Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy†
Zhongjian
Xie‡
a,
Dou
Wang‡
bc,
Taojian
Fan‡
a,
Chenyang
Xing
a,
Zhongjun
Li
a,
Wei
Tao
 *d,
Liping
Liu
*b,
Shiyun
Bao
*b,
Dianyuan
Fan
a and
Han
Zhang
*d,
Liping
Liu
*b,
Shiyun
Bao
*b,
Dianyuan
Fan
a and
Han
Zhang
 *a
*a
aShenzhen Engineering Laboratory of Phosphorene and Optoelectronics and Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, Shenzhen University, Shenzhen 518060, China. E-mail: hzhang@szu.edu.cn
bDepartment of Hepatobiliary and Pancreatic Surgery, the 2nd Clinical Medicine College (Shenzhen People's Hospital) of Jinan University, Shenzhen 518020, China. E-mail: baomi94@163.com; leoliping@aliyun.com
cIntegrated Chinese and Western Medicine Postdoctoral Research Station, Jinan University, Guangzhou 510632, China
dCenter for Nanomedicine and Department of Anesthesiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA. E-mail: wtao@bwh.harvard.edu
First published on 5th June 2018
Abstract
Two-dimensional (2D) inorganic nanomaterials for biomedical applications still face the challenge of simultaneously offering a high photothermal conversion efficiency (PTCE), efficient drug delivery, biocompatibility and biodegradability. Herein, cancer treatment using tin sulfide nanosheet (SnS NS)-based dual therapy nano-platforms (SDTNPs), including photothermal- and chemo-therapy, is demonstrated. SnS, a black phosphorus (BP) analogue binary IV–VI compound, was synthesized using liquid phase exfoliation. SnS NSs comprising 2–4 layers exhibited good biocompatibility and a high PTCE of 39.3%, which is higher than other popular 2D materials. The SnS NSs showed a stable photothermal performance over 2 h of laser irradiation and exhibited ∼14% degradation after 10 h of irradiation. It was also found that SnS NSs show high loading of small molecules such as doxorubicin (DOX) (up to ∼200% in weight). Consequently, the SDTNPs achieved notable tumor therapy through the combination of photothermal- and chemo-therapy both in vitro and in vivo. Our study may pave the way for the biomedical application of SnS and other IV–VI compound-based 2D nanomaterials. Compared with traditional therapies, SnS NS-based laser therapy is green and efficient, due to its biocompatibility, photo-degradability, high efficiency photothermal properties and high drug loading.
1. Introduction
Since the discovery of single-layer graphene in 2004,1 significant effort has been devoted to exploring new two-dimensional (2D) nanomaterials (NMs) with useful physicochemical properties, such as transition metal dichalcogenides (TMDs),2 metal–organic frameworks (MOFs),3 hexagonal boron nitride (hBN),4 covalent–organic frameworks (COFs),5 layered dual hydroxides (LDHs)6 and black phosphorus (BP).7–11 These materials have various applications in optoelectronics, energy storage and conversion, and biomedicine.Photothermal therapy (PTT), a therapeutic strategy in which light energy is converted into heat, has been widely studied in effective cancer therapy. Due to the minimal invasiveness and high selectivity of light irradiation, PTT is considered a promising alternative to conventional clinical cancer treatments, for example surgery, radiotherapy, and chemotherapy. In addition to well-known photothermal converters, such as noble metal nanoparticles,12,13 and carbon nanotubes,14 2D NMs have emerged as an important family of potential photothermal converters, as demonstrated in several reports on graphene oxide (GO),15 MoS2,16 BP,17 palladium metal (Pd),18 and antimonene quantum dots (AMQDs).19
Ti3C2 MXenes were recently reported as an effective photothermal agent under sun irradiation.20 The ultrathin Ti3C2 MXenes showed strong optical absorption in the NIR region with an extinction coefficient as high as 29.1 L g−1cm−1.21 This was demonstrated for highly effective photothermal ablation of tumors.22 Although Ti3C2 MXenes exhibit high PTT performance, their fabrication is still a problem since it is hazardous and time-consuming using HF pretreatment. Fortunately, a simple fluorine-free fabrication method was proposed to solve this problem.23 Besides the Ti3C2 MXenes, another newly developed Nb2C MXenes exhibited high photothermal conversion efficiency (36.4% at 808 nm and 45.65% at 1064 nm). The Nb2C MXene intrinsically features unique enzyme-responsive biodegradability and high biocompatibility. Importantly, the surface-engineered Nb2C MXenes present highly efficient in vivo photothermal ablation of tumors.24 For in vivo theranostics, the Ta4C3 MXenes exhibit unique dual-mode photoacoustic/computed tomography imaging and are highly effective for in vivo photothermal ablation of tumors.25
BP has also recently attracted significant attention. It has a layer-dependent band gap of 0.3–2 eV, highly accurate optical response, and anisotropic charge transport, resulting in interesting electronic and photoelectronic applications.26–28 Recently, several studies have shown the potential of BP NMs in biomedical applications. BP quantum dots, BP nanoparticles and even BP nanosheet-based hybrid aerogels have been shown to be efficient NIR photothermal agents.8,17,29,30 Moreover, the potential of using a BP-based theranostic delivery platform has also been demonstrated.10 It has a high drug loading capacity because of its atomically thin 2D structure, and a relatively large surface area owing to its wrinkled topography.31,32
Tin sulfide (SnS), a BP analogue binary IV–VI compound, not only crystallizes in layered structures via van der Waals forces, but also has a similar wrinkled structure to BP.33 SnS exhibits strong anisotropic optoelectronic and mechanical properties,34–36 as well as dual indirect and direct band gaps. The indirect band gap of SnS varies from 1.07 to 1.25 eV and the direct band gap varies from 1.30 to 1.39 eV, which lies in between those of Si (1.12 eV) and GaAs (1.43 eV).36 Correspondingly, it has large absorption coefficients (>104 cm−1) across the ultraviolet, visible, and NIR regions of the spectrum,33,37 which are substantially higher than those of materials such as cadmium telluride (CdTe), copper indium selenide (CIS) and copper indium gallium selenide (CIGS).36 In contrast to the scarcity of In and Ga, the constituent elements of SnS are relatively abundant. And compared with the toxicity of Cd and Se, Sn and S are safer for the environment. Therefore, a suitable band gap and a large absorption coefficient of SnS in the NIR region may satisfy the requisite absorption for NIR photothermal transduction, and its wrinkled structure could lead to a high drug loading ratio similar to BP.31,32
SnS is also biocompatible and relatively stable, which are important attributes for biomedical applications.38 Specifically, tin is one of the microelements necessary for human health, indicating its potential biocompatibility.39 Inorganic tin compounds are poorly absorbed by the human body and are rapidly excreted in faeces, which accounts for their low toxicity.40 Human volunteers showed mild signs of tin toxicity when given a high concentration dose of 1400 mg L−1.40 Interestingly, long-term animal carcinogenic studies showed fewer malignant tumors in animals exposed to tin than in controls.40 Furthermore, a tin compound exhibited interesting anti-proliferative behavior in human ovarian tumors.41 More recently, it has been reported that nanoparticles with a greater density achieve more efficient passive tumor targeting and longer retention times in highly permeable organs.42 The density of SnS is higher than that of some typical 2D materials, such as BP, graphene and MoS2, which may result in SnS showing more efficient tumor targeting and longer retention times.
Currently, few photothermal agents simultaneously meet the requirements of a suitable absorption spectrum in the biological NIR region, high efficiency drug delivery, biodegradability and biocompatibility, as outlined above. Therefore, the development of novel theranostic agents combining all of these ideal properties has significant clinical value. Based on the similarity of its properties to BP, SnS warrants the investigation of its potential as a photothermal and drug delivery agent. Its physical (suitable band gap, large absorption coefficient in the biological NIR region and potential efficient drug loading due to its wrinkled structure) and biological (non-toxic Sn and S elements, potential biocompatibility and potential anti-cancer effects) features show significant promise.
Herein, we designed a dual-function theranostic platform based on the photothermal properties and drug loading potential of 2D SnS NSs (Fig. 1), and applied this 2D SnS platform in cancer theranostics. SnS NSs were synthesized from bulk SnS using the liquid exfoliation method and were then functionalized with targeting and imaging agents. The functionalized SnS NSs were then used as theranostic agents. The SnS NSs display excellent biocompatibility, while exhibiting efficient photothermal behavior. Furthermore, a high drug loading ratio (DOX/SnS NSs = ∼200% in weight) can be obtained due to its puckered structure. This theranostic platform can achieve both photothermal- and chemo-therapy, and exhibited an enhanced antitumor effect both in vitro and in vivo without observable side effects or toxicity to healthy tissue. Our study demonstrated the promising application of SnS NSs as an innovative 2D platform for PTT and theranostic delivery in the treatment of tumors. Owing to its promising therapeutic performance and good biocompatibility, we expect to shed new light on IV–VI compounds for their potential in biomedical applications.
 | ||
| Fig. 1 Schematic representation of the SDTNP for in vitro and in vivo experiments. (1) DOX (therapeutic agent), (2) PEG-FA (targeting agent), and (3) PEG-FITC (fluorescent imaging agent). | ||
2. Experimental
2.1. Materials
The bulk SnS was purchased from J&K Scientific. PEG-NH2, FITC-PEG-NH2 and FA-PEG-NH2 were purchased from Nanocs Inc. (New York, USA). The molecular weight (MW) of PEG applied in this study is 2 kDa. Phosphate buffered saline (PBS) (pH 7.4), trypsin–EDTA, fetal bovine serum (FBS), DMEM/F12(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RPMI-1640 medium, and penicillin/streptomycin were purchased from Gibco Life Technologies (AG, Switzerland). Doxorubicin hydrochloride, paraformaldehyde, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). AO/PI assay kit was obtained from Logos Biosystems. Hypure water (18.25 MΩ cm, 25 °C) was used to prepare water-based dispersions. All other chemicals used in this work were of analytical reagent grade.
1), RPMI-1640 medium, and penicillin/streptomycin were purchased from Gibco Life Technologies (AG, Switzerland). Doxorubicin hydrochloride, paraformaldehyde, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). AO/PI assay kit was obtained from Logos Biosystems. Hypure water (18.25 MΩ cm, 25 °C) was used to prepare water-based dispersions. All other chemicals used in this work were of analytical reagent grade.
2.2. Synthesis of SnS NSs
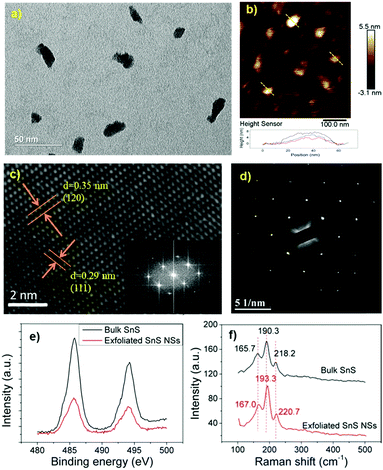
SnS NSs were prepared by liquid exfoliation. Briefly, 100 mg of the obtained SnS was added to 50 mL of isopropyl alcohol (IPA). The liquid exfoliation involves probe sonication for 10 h and bath sonication for 10 h. The power of probe sonication is 240 W with the on/off cycle of 2/4 s. The power of bath sonication is 360 W. Both the temperatures of probe sonication and bath sonication are controlled below room temperature. In order to get monosized SnS NSs, the range of centrifugation speed was narrowed. Firstly, a centrifugation speed of 4000 rpm was used to remove large non-exfoliated SnS particles. Then, the supernatant was centrifuged at 6000 rpm and the obtained SnS NSs in the sediment are shown in Fig. 2a and b.2.3. Characterization techniques
Transmission electron microscopy (TEM, JEM 1230) and atomic force microscopy (AFM, Bruker, Dimension Fastscan) were used to characterize the morphology and height of the SnS nanosheets (NSs). To confirm the crystal feature of the SnS NSs, high-resolution transmission electron microscopy (HRTEM, Tecnai G2 F30) was used to characterize the crystal lattice and selected-area electron diffraction (SAED) of SnS NSs. For the AFM sample, the SnS NSs were dispersed on a Si substrate by the drop-casting method and the images were scanned at 512 pixels per line. The UV-Vis-NIR absorption spectra of SnS NSs were measured in the range of 200–1100 nm with a step of 1 nm by using a UV-Vis absorbance spectrometer (Cary 60, Agilent). Raman tests were performed using a high-resolution confocal Raman microscope (HORIBA LabRAM HR800) at room temperature. To avoid disturbance, the Raman tests were excited at wavelengths of 532 nm and 638 nm, respectively. X-ray diffractometer (XRD, Ultima IV) was used to confirm the crystal structure of SnS NSs.2.4. Cell culture assays
SMCC-7721 (Human hepatocellular carcinoma), A549 (human lung carcinoma), B16 (Mouse melanoma) and HeLa (Human cervical cancer) cell lines were originally obtained from the American Type Culture Collection (ATCC, Rockville, MD). A549, B16 and HeLa cells were cultured in the RPMI-1640 medium with 10% FBS and 1% penicillin/streptomycin at 37 °C with 5% CO2, and SMCC-7721 cells were cultured in the DMEM/F12 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium under the same conditions.
1) medium under the same conditions.
2.5. In vitro toxicity
SMCC-7721, A549, B16 and HeLa cells were seeded at a density of 8 × 103 cells per well into 96-well plates and then incubated for 10 h. Afterwards, the cells were incubated with SnS NSs at different SnS NS concentrations (10, 25, 50, and 100 ppm) for 48 h (fresh medium with the same SnS NS concentrations was changed every day). Three multiple wells in 96-well plates were set for every sample. The medium containing SnS NSs was changed with CCK-8-contained medium and an additional 90 min-incubation was conducted for the cells. Subsequently, a microplate reader (ELx808, BioTek, USA) was used to measure the absorbance at 450 nm.2.6. In vitro photothermal therapy study
SMCC-7721 cells were incubated with SnS NSs (0, 25, 50 and 100 ppm) for 6 h at 37 °C under the same conditions and then irradiated with an 808 nm laser for 10 min. For each concentration of SnS NSs, three multiple wells were set in 96-well plates. The distance from the laser tip to 96-well plates was 0.5 cm. After additional incubation for 12 h, the relative cell viabilities were then measured by the CCK-8 cell cytotoxicity assay. The cell viability was normalized using the control sample without treatment.2.7. In vitro chemotherapy study
The SMCC-7721 cells were seeded into 96-well plates at a density of 8 × 103 cells per well and incubated for 12 h. Then the culture medium in each well was replaced with 100 μL of SnS (20, 30, 40 ppm), SnS (as PTT group; 20, 30, and 40 ppm), SnS-FA (as PTT group; 20, 30, and 40 ppm for SnS), SnS-PEG-FA-DOX (as chemotherapy group; 20, 30, and 40 ppm for SnS and 10, 15, and 20 ppm for DOX), and SnS-PEG-FA-DOX (as chemotherapy + PTT group; 20, 30, and 40 ppm for SnS and 10, 15, and 20 ppm for DOX). After additional incubation for 6 h, the cells in the PTT therapy group and the chemotherapy + PTT group were irradiated with an 808 nm laser for 10 min. After additional incubation for 12 h, the relative cell viabilities were then measured by the CCK-8 cell cytotoxicity assay.2.8. DAPI staining assay
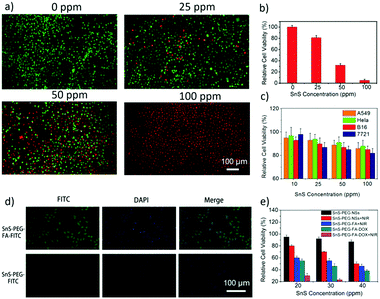
SMCC-7721 cells were seeded at a density of 8 × 103 cells into the 96-well plates and treated with SnS-PEG-FITC and SnS-PEG-FA-FITC for 6 h. SMCC-7721 cells were fixed with 4% paraformaldehyde for 15 minutes and 0.2% Triton X-100 for 20 min, and then the cells were stained with 1 μg mL−1 DAPI for 5 minutes, followed by observation under a fluorescence microscope.2.9. In vivo photothermal assay
Tumor bearing nude mice were injected with 100 μL of SnS NSs (100 ppm). After 1 h injection, the nude mice were irradiated with an 808 nm laser at 1 W cm−2 for 5 min. During the irradiation process, an IR thermal camera (FLIR E50, USA) was used to monitor the temperature changes of the tumor sites.2.10. In vivo anticancer therapy
Female BALB/c (nu/nu) mice (6 weeks old) used in this research were all purchased from the Guangdong Medical Laboratory Animal Center (Guangzhou, China), and all the animal researches were conducted in compliance with the guidelines approved by the Animal Welfare and Research Ethics Committee at Shenzhen University (ID: 2017003). The tumor model was generated by injecting SMCC-7721 cells (3.5 × 106 per mouse) into the left axilla of each mouse. When the tumor volumes reached about 100 mm3, the mice were randomly divided into 5 groups (5 mice for each group). Groups 1–5 were injected in veins with saline (200 μL), SnS (10 mg kg−1), SnS-PEG-FA-DOX (10 mg kg−1 for SnS and 6 mg kg−1 for DOX), and SnS-PEG-FA-DOX (10 mg kg−1 for SnS and 6 mg kg−1 for DOX), respectively. The mice in groups 2, 3 and 5 were anesthetized and irradiated with an 808 nm laser at 1 W cm−2 for 10 min after 12 h injection at the tumor site. Subsequently, the body weight and tumor growth were monitored using a balance and a caliper every other day. The tumor volume (V) was calculated according to the formula: V = 4π/3 × (tumor length/2) × (tumor width/2)2. The relative tumor size was calculated as V/V0, where V0 is the initial tumor volume of the treatment. The mice were humanely sacrificed after two weeks of treatment.2.11. Histology examination
Finally, the mice were euthanized. The heart, liver, spleen, lung, kidney and tumor tissues were sliced and dehydrated successively and embedded in liquid paraffin for hematoxylin and eosin (H&E) staining.3. Results and discussion
3.1. Characterization
The SnS NSs used in this study were prepared using a liquid exfoliation method in isopropyl alcohol (IPA). Previous work has shown that SnS NSs could be exfoliated using N-methyl-2-pyrrolidone (NMP).37 IPA has a lower boiling point than NMP and can therefore readily substitute it to avoid potential toxicity to healthy tissue.To obtain SnS NSs with the desired dimensions, dispersions of SnS NSs in IPA at different centrifugal speeds were characterized using transmission electron microscopy (TEM) and atomic force microscopy (AFM). Fig. 2a shows the dimensions of the SnS NSs obtained from the supernatant after centrifugation at 5000 rpm. The TEM image shows a typical lateral SnS NS size of less than 50 nm. The AFM image (Fig. 2b) shows that the thickness of the SnS NSs falls within the range of 4–8 nm. Clear lattice fringes with inter-atomic d-spacings of 0.29 nm and 0.35 nm for SnS NSs shown in Fig. 2c can be ascribed to the (111) and (120) planes, respectively. Fast Fourier transformation (FFT) of the high-resolution TEM (HRTEM) image demonstrates the crystallinity of the SnS NSs, showing the expected crystallographic lattice reflections. Furthermore, the selected-area electron diffraction (SAED) image in Fig. 2d suggests that the highly crystalline features of the SnS NSs were not destroyed during the liquid exfoliation procedure.
The chemical composition and crystal structure of bulk SnS and SnS NSs were characterized using X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). In Fig. 2e, the two strong peaks at 485.7 and 494.2 eV are ascribed to the 3d5/2 and 3d3/2 orbitals for both bulk SnS and SnS NSs. No other impurities, such as SnS2, Sn or S, were detected, indicating the high purity of the starting material and the final exfoliated SnS NSs. The unsymmetrical XPS peak may indicate the impurity of SnO. As shown in Fig. S1 (ESI†), both the bulk SnS and the exfoliated SnS NSs exhibited typical crystalline diffraction.
The bulk SnS and the exfoliated SnS NSs were also characterized by Raman spectroscopy (Fig. 2f). For bulk SnS, three distinct peaks were observed in the range of 100–500 cm−1. The peak at 165.7 cm−1 corresponds to the B3g mode, and the Raman peaks at 190.3 and 218.2 cm−1 can be assigned to the Ag mode.43,44 For exfoliated SnS NSs, three similar peaks were observed at 167, 193.3 and 220.7 cm−1, respectively. This indicates a Raman shift for the exfoliated SnS NSs compared with the bulk SnS. It can be concluded that, of the two vibrational modes, the Ag mode is more sensitive to the thickness of the SnS NS layers. All characterization methods confirm the purity and crystallinity of the exfoliated SnS NSs. Consequently, the as-prepared SnS NSs with a relatively small size (<50 nm) could be useful in biomedical applications.
3.2. Photothermal response
The stability of the SnS NSs in deionized water was characterized over ten days. After this time the absorbance of the SnS NSs showed a slight decrease, which indicated that the SnS NSs were stable for at least ten days following synthesis (Fig. S2, ESI†). Although the SnS NSs did not degrade obviously when dispersed in water, the aggregation of the NSs in a physiological medium is a further concern. Therefore, the stability of the SnS NSs in a physiological medium was also characterized. It was found that the SnS NSs readily aggregated in the presence of salts when dispersed in phosphate buffered saline (PBS) (Fig. 3b). Positively charged monofunctional poly(ethylene glycol) amine (PEG-NH2) was thus used to coat the surface of the SnS NSs by electrostatic adsorption to give SnS-PEG NSs. The success of this coating was validated by STEM-EDS, which showed the co-localization of four different elements; Sn and S from the SnS NSs, and N and O from the surface coating of PEG-NH2 (Fig. 3a). After surface modification, the zeta potential of the SnS NSs changed from −23 to −15 mV. Fig. 3b shows the coating halo of PEG on SnS NSs. Even after the coating, it can be observed that the size of SnS NSs was ∼50 nm, which was favorable for bio-experiments. Consequently, the SnS-PEG NSs exhibited enhanced stability in PBS (Fig. 3c and d). The surface coating of FITC-PEG-FA on SnS NSs was further confirmed by absorbance (Fig. S4, ESI†).The optical absorption spectra of SnS NSs dispersed in water are shown in Fig. 3e. They show a broad absorption band spanning the UV and NIR regions, and are similar to those of other 2D NMs, such as GO,45 WS246 and BP.8 The concentration (C) of the SnS NSs was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The normalized absorbance over the characteristic length of the cuvette (A/L, where A is the absorbance and L is the light path) at 808 nm for different concentrations of SnS NSs could be determined (Fig. 3e). The extinction coefficient at 808 nm was estimated to be 3.4 L g−1 cm−1, according to the Lambert–Beer law (A/L = kC, where k is the extinction coefficient).
The photothermal properties of the SnS NSs were further evaluated. Different concentrations of SnS NSs suspended in water were exposed to an 808 nm NIR laser (power density: 2.0 W cm−1) and the temperature of the suspension was measured as a function of time (Fig. 3f). At an SnS concentration of 100 ppm, the temperature increased from 25 °C to 54 °C after irradiation for 600 s, indicating that the SnS NSs can efficiently convert NIR light into thermal energy. Furthermore, by using a reported method,47 the photothermal conversion efficiency of the SnS NSs was determined to be 39.3% (Fig. S6, ESI†), higher than those of popular photothermal agents, such as copper selenide (22%), Au nanoparticles (21%),48,49 and black phosphorus quantum dots (BPQDs, 28.4%),8 but lower than that of MoS2 (53.3%).50
The photothermal stability of the SnS NSs was examined further. Fig. 3f shows six photothermal cycles for different SnS NS concentrations. One photothermal cycle consisted of sample irradiation using an 808 nm laser for 10 min, followed by a further 10 min period with the laser turned off. It was found that the temperature initially increased and then decreased to room temperature by natural cooling. The highest photothermal temperature throughout the six cycles was approximately consistent, shown by the dotted line. This indicates that the SnS NSs did not appreciably deteriorate under NIR irradiation. For the 100 ppm sample, it can be observed that there was a slight increase of ΔT as the irradiation cycle number increased, which was due to the increasing concentration of the SnS NSs with the evaporation of the water dispersant. For the 50 and 25 ppm samples, there was a slight decrease of the highest temperature as the irradiation cycle number increased, which may be due to the degradation of the SnS NSs under laser irradiation. To confirm this hypothesis, the 50 ppm sample was irradiated for a further 10 h. After 10 h of irradiation, both the absorbance and ΔT decreased by ∼14%, showing the photodegradability of the SnS NSs (Fig. 3g and h). This can also be proved by the Raman spectra (Fig. S3, ESI†). When irradiated with the laser with strong power, a new phase of SnS2 appeared.
Based on these observations, it can be concluded that the SnS NSs have good photostability under several hours of laser irradiation despite being ultimately photodegradable. This slow degradation profile could make SnS NSs a promising material for PTT.
3.3. Drug delivery platform
2D nanomaterials have been widely used as drug carriers in view of their relatively large surface areas, for example the reported GO,51 MoS252 and BP materials.10 In the present study, the as-prepared SnS NSs were employed as carriers for DOX, a prevalent anti-cancer drug in clinical application.To load DOX onto SnS NSs, a dispersion of SnS NSs was mixed with DOX at different DOX/SnS NS feeding ratios and then stirred. To characterize the drug loading process, UV-Vis and fluorescence spectra were measured after stirring for 0, 9 and 24 h. As shown in Fig. 4a, the absorbance peaks at ∼490 nm were attributed to DOX. It was found that the UV-Vis absorbance decreased significantly from 0 h to 9 h due to the enclosure of DOX in the SnS NSs. The spectra then remained essentially stable from 9 h to 24 h, indicating that drug loading was almost complete after 9 h. For absorbance spectra, base lines were built at the DOX peak (black dotted line). The decrease in absorbance was mainly exhibited by the angular rotation of the base line (Fig. 4a). The vertical distance from the DOX absorbance peak to the base line was defined as the peak height. The peak heights for 0 h (Peak 1) and 9 h (Peak 2, Fig. 4a) were the same, despite having different base lines. Moreover, the spectrum at 9 h was the same as that of pure DOX at the same concentration. This indicates that the drug loading process decreases the absorbance of SnS NSs but not that of DOX. The loading process of naked SnS NSs and SnS-PEG NSs was almost the same (Fig. S7, ESI†), indicating that the PEG coating does not influence the drug loading of SnS NSs.
Sample fluorescence was measured at the same time points. Fluorescence was measured immediately upon mixing of the SnS NSs and DOX. Compared with pure DOX (same concentration), significant fluorescence quenching at ∼600 nm was observed. This was due to the strong absorption of SnS NSs at ∼600 nm. After stirring for 9 h, a marked increase in fluorescence was observed which was the same as that of pure DOX at the same concentration. Based on these absorbance and fluorescence observations, it can be reasonably hypothesized that when SnS NSs were DOX loaded the absorbance of SnS NSs decreased and the fluorescence of DOX could be released.
Furthermore, the successful drug loading can be characterized by zeta potential measurements. It was found that the SnS NSs have a negative potential while they became positive when loading DOX (Fig. 4c). This also confirms that the interaction between SnS NSs and DOX is electrostatic absorption. The drug-loading ratio of the SnS NSs was measured using the dialysis method. The SnS NSs and the DOX mixture were stirred for 24 h and then dialyzed against deionized water. Free DOX was dialyzed against water and its concentration can be determined using the normalized absorbance or fluorescence spectra (Fig. S6, ESI†). Our testing conditions showed that the saturation of DOX loading can be up to ∼200% (Fig. 4d).
As a BP-analogue material, the photothermal performance of SnS is compared with that of BP. The extinction coefficient (3.4 L g−1 cm−1) of SnS is lower than that of BP8 (14.8 L g−1 cm−1) but both the drug loading ratio (∼200%) and PTCE (39.3%) of SnS are higher than those of BP (drug loading ratio ∼108%10 and PTCE of 28.4%8). Moreover, the cost of SnS is much lower than that of BP, indicating its promising potential in clinical application.
3.4. In vitro experiments
Nanomaterials used in biomedicine must be sufficiently biocompatible; therefore, the cytotoxicity of the PEGylated SnS NSs, namely SnS-PEG NSs, to several types of cells was therefore examined. Cell cytotoxicity assay using the standard Cell Counting Kit-8 (CCK-8) was carried out to determine the relative viabilities of A549 (human lung carcinoma cells), HeLa (human cervical cancer cells), B16 (mouse melanoma cells) and SMCC-7721 (human hepatocellular carcinoma cells) cells after incubation with the SnS-PEG NSs at different concentrations (10, 25, 50, and 100 ppm) for 48 hours. No obvious cytotoxicity could be observed for the four cell types, even at a concentration of 100 ppm, which can lead to high photothermal performance. This suggests good biocompatibility and suitability of SnS-PEG NSs for biomedical applications (Fig. 5c).The photothermal killing effect of the SnS-PEG NSs in cancer cells was then investigated. SMCC-7721 cancer cells were incubated with the SnS-PEG NSs for 6 h, and the cells were irradiated with an NIR laser of 808 nm for 10 min. The live/dead cells were differentiated using acridine orange (AO, live cells, green fluorescence) and propidium iodide (PI, dead cells, red fluorescence) and co-stained after the PTT. A SnS-PEG NS-dose-dependent photothermal killing effect was observed for SMCC-7721 cells (Fig. 5a). It should be noted that almost all cells were killed after incubation with 100 ppm of the SnS-PEG NSs and exposure to the NIR laser (Fig. 5b). These results clearly demonstrate the photothermal efficiency of the SnS-PEG NSs in promoting cancer cell death. The irradiation conditions were also much less intensive than those used with Au nanoshells (40 W cm−2, 5 min)53 and copper selenide nanocrystals (33 W cm−2, 5 min)54 for in vitro photothermal cell destruction.
To achieve an actively targeted DOX delivery system, we modified SnS-PEG NSs with FA to give SnS-PEG-FA-DOX, based on the specific binding between FA and the FA receptors55 over expressed on SMCC-7721 cancer cells (Fig. 5d). The tumor cell killing effect of the SDTNPs was then investigated. SMCC-7721 cells were incubated with SnS-PEG NSs and SnS-PEG-FA-DOX, in the presence and in the absence of laser irradiation. The CCK-8 and live/dead assays were then performed to evaluate therapeutic efficacy in vitro (Fig. 5e). For the cells treated with SnS and irradiated with laser light, more than 45% of cells survived at the 40 ppm concentration. For the cells treated with SnS-PEG-FA-DOX without laser irradiation, 60% were killed. Compared with chemotherapy alone (SnS-PEG-FA-DOX in the dark), and PTT with SnS under 808 nm laser irradiation, the combined dual treatment of SDTNPs offers the most effective cancer cell killing, where nearly all of the cells were killed. This demonstrates the tumor cell killing efficacy of the synergistic photothermal- and chemo-therapy of SDTNPs (Fig. 5e).
3.5. In vivo tumor eradication
Based on the in vitro therapeutic effects observed, we carried out an in vivo antitumor evaluation to validate the enhanced therapeutic effects of the SDTNPs. Firstly, we studied their in vivo photo-thermal response. After intratumoral injection of PBS and SnS-PEG NSs, respectively, animal NIR images and ΔT were monitored using a thermal camera over a 5 min irradiation period. ΔT of the PBS treated mice was only ∼5 °C, while that for the SnS-PEG NS treated animals was more than 23 °C, reaching more significant temperature increase throughout the irradiation period (Fig. 6b and c). Based on the in vivo photothermal effect, we performed an in vivo antitumor evaluation to validate the enhanced therapy of cancer. The SMCC-7721 tumor-bearing nude mice were treated with Group 1: saline (control), Group 2: laser irradiation only, Group 3: SnS-PEG NSs with laser irradiation, Group 4: SnS-PEG-FA-DOX without laser irradiation, and Group 5: SnS-EPG-FA-DOX with laser irradiation. The tumor volumes were calculated by measuring the width and length every two days. At the end of the experiment, all nude mice were euthanized and tumors were collected. In line with the results of the tumor growth curve shown in Fig. 6d, Group 3 showed decreasing tumor growth indicating the promising application of SnS NSs as photothermal agents. Moreover, a significant therapeutic effect could be found in Group 5, demonstrating that an enhanced antitumor effect could be achieved using the combined therapy strategy of SDTNPs. The body weight of the nude mice was not found to be significantly affected by treatment, demonstrating that the combined therapy had no acute side effects (Fig. 6e). To further evaluate the in vivo toxicity of SnS NSs, the major organs of the mice were dissected and stained using hematoxylin and eosin (H&E) for histological analysis. As shown in Fig. 6f, the treated mice, which were euthanized two weeks after the injection of SnS-PEG-FA-DOX with NIR irradiation, exhibited no significant damage to normal tissues, including the heart, liver, spleen, lung, and kidney. This indicates that the SDTNP treatment had no observable side effects or toxicity towards healthy tissue.4. Conclusion
In summary, the treatment of cancer cells and tumors using SDTNPs, including photothermal- and chemo-therapy, was demonstrated. Apart from exhibiting excellent biocompatibility, SDTNPs also showed high photothermal performance with a photothermal conversion efficiency of 39.3% at 808 nm. Furthermore, SnS NSs showed stable photothermal performance over short periods of laser irradiation (2 h) whilst demonstrating photodegradability over a longer exposure period. As a drug carrier, SnS NSs can accommodate a high DOX/SnS NS ratio of ∼200% (w/w%) due to their wrinkled structure, similar to BP. Consequently, SnS NSs composed of only a few layers can be developed as both a high efficiency and stable photothermal agent, and efficient drug carrier with degradable characteristics.Furthermore, we demonstrated the efficacy of SDTNPs in several carcinoma cell lines as well as in nude mice subcutaneous xenograft tumors. In in vitro experiments, SDTNPs showed an enhanced therapeutic effect through the combination of photothermal- and chemo-therapy. Moreover, this enhanced therapeutic effect was also demonstrated in vivo, and no observable side effects or toxicity was detected in healthy tissue. Consequently, 2D SnS NSs, a BP-analogue binary IV–VI compound, have been demonstrated to be an innovative 2D nano-platform for photothermal therapy and theranostic drug delivery for the treatment of tumors. This may pave the way for the biomedical application of other IV–VI compounds, such as GeS and SnSe, due to their low cost and biocompatibility.
Compared with traditional treatments, this dual therapy nano-platform based on SnS NSs is a green and efficient approach, using low power laser irradiation due to its safety, limited side effects and no trauma; and using SnS NSs due to their high efficiency photothermal and drug loading features, and photodegradability. Its clinical application could have a significant effect on the future treatment of cancer.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The research is partially supported by the National Natural Science Fund (Grant No. 61435010, 61575089 and 81470868), Science and Technology Innovation Commission of Shenzhen (KQTD2015032416270385 and JCYJ20150625103619275), the Science and Technology Planning Project of Guangdong Province (Grant No. 2016B050501005), the Educational Commission of Guangdong Province (Grant No. 2016KCXTD006) and the China Postdoctoral Science Foundation (Grant No. 2017M612730). We thank Sarah Dodds, PhD, from Liwen Bianji, Edanz Editing China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef PubMed.
- Q. H. Wang, K. Kalantarzadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. Mcdonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2017, 112, 724–781 CrossRef PubMed.
- J. Xue, J. Sanchez-Yamagishi, D. Bulmash, P. Jacquod, A. Deshpande, K. Watanabe, T. Taniguchi, P. Jarillo-Herrero and B. J. Leroy, Nat. Mater., 2011, 10, 282–285 CrossRef PubMed.
- H. Xu, J. Gao and D. Jiang, Nat. Chem., 2015, 7, 905 CrossRef PubMed.
- A. I. Khan and D. O’Hare, ChemInform, 2002, 12, 3191–3198 Search PubMed.
- H. Wang, X. Yang, W. Shao, S. Chen, J. Xie, X. Zhang, J. Wang and Y. Xie, J. Am. Chem. Soc., 2015, 137, 11376–11382 CrossRef PubMed.
- Z. Sun, H. Xie, S. Tang, X. F. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., 2015, 54, 11526 CrossRef PubMed.
- L. Li, Y. Yu, G. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef PubMed.
- W. Tao, X. Zhu, X. Yu, X. Zeng, Q. Xiao, X. Zhang, X. Ji, X. Wang, J. Shi, H. Zhang and L. Mei, Adv. Mater., 2017, 29, 1–9 Search PubMed.
- W. Chen, J. Ouyang, H. Liu, M. Chen, K. Zeng, J. Sheng, Z. Liu, Y. Han, L. Wang, J. Li, L. Deng, Y. N. Liu and S. Guo, Adv. Mater., 2017, 29, 1–7 Search PubMed.
- Z. Qin and J. C. Bischof, Chem. Soc. Rev., 2012, 41, 1191–1217 RSC.
- R. Bardhan, S. Lal, A. Joshi and N. J. Halas, Acc. Chem. Res., 2011, 44, 936–946 CrossRef PubMed.
- C. Liang, S. Diao, C. Wang, H. Gong, T. Liu, G. Hong, X. Shi, H. Dai and Z. Liu, Adv. Mater., 2014, 26, 5646–5652 CrossRef.
- M. Li, X. Yang, J. Ren, K. Qu and X. Qu, Adv. Mater., 2012, 24, 1621 CrossRef.
- S. S. Chou, B. Kaehr, J. Kim, B. M. Foley, M. De, P. E. Hopkins, J. Huang, C. J. Brinker and V. P. Dravid, Angew. Chem., 2013, 52, 4160–4164 CrossRef PubMed.
- J. Shao, H. Xie, H. Huang, Z. Li, Z. Sun, Y. Xu, Q. Xiao, X. F. Yu, Y. Zhao and H. Zhang, Nat. Commun., 2016, 7, 12967 CrossRef PubMed.
- X. Huang, S. Tang, B. Liu, B. Ren and N. Zheng, Adv. Mater., 2011, 23, 3420 CrossRef PubMed.
- W. Tao, X. Ji, X. Xu, M. A. Islam, Z. Li, S. Chen, P. E. Saw, H. Zhang, Z. Bharwani, Z. Guo, J. Shi and O. C. Farokhzad, Angew. Chem., 2017, 11896–11900 CrossRef PubMed.
- R. Li, L. Zhang, L. Shi and P. Wang, ACS Nano, 2017, 11, 3752–3759 CrossRef PubMed.
- J. Xuan, Z. Wang, Y. Chen, D. Liang, L. Cheng, X. Yang, Z. Liu, R. Ma, T. Sasaki and F. Geng, Angew. Chem., 2016, 55, 14569 CrossRef PubMed.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384 CrossRef PubMed.
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681 RSC.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef PubMed.
- H. Lin, Y. Wang, S. Gao, Y. Chen and J. Shi, Adv. Mater., 2018, 30, 1–11 Search PubMed.
- F. Xia, H. Wang and Y. Jia, Nat. Commun., 2014, 5, 4458 CrossRef PubMed.
- A. Castellanosgomez, L. Vicarelli, E. Prada, J. O. Island, K. L. Narasimhaacharya, S. I. Blanter, D. J. Groenendijk, M. Buscema, G. A. Steele and J. V. Alvarez, 2D Mater., 2014, 1, 25001 CrossRef.
- X. Ling, H. Wang, S. Huang, F. Xia and M. S. Dresselhaus, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4523–4530 CrossRef PubMed.
- C. Sun, L. Wen, J. Zeng, Y. Wang, Q. Sun, L. Deng, C. Zhao and Z. Li, Biomaterials, 2016, 91, 81 CrossRef PubMed.
- C. Xing, G. Jing, X. Liang, M. Qiu, Z. Li, R. Cao, X. Li, D. Fan and H. Zhang, Nanoscale, 2017, 9, 8096–8101 RSC.
- J. Kim, S. S. Baik, S. H. Ryu, Y. Sohn, S. Park, B.-G. Park, J. Denlinger, Y. Yi, H. J. Choi and K. S. Kim, Science, 2015, 349, 723–726 CrossRef PubMed.
- J. W. Jiang, Nanotechnology, 2015, 26, 365702 CrossRef PubMed.
- C. Xin, J. Zheng, Y. Su, S. Li, B. Zhang, Y. Feng and F. Pan, J. Phys. Chem. C, 2016, 120, 22663–22669 CrossRef.
- S. M. Herron, J. T. Tanskanen, K. E. Roelofs and S. F. Bent, Chem. Mater., 2014, 26, 7106–7113 CrossRef.
- R. E. Banai, L. A. Burton, S. G. Choi, F. Hofherr, T. Sorgenfrei, A. Walsh, B. To, A. Cröll and J. R. S. Brownson, J. Appl. Phys., 2014, 116, 7363 CrossRef.
- N. K. Reddy, M. Devika and E. S. R. Gopal, Crit. Rev. Solid State Mater. Sci., 2015, 40, 1–40 CrossRef.
- J. R. Brent, D. J. Lewis, T. Lorenz, E. A. Lewis, N. Savjani, S. J. Haigh, G. Seifert, B. Derby and P. O’Brien, J. Am. Chem. Soc., 2015, 137, 12689 CrossRef PubMed.
- D. Avellaneda, M. T. S. Nair and P. K. Nair, Thin Solid Films, 2009, 517, 2500–2502 CrossRef.
- S. V. De Azevedo, F. R. Moreira and R. C. Campos, Clin. Biochem., 2013, 46, 123 CrossRef PubMed.
- K. A. Winship, Adverse Drug React. Acute Poisoning Rev., 1988, 7, 19–38 Search PubMed.
- M. Cagnoli, A. Alama, F. Barbieri, F. Novelli, C. Bruzzo and F. Sparatore, Anticancer Drugs, 1998, 9, 603 CrossRef PubMed.
- S. Tang, C. Peng, J. Xu, B. Du, Q. Wang, R. D. Vinluan III, M. Yu, M. J. Kim and J. Zheng, Angew. Chem., 2016, 128, 16039–16043 CrossRef PubMed.
- J. Chao, Z. Wang, X. Xu, Q. Xiang, W. Song, G. Chen, J. Hu and D. Chen, RSC Adv., 2012, 3, 2746–2753 RSC.
- A. M. Tripathi and S. Mitra, RSC Adv., 2014, 4, 10358–10366 RSC.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, D. Vinh and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu and H. Xing, Adv. Mater., 2014, 26, 1886–1893 CrossRef PubMed.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636 CrossRef PubMed.
- B. Wang, J. H. Wang, Q. Liu, H. Huang, M. Chen, K. Li, C. Li, X. F. Yu and P. K. Chu, Biomaterials, 2014, 35, 1954–1966 CrossRef PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef PubMed.
- Z. Huang, Y. Qi, D. Yu and J. Zhan, RSC Adv., 2016, 6, 31031–31036 RSC.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876–10877 CrossRef PubMed.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Shi, L. Feng, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433–3440 CrossRef PubMed.
- M. P. Melancon, W. Lu, Z. Yang, R. Zhang, Z. Cheng, A. M. Elliot, J. Stafford, T. Olson, J. Z. Zhang and C. Li, Mol. Cancer Ther., 2008, 7, 1730 CrossRef PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560 CrossRef PubMed.
- W. Tao, J. Zhang, X. Zeng, D. Liu, G. Liu, X. Zhu, Y. Liu, Q. Yu, L. Huang and L. Mei, Adv. Healthcare Mater., 2015, 4, 1203–1214 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The stability of SnS NSs; the fluorescence of FITC and DOX; and the photodynamic study of SnS NSs. See DOI: 10.1039/c8tb00729b |
| ‡ Z. Xie, D. Wang and T. Fan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |