Promoting magnesium sulfite oxidation via partly oxidized metal nanoparticles on graphitic carbon nitride (g-C3N4) in the magnesia desulfurization process†
Abstract
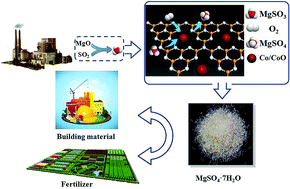
Sulfur dioxide (SO2) emission has become a crucial problem, especially in developing countries since it is one of the main reasons for the formation of acid precipitation and the secondary formation of fine particulate matter (PM2.5). Wet magnesia desulfurization stands out as a widely used practical approach for the control of SO2 due to its high removal efficiency, low operating cost and potential valuable by-products. However, the effluent of magnesium sulfite (main by-product) can reemit SO2 and cause water pollution. Thus, it is important, but challenging, to enhance the oxidation rate to improve the utilization of MgSO4 for sustainable resources and meanwhile downsize the absorbing tower. Herein, we report a series of graphitic carbon nitride (g-C3N4)-assembled transition metal catalysts (M–C3N4) for the first time via a facile and environmentally friendly synthetic route as a new generation of catalysts for the oxidation of magnesium sulfite. We have performed both theoretical evaluation and deep experimental validation of the series of M–C3N4 materials, and in particular, the cobalt–C3N4 material since it possesses the best catalytic activity reported to date, which is comparable to that of aqueous precious metal benchmarks but only with a 2.4 wt% cobalt loading. Additionally, the ability to recycle the Co–C3N4 complex is investigated to facilitate its practical application. This fabrication method is expected to provide a new concept for the construction of multifunctional nanocomposites for a large variety of industrially relevant desulfurization applications.


 Please wait while we load your content...
Please wait while we load your content...