Enhanced hydrogen production performance through controllable redox exsolution within CoFeAlOx spinel oxygen carrier materials†
Abstract
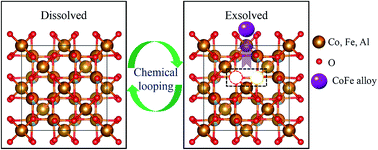
The high reaction temperature required for chemical looping in water splitting presents challenges in terms of producing stable oxygen carrier materials (OCMs). The currently available materials are generally prepared by a deposition method through which iron oxides and refractory supports are spatially separated, thus having low activity and stability. Here, we report CoFeAlOx as an OCM, in which the active phase of the CoFe alloy and the parent spinel support are homogeneously mixed into a solid solution. Using a variety of characterization techniques, we studied the exsolution and dissolution effects of the CoFe alloy on the spinel support in a chemical looping redox cycle and found that the exsolution process can be tailored by the reduction level, which determines the interface structure. When reduced by CO, the exsolved particles were similar to the deposited analogues, showing obvious sintering. However, for the sample reduced by CO and CO2, the exsolved CoFe alloy can be embedded into the support, with it re-emerging fresh and confined at each reduction period. After 20 cycles, it showed a high hydrogen production rate and outstanding stability, demonstrating that the controllable exsolution could significantly improve the high temperature redox performance. Based on these results, this study also provides a new dimension for designing improved redox materials for chemical looping, calcium looping, or solar-driven thermochemical applications.


 Please wait while we load your content...
Please wait while we load your content...