. 1). The morphology, stability, in vitro release profile and hemolysis of the as-prepared nanogels were characterized. Furthermore, the intracellular uptake, in vivo blood circulation and drug biodistribution were measured. Lastly, mouse transplanted tumor experiments were performed to evaluate the in vivo combination therapy efficiency.
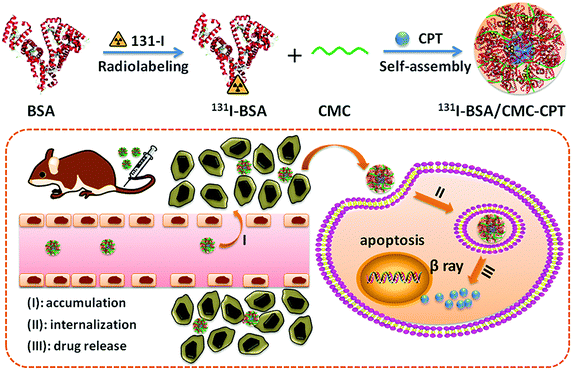 | ||
| Fig. 1 Schematic illustration to show the preparation of 131I-BSA/CMC-CPT nanogels for in vivo combined chemo-radioisotope therapy. | ||
2. Materials and methods
2.1 Materials
Radionuclide 131I was produced by Beijing atomic Hi-tech Co., Ltd. Bovine Serum Albumin (BSA) and sodium carboxymethyl cellulose (CMC) were obtained from Sigma-Aldrich. Camptothecin (CPT) was purchased from Shanghai Macklin Biochemical Co., Ltd. Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), antibiotics (penicillin and streptomycin) and trypsin were provided by Gibco. All other regents were obtained from Sigma-Aldrich.Mouse lung cancer cells (LLC) were provided by BeNa Culture Collection (BNCC) and cultured in DMEM containing 10% FBS and 1% antibiotics. The cultures were maintained in a humidified incubator with 95% atmosphere and 5% CO2 at 37 °C.
Female C57BL/6 mice of age 6–9 weeks were purchased from Beijing HuaFuKang Biotechnology Co., Ltd and kept in a pathogen-free environment. The procedures for animal experiments were performed according to National Institutes of Health guidelines and approved by the Ethics Committee of Peking University.
2.2 Fabrication of BSA/CMC and 131I-BSA/CMC nanogels
BSA was first labeled with radionuclide 131I by the commonly used chloramine-T method.33 Briefly, BSA (2 mg) and 131I (400 μCi) were dissolved in 1.0 mL of phosphate buffer solution (pH 7.4). Chloramine-T (1 mL, 2 mg mL−1) was then dropwise added to the mixture solution under gentle stirring. After reaction for 2 min, the mixture solution was sealed into dialysis bag (Mw = 3500 Da) and dialyzed against phosphate buffer to remove unreacted reagents.BSA (1.0 mg), 131I-BSA (1.0 mg) and CMC (2.0 mg) were separately dissolved in 1.0 mL of deionized water. Then, the pH value of the BSA solution and the CMC solution were adjusted to 3.5 and 7.0, respectively. After stirring for 30 min, the CMC solution was added dropwise into BSA solution until BSA/CMC (w/w) reached at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Following this, the pH value of the mixture solution was adjusted to the isoelectric point of BSA (∼5.0). Nanogels were produced by heating the mixture solution for 60 min at 70 °C. The stability of the nanogels was investigated after its storage at 4 °C for a period of time.
1. Following this, the pH value of the mixture solution was adjusted to the isoelectric point of BSA (∼5.0). Nanogels were produced by heating the mixture solution for 60 min at 70 °C. The stability of the nanogels was investigated after its storage at 4 °C for a period of time.
2.3 CPT loading and release
First, 5.0 mg of CPT was dissolved in 1 mL of ethanol, and mixed with the CMC solution (2.0 mg mL−1). The mixture solution was added dropwise into the BSA solutions until the BSA/CMC ratio (w/w) was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Following this, the pH value of mixture solution was adjusted to 5.0 and the mixture was stirred for 30 min. The nanogels were obtained by heating the mixture for 60 min at 70 °C. Excess CPT was removed by dialysis against deionized water (Mw = 3500 Da). The drug loading capacity (DLC) and encapsulation efficiency (EE) of CPT in the nanogels were determined by a HPLC (Agilent 1200) at 232 nm and calculated as follows:
1. Following this, the pH value of mixture solution was adjusted to 5.0 and the mixture was stirred for 30 min. The nanogels were obtained by heating the mixture for 60 min at 70 °C. Excess CPT was removed by dialysis against deionized water (Mw = 3500 Da). The drug loading capacity (DLC) and encapsulation efficiency (EE) of CPT in the nanogels were determined by a HPLC (Agilent 1200) at 232 nm and calculated as follows:| DLC (wt%) = the weight of loaded CPT in nanogels/the weight of nanogels × 100% |
| EE (%) = the weight of loaded CPT in nanogels/the weight of CPT added initially × 100% |
For the measurement of drug release profiles, BSA/CMC-CPT solution was sealed in dialysis bags (Mw = 3500 Da) and immersed in PBS at pH 5.0 and 7.4 at 37 °C with gentle stirring. At certain time intervals, samples were collected and measured by HPLC (Agilent 1200). All experiments were performed in triplicate.
2.4 Characterization of nanogels
The particle size, ζ potential and polydispersity index (PDI) of the nanogels were analyzed by a laser light scattering instrument (Nicomp 380 Z3000, PSS, USA). To observe its morphology, the nanogel was placed on carboncoated copper grids, air-dried for 10 min, and then observed by a transmission electron microscope (JEM-1400, JEOL, Japan).2.5 Radiolabeling stability of 131I-labeled nanogels
In order to examine the stability of radionuclide, mouse serum (∼200 μL) was treated with 131I-BSA/CMC or 131I-BSA/CMC-CPT for different periods of time at 37 °C. Serum samples (10 μL) were taken out and centrifuged to remove the detached 131I. Following this, the radioactivity of the samples were measured using an automatic gamma counter (WIZARD2 2480, PerkinElmer Instruments Inc, USA).2.6 Hemolysis study
The hemolytic property of nanogels was analyzed as previously reported.34 In brief, fresh blood samples were collected from the cardiac region of mice and stirred for 10 min to remove the fiber protein. Following this, 100 mL of physiological saline was added and centrifuged for 15 min at 1000 rpm to precipitate the red blood cells (RBCs). The obtained RBCs were washed with PBS until the supernatant was clear. Following this, RBCs were formulated as 2% suspension with 0.9% NaCl solution for hemolysis analysis. BSA/CMC, BSA/CMC-CPT, PEI25K (0.1 mg mL−1 or 1 mg mL−1), PBS (negative control) and 1% Triton X-100 (positive control) were separately added to RBCs suspension, and incubated for 2 h at 37 °C with gentle stirring. After centrifugation for 15 min at 1000 rpm, hemoglobin release was analyzed by an infinite microplate spectrophotometer at 540 nm (M200, Tecan, Switzerland) and calculated as follows: (OD2 − OD0)/(OD1 − OD0) × 100%, where OD0, OD1, and OD2 represent the optical density of negative control, positive control and samples, respectively. Three independent experiments were performed for hemoglobin release.2.7 In vitro cytotoxicity study
To investigate the potential toxicity of our nanogels to cancer cells, LLC cells (2 × 105 cells per well) were first cultured in 96-well plates for 24 h, and then treated with different concentrations of BSA/CMC, free 131I, 131I-BSA/CMC, free CPT, BSA/CMC-CPT and 131I-BSA/CMC-CPT. After treatment for 24 h, relative cell viabilities were measured at 450 nm by an infinite microplate spectrophotometer (M200, Tecan, Switzerland).2.8 Intracellular uptake study
For intracellular uptake study, BSA was first labeled with FITC to prepare FITC-labeled BSA/CMC-CPT nanogels. LLC cells were seeded into 24-well plates (2 × 105 cell per well) and cultured overnight. Following this, the cells were treated with FITC-labeled BSA/CMC-CPT nanogels for 1, 2, 3 and 6 h, and washed several times with PBS to completely remove the BSA/CMC-CPT nanogels. The fluorescence intensity was detected by a flow cytometer (Beckman Coulter, USA).For confocal images, LLC cells were first seeded into 12-well plates (2 × 105 cells per well) and cultured for 24 h. FITC-labeled BSA/CMC-CPT nanogels were then added to each well and treated for 6 h. Following this, the cells were washed several times with PBS to completely remove the BSA/CMC-CPT nanogels, fixed with 4% formaldehyde (20 min), and stained with 4′,6-diamidino-2-phenylindole (DAPI). The corresponding confocal images were observed by a confocal fluorescence microscope (CLSM, Leica TCS SP8).
2.9 Blood circulation and biodistribution study
Normal C57BL/6 mice were i.v. injected with different drug formulations: (1) free 131I (300 μCi), (2) 131I-BSA/CMC and (3) 131I-BSA/CMC-CPT (300 μCi of 131I). At certain time intervals, ∼20 μL of blood samples were collected from orbital venous plexus of mice. The radioactivity in blood samples were detected using an automatic gamma counter (WIZARD2 2480, PerkinElmer Instruments Inc, USA).To detect the tumor uptake of 131I-BSA/CMC-CPT, LLC tumor-bearing mice were i.v. injected with free 131I (300 μCi), 131I-BSA/CMC and 131I-BSA/CMC-CPT (300 μCi of 131I) and sacrificed at 24 h, 48 h or 72 h post-injection. The radioactivity in major organs (heart, liver, spleen, lung and kidney) and tumors were detected by an automatic gamma counter (WIZARD2 2480, PerkinElmer Instruments Inc, USA).
2.10 In vivo combination therapy
LLC tumor-bearing C57BL/6 mice were constructed by subcutaneously injecting the LLC cells (2 × 106) into the right abdomen of mice. When the tumor volume reached about 120 mm3, mice were divided into six groups randomly and i.v. injected with different formulations: (1) PBS, (2) BSA/CMC (54 mg kg−1), (3) BSA/CMC-CPT (10 mg kg−1 of CPT), (4) 131I-BSA/CMC (8 mCi kg−1 of 131I), (5) the mixture of CPT and 131I (10 mg kg−1 of CPT, 8 mCi kg−1 of 131I), and (6) 131I-BSA/CMC-CPT (10 mg kg−1 of CPT, 8 mCi kg−1 of 131I). Tumor sizes were measured every two days. Additionally, body weights were also measured for evaluating systemic toxicity. In vivo anticancer efficacy indices, such as tumor volume (TV), relative tumor volume (RTV) and tumor growth inhibition value (TGI) were calculated as follows:| TV = length of tumor diameter × width of tumor diameter2/2 |
| RTV = TV of treatment time/TV of initial time |
| TGI = (1 − RTV of treatment group/RTV of control group) × 100% |
2.11 Blood chemistry analysis
Two groups (n = 6) of healthy C57BL/6 mice were i.v. injected with PBS or 131I-BSA/CMC-CPT. After 50 days post injection, mice were sacrificed and blood samples were collected for blood biochemistry analysis.2.12 Statistical analysis
Statistical analysis was conducted using SPSS 19.0 (IBM, USA). The student's t-test or one-way ANOVA were used for two-group comparison or multiple-group (more than two groups) comparison. In all experiments, p < 0.05 was considered significant.3. Results and discussion
3.1 Fabrication of BSA/CMC and 131I-BSA/CMC nanogels
BSA/CMC hybrid nanogels were formed by simple self-assembly of BSA and CMC under suitable conditions. The optimum conditions for forming nanogels were explored and listed in Table S1 (ESI†). As revealed by dynamic light scattering (DLS) data, the most satisfying nanogels were formed at pH 5.0 with a weight ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (BSA/CMC) and heating temperature at 70 °C for 60 min. As shown in Fig. 2a and c, BSA/CMC hybrid nanogels display a regular spherical shape (∼90 nm) with a narrow size distribution (PDI < 0.2).
1 (BSA/CMC) and heating temperature at 70 °C for 60 min. As shown in Fig. 2a and c, BSA/CMC hybrid nanogels display a regular spherical shape (∼90 nm) with a narrow size distribution (PDI < 0.2).
The mechanism of BSA and CMC self-assembly into hybrid nanogels was preliminary discussed. As shown in Fig. 3, BSA and CMC have opposite ζ-potentials in the range of pH from 3.0 to 5.0, where the electrostatic attraction between BSA and CMC would occur; thus, electrostatic attraction plays a key role in the formation of nanogels. When pH values were in the range of 5.0–8.0, BSA and CMC had the same charge. However, electrostatic repulsion can also induce changes in the interaction between BSA and CMC. Moreover, the anionic CMC can bind to the cation region of the BSA surface along with the possible van der Waals forces and hydrogen bonds, to form nanogels (Table S1, ESI†). Moreover, the gelation property of protein is also crucial for the formation of nanogels. The intermolecular hydrophobic association would occur when the heat treatment temperature is greater than the protein's denaturation temperature, which may promote the association of BSA and CMC. When these forces reach equilibrium, a uniform and stable nanogel could be formed.
3.2 Loading of CPT into nanogels
BSA, as a biodegradable and biocompatible material, has been reported as an ideal carrier for hydrophobic molecules. Hydrophobic drug CPT could interact with the BSA by hydrophobic interactions. Therefore, CPT could be encapsulated into hybrid nanogels by hydrophobic attractions. Such hybrid BSA/CMC nanogels exhibited high CPT encapsulation efficiency (∼89.42%) and high drug loading capacity (∼16.72 wt%) (Table S2, ESI†). As revealed by TEM images and DLS in Fig. 2b and d, CPT-loaded BSA/CMC nanogels had regular spherical shape with narrow size distribution (PDI < 0.2). However, the particle sizes of BSA/CMC-CPT nanogels were slightly increased compared with BSA/CMC (Table S2, ESI†), suggesting the successful loading of CPT into the BSA/CMC nanogel.3.3 Characterization of nanogels
The in vitro stability of the nanogels in terms of ζ-potential and particle size was investigated by a storage method. As shown in Fig. 2e and f, there was no significant change in ζ-potential and particle size of nanogels after 96 h-storage, suggesting good stability. Besides, the stability of 131I-BSA/CMC-CPT nanogels in different pH values was also evaluated, and the results showed that there was little change in particle size and ζ-potential in the range of pH 3.0–8.0 (Table S3, ESI†). The radioactive stability of the nanogels in mouse serum was also investigated at 37 °C. As clearly depicted in Fig. 2g, nanogels still maintained a high level of radioactivity (>80%) after incubation for 48 h.3.4 In vitro drug release profile
The in vitro release profiles of CPT from 131I-BSA-CMC/CPT nanogels were investigated using a dialysis method.35 Nanogels were maintained in PBS solution with pH 7.4 and 5.0 to simulate the physiological and tumor environment, respectively. As shown in Fig. 2h, 131I-BSA-CMC/CPT nanogels displayed a sustained release profile without burst release behavior at both pH 7.4 and 5.0. The cumulative release of CPT from nanogels at pH 5.0 and pH 7.4 was 63.4% and 35.2% within 72 h, respectively. Interestingly, 131I-BSA/CMC-CPT nanogels displayed a priority release behavior at pH 5.0 than at pH 7.4, which might be attributed to the decreased electrostatic repulsions and hydrophobic interactions between the nanogels and CPT at acidic pH. This acid-facilitated release behavior was favorable for reducing damage to normal tissues.3.5 Hemolysis study
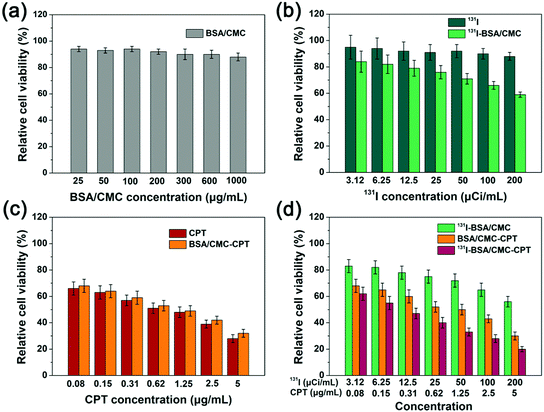
For an injectable drug formulation, high hemolysis (>5%) must be avoided. To examine whether our drug formulations could effectively avoid intravascular or extravascular hemolysis, we next conducted a hemolysis experiment. As shown in Fig. 4, our nanogels exhibited <5% hemoglobin release at different concentrations, which was significantly lower than that of PEI25K at the same dose, reflecting the high safety of our nanogels.3.6 In vitro cytotoxicity study
To quantitatively compare the cytotoxicity of different drug formulations, a CCK-8 assay was carried out. As shown in Fig. 5a, there was no significant toxicity of the BSA/CMC treatment group even at the concentration of 1000 μg mL−1, reflecting its good biocompatibility. Furthermore, 131I-BSA/CMC showed higher toxicity at the same radiation concentration than free 131I (Fig. 5b), which might be attributed to the enhanced cellular uptake of 131I-BSA/CMC. We further compared the potential toxicity of BSA/CMC-CPT and free CPT. As depicted in Fig. 5c, there was no significant difference in the toxicity between BSA/CMC-CPT and free CPT under the same dose of CPT. As for the 131I-BSA/CMC-CPT treatment group, the combination of chemotherapy and radiotherapy exhibited higher toxicity than the single treatment group of 131I-BSA/CMC or BSA/CMC-CPT (Fig. 5d). Therefore, the BSA/CMC nanogel would be a promising candidate for co-delivery of 131I and CPT to achieve combination of chemotherapy and radiotherapy.3.7 Intracellular uptake study
To investigate whether BSA/CMC nanogels could effectively deliver CPT to tumor cells, we next performed intracellular uptake study by flow cytometry and CLSM. As shown in Fig. 6a, a large amount of green fluorescence came from FITC-BSA/CMC-CPT accumulated in the cytoplasm after incubation for 6 h, reflecting the efficient uptake of these nanogels by LLC cells. This phenomenon was further confirmed by flow cytometry analysis (Fig. 6b and c), which was favorable for promoting the therapy effect.3.8 In vivo blood circulation and biodistribution
In order to further understand the in vivo behaviors of 131I-BSA/CMC-CPT nanogels, we next investigated the blood circulation and biodistribution of 131I-BSA/CMC-CPT nanogels. LLC tumor-bearing mice were injected with free 131I, 131I-BSA/CMC or 131I-BSA/CMC-CPT nanogels (300 μCi of 131I) through veins. At certain time points, blood samples were collected and analyzed by an automatic gamma counter (WIZARD2 2480, PerkinElmer Instruments Inc, USA). As shown in Fig. 7a and b, the half-life of free 131I was very short (t1/2α = 0.39 h, t1/2β = 12.2 h) owing to rapid renal filtration. Therefore, high doses of 131I are often needed for radiotherapy, which easily lead to unoptimistic therapeutic outcomes and severe damage to normal tissues adjacent to tumors under exposure to radiation beams. As expected, 131I-BSA/CMC-CPT exhibited prolonged blood circulation half-life (t1/2α = 1.23 h, t1/2β = 27.4 h) than that of free 131I, which might be because the nanogel formulation could effectively prevent rapid metabolism by EPR effect.36–39 As a result, the doses and frequencies of 131I could be reduced and thus, the damage to normal tissues and side effects were significantly reduced.To investigate in vivo biodistribution of different drug formulations, major organs and tumors of LLC tumor-bearing mice were collected after 24, 48 and 72 h post injection. The radioactivity in these organs and tumors was measured using an automatic gamma counter. As shown in Fig. 7c, strong tumor radioactivity was detected in the 131I-BSA/CMC-CPT group, which was significantly higher than the free 131I group. Interestingly, the majority of radioactivity in normal organs could be efficiently excreted 72 h post injection, and its clearance from the tumor was found to be relatively slower due to the EPR effect (Fig. 7d), which was favorable to reduce the damage to normal organs and enhance the anticancer effect.40–44
3.9 In vivo combination therapy
Encouraged by the above experimental results, we then evaluated the in vivo therapy effects of 131I-BSA/CMC-CPT nanogels (Table 1). C57BL/6 mice bearing LLC tumors were divided into six groups (n = 6) and i.v. injected with different drug formulations: (1) PBS, (2) BSA/CMC, (3) BSA/CMC-CPT, (4) 131I-BSA/CMC, (5) the mixture of free CPT and 131I, and (6) 131I-BSA/CMC-CPT every two days five times. As shown in Fig. 8b, the tumor bearing mice after radiotherapy with 131I-BSA/CMC or chemotherapy with BSA/CMC-CPT exhibited weakly inhibited tumor growth compared with PBS or BSA/CMC treatment group. In contrast, mice that underwent treatment with 131I-BSA/CMC-CPT showed significantly improved therapeutic efficacy in inhibiting the tumor growth, which was superior to that of the simple mixture of free CPT and 131I under the same doses. As expected, the survival time of mice in the 131I-BSA/CMC-CPT treatment group was significantly extended compared with other groups (Fig. 8c). Moreover, there was no significant change in body weight of mice, reflecting the reliable safety (Fig. 8d).| Samples | TVa (mm3) | RTVa | TGIa (%) | Curesa (%) |
|---|---|---|---|---|
| a TV, RTV, TGI and cures were measured at day of 20. | ||||
| PBS | 3048 ± 946 | 24.1 ± 9.6 | — | 0 |
| BSA/CMC | 2864 ± 732 | 22.7 ± 9.8 | 5.8 | 0 |
| BSA/CMC-CPT | 1760 ± 548 | 14.2 ± 4.2 | 41.1 | 50 |
| 131I-BSA/CMC | 2147 ± 633 | 17.6 ± 5.4 | 26.9 | 33.3 |
| Free CPT + 131I | 2388 ± 694 | 19.9 ± 6.1 | 17.4 | 16.7 |
| 131I-BSA/CMC-CPT | 646 ± 286 | 5.3 ± 1.8 | 78.2 | 83.3 |
 | ||
| Fig. 8 (a) Schematic illustration of 131I-BSA/CMC-CPT in vivo therapy. (b) Tumor growth curves, (c) survival time, and (d) body weights of mice with different treatments. | ||
3.10 Blood chemistry analysis
Although potent therapy efficiency of our nanogels was proved, toxic side effects remain a very important problem that cannot be ignored. Therefore, we next investigated the toxic side effects of 131I-BSA/CMC-CPT to treated mice. Normal C57BL/6 mice (n = 6) were i.v. injected with PBS or 131I-BSA/CMC-CPT every two days five times. After 50 days post-injection, blood samples were collected and analyzed for blood biochemistry. As shown in Fig. 9a–d, the major liver and kidney function indicators (ALT, AST, ALP, ALB, BUN, and A/G) showed no significant differences compared with the PBS group. Additionally, various parameters were measured for routine blood examination and it was found that the values fluctuated in a normal range compared with the PBS group, suggesting the good safety of 131I-BSA/CMC-CPT within 50 days post-injection.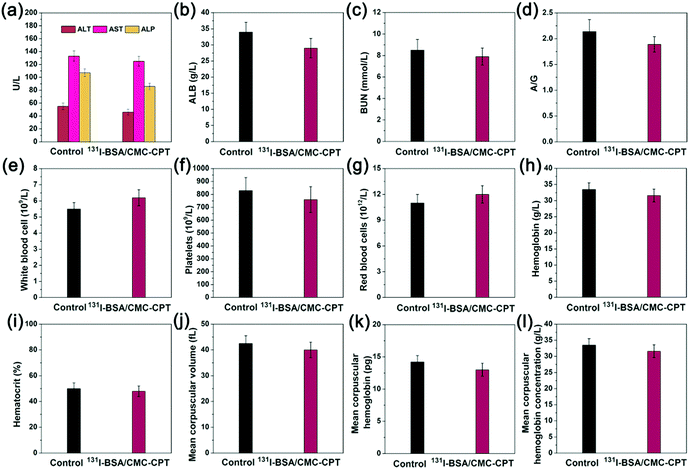 | ||
| Fig. 9 In vivo toxicology evaluation of 131I-BSA/CMC-CPT nanogels. (a–d) Liver and kidney function indicators, and (e–l) hematology measurements. | ||
4. Conclusion
In this study, we have developed a novel biocompatible and biodegradable hybrid nanogel platform via the self-assembly of BSA and CMC. It was facile to realize the efficient co-delivery of radionuclide 131I and chemotherapeutic drug CPT to achieve the combined chemo-radioisotope therapy of cancer. Notably, the preparation process was both simple and green, which make it easy to scale up. The favorable particle size and size distribution, robust stability, excellent biocompatibility and low hemolysis of the as-fabricated 131I-BSA/CMC-CPT nanogels illustrate its promising potential for delivery of therapeutic agents. Interestingly, nanogels exhibited a pH-dependent drug release profile, which was favorable to reduce the damage caused to normal tissues. Furthermore, such nanogels showed enhanced accumulation in tumor tissue and prolonged blood circulation time owing to the ERP effect. As a result, excellent in vivo anticancer efficacy was achieved by chemo-radioisotope combined treatment with 131I-BSA/CMC-CPT, which was significantly superior to single radiotherapy with 131I-BSA/CMC or chemotherapy with BSA/CMC-CPT. Overall, this study presents a simple and green approach for development of a novel biocompatible and eco-friendly hybrid nanogel platform, thus offering a new alternative opportunity for combined chemo-radioisotope therapy of cancer.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by National Key R&D Program of China (2017YFF0207804), The Chinese Central Level Public Welfare Scientific Research Institutes Foundation for Basic Research & Development (562016Y-4687) and the National Natural Science Foundation of China (No. 21576029; 21406013).Notes and references
- L. S. Rebecca, K. D. Kimberly and J. Ahmedin, Ca-Cancer J. Clin., 2018, 68, 7–30 CrossRef PubMed.
- K. D. Miller, R. L. Siegel, C. C. Lin, A. B. Mariotto, J. L. Kramer, J. H. Rowland, K. D. Stein, R. Alteri and J. Ahmedin, Ca-Cancer J. Clin., 2016, 66, 271–289 CrossRef PubMed.
- E. Pérezherrero and A. Fernándezmedarde, Eur. J. Pharm. Biopharm., 2015, 93, 52–79 CrossRef PubMed.
- G. Sersa, M. Cemazar and Z. Rudolf, Cancer Ther., 2003, 1, 133–142 Search PubMed.
- J. D. Bradley, R. Paulus, R. Komaki, G. Masters, G. Blumenschein, S. Schild, J. Bogart, C. Hu, K. Forster and A. Magliocco, Lancet Oncol., 2015, 16, 187–199 CrossRef PubMed.
- H. A. Maas, V. E. Lemmens, P. H. Nijhuis, I. H. de Hingh, C. C. Koning and M. L. Janssen-Heijnen, Eur. J. Surg. Oncol., 2013, 39, 1087–1093 CrossRef PubMed.
- X. Xu, W. Ho, X. Zhang, N. Bertrand and O. Farokhzad, Trends Mol. Med., 2015, 21, 223–232 CrossRef PubMed.
- J. B. Fitzgerald, B. Schoeberl, U. B. Nielsen and P. K. Sorger, Nat. Chem. Biol., 2006, 2, 458 CrossRef PubMed.
- K. S. Albain, S. R. Suzanne, V. W. Rusch, A. T. Turrisi, F. A. Shepherd, S. Colum, C. Yuhchyau, R. B. Livingston, R. H. Feins and D. R. Gandara, Lancet, 2009, 374, 379–386 CrossRef.
- C. R. De, E. Baudin, A. Bachelot, S. Leboulleux, J. P. Travagli, B. Caillou and M. Schlumberger, Int. J. Radiat. Oncol., Biol., Phys., 2004, 60, 1137 CrossRef PubMed.
- W. Rebecca and M. Richard, Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus, John Wiley & Sons, Ltd, 2003 Search PubMed.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1–18 CrossRef PubMed.
- N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J. S. Guthi, S. F. Chin, A. D. Sherry, D. A. Boothman and J. Gao, Nano Lett., 2006, 6, 2427–2430 CrossRef PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef PubMed.
- A. K. Patri, I. J. Majoros and J. R. Baker, Curr. Opin. Chem. Biol., 2002, 6, 466–471 CrossRef PubMed.
- W. Wu, S. Jing, P. Banerjee and S. Zhou, Biomaterials, 2010, 31, 7555–7566 CrossRef PubMed.
- N. Morimoto, T. Endo, M. Ohtomi, Y. Iwasaki and K. Akiyoshi, Macromol. Biosci., 2005, 5, 710–716 CrossRef PubMed.
- Z. Lin, J. Pan, S. Dong and Z. Li, J. Drug Targeting, 2017, 25, 673–684 CrossRef PubMed.
- C. Chen, S. Li, K. Liu, G. Ma and X. Yan, Small, 2016, 12, 4719–4725 CrossRef PubMed.
- L. He, H. Liang, L. Lin, B. R. Shah, Y. Li, Y. Chen and B. Li, Colloids Surf., B, 2015, 126, 288–296 CrossRef PubMed.
- J. Li and P. Yao, Langmuir the Acs Journal of Surfaces & Colloids, 2009, 25, 6385 Search PubMed.
- R. Bhattacharyya and S. K. Ray, Polym. Eng. Sci., 2013, 53, 2439–2453 CrossRef.
- K. C. Cheng, J. M. Catchmark and A. Demirci, Biomacromolecules, 2011, 12, 730–736 CrossRef PubMed.
- A. S. Turaev, Chem. Nat. Compd., 1995, 31, 254–259 CrossRef.
- K. Pal, A. K. Banthia and D. K. Majumdar, Biomed. Mater., 2006, 1, 85 CrossRef PubMed.
- M. J. Ernsting, W. L. Tang, N. Maccallum and S. D. Li, Bioconjugate Chem., 2011, 22, 2474 CrossRef PubMed.
- C. V. Uglea, A. Pârv, M. Corjan, A. D. Dumitriu and R. M. Ottenbrite, J. Bioact. Compat. Polym., 2005, 20, 571–583 CrossRef.
- J. Ge, E. Neofytou, J. Lei, R. E. Beygui and R. N. Zare, Small, 2012, 8, 3573–3578 CrossRef PubMed.
- M. M. Rahimnejad, M. Jahanshahi and G. D. Najafpour, Afr. J. Biotechnol., 2006, 5, 1918–1923 Search PubMed.
- D. Zhao, X. Zhao, Y. Zu, J. Li, Y. Zhang, R. Jiang and Z. Zhang, Int. J. Nanomed., 2010, 5, 669 Search PubMed.
- Z. Yu, M. Yu, Z. Zhang, G. Hong and Q. Xiong, Nanoscale Res. Lett., 2014, 9, 343 CrossRef PubMed.
- P. J. Mcconahey and F. J. Dixon, Methods Enzymol., 1980, 70, 210 Search PubMed.
- L. Dai, R. Liu, L. Hu, Z. Zou and C. Si, ACS Sustainable Chem. Eng., 2017, 5, 8241–8249 CrossRef.
- S. Modi and B. D. Anderson, Mol. Pharmaceutics, 2013, 10, 3076 CrossRef PubMed.
- V. Trieu, J. Cordia, A. Yang, B. Beals, S. Ci, L. Louie and N. Desai, Cancer Res., 2008, 68, 5747 Search PubMed.
- S. D. Li and L. Huang, Mol. Pharmaceutics, 2008, 5, 496 CrossRef PubMed.
- M. D. Blaufox, M. Aurell, B. Bubeck, E. Fommei, A. Piepsz, C. Russell, A. Taylor, H. S. Thomsen and D. Volterrani, J. Nucl. Med., 1996, 37, 1883–1890 Search PubMed.
- M. D. Blaufox and A. Cohen, Am. J. Physiol., 1970, 218, 542–544 Search PubMed.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271–284 CrossRef PubMed.
- M. He, Y. Hue, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef PubMed.
- A. N. Lukyanov, Z. Gao, L. Mazzola and V. P. Torchilin, Pharm. Res., 2002, 19, 1424–1429 CrossRef.
- Y. Matsumura, Adv. Drug Delivery Rev., 2008, 60, 899–914 CrossRef PubMed.
- L. Tian, Q. Chen, X. Yi, G. Wang, J. Chen, P. Ning, K. Yang and Z. Liu, Theranostics, 2017, 7, 614 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tb01295d |
| This journal is © The Royal Society of Chemistry 2018 |