 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Charge polarization, local electroneutrality breakdown and eddy formation due to electroosmosis in varying-section channels†
Mauro
Chinappi
 *a and
Paolo
Malgaretti
*a and
Paolo
Malgaretti
 *bc
*bc
aDipartimento di Ingegneria Industriale, Università di Roma Tor Vergata, via del Politecnico 1, 00133 Roma, Italia. E-mail: mauro.chinappi@uniroma2.it
bMax-Planck-Institut für Intelligente Systeme, Heisenbergstr. 3, D-70569 Stuttgart, Germany. E-mail: malgaretti@is.mpg.de
cIV Institute for Theoretical Physics, University of Stuttgart, Pfaffenwaldring 57, 70569 Stuttgart, Germany
First published on 1st November 2018
Abstract
We characterize the dynamics of an electrolyte embedded in a varying-section channel under the action of a constant external electrostatic field. By means of molecular dynamics simulations we determine the stationary density, charge and velocity profiles of the electrolyte. Our results show that when the Debye length is comparable to the width of the channel bottlenecks a concentration polarization along with two eddies sets inside the channel. Interestingly, upon increasing the external field, local electroneutrality breaks down and charge polarization sets leading to the onset of net dipolar field. This novel scenario, that cannot be captured by the standard approaches based on local electroneutrality, opens the route for the realization of novel micro and nano-fluidic devices.
The transport of ions, molecules and polymers across constrictions such as pores, membranes or varying-section micro- nano-channels is crucial for several biological as well as synthetic systems. For example, in biological cells ion channels control the uptake of ions from the environment1 whereas, in resistive pulse sensing techniques, the interactions of colloidal particles2 or macromolecules3–7 with the nano- or micro-pore are measured from the variation of the electric conductance of the pore induced by the presence of the particle. Moreover, electro-osmotic flux can play a crucial role in molecule capturing in nanopores.8–10 Recent studies have shown that ionic transport and electro-osmotic flow in micro- and nano-fluidic circuitry can be controlled by tuning the geometry of the micro–nano-channel.11–13 Indeed, conical14–16 or heterogeneously charged17 pores have been used to realize nano-fluidic diodes and to rectify electro-osmotic flows.18,19 Moreover, periodic varying-section channels have been used to realize nanofluidic transistors.20 Similarly, recent contributions have shown that the shape of the confining vessel and the boundary conditions therein imposed can be exploited to control the flow. Indeed, electro-osmotic transport can be strongly enhanced by grafting charged brushes on the channel walls21 and by hydrophobic surfaces.22
In this contribution, we study, via molecular dynamics simulations the electro-osmotic flow of an electrolyte embedded in a varying-section channel. The advantage of our approach, as compared to others based on the solution of some continuum models,23–27 is that the ionic densities are left free to relax according to the interactions among ions and between ions and the channel walls. Therefore our approach allows us to critically discuss, for example, the local electroneutrality assumption and its possible breakdown. Our results show that when the systems is driven by a constant electrostatic field acting along the longitudinal axis of the channel, inhomogeneous ionic and charge densities are induced due to the variations of local channel section. This phenomenon is similar to concentration polarization (CP) reported for electrolytes transported across ionic-selective membranes23,25,28 (see also ref. 29 and 30 for recent reviews) i.e., in open circuit conditions and under severe modulations of channel section.25,27,28 Interestingly, our results show that CP can be obtained also for close circuit conditions and for smooth variations of channel section. In particular, we observe CP when the Debye length is comparable with the channel bottlenecks, i.e., in the entropic electrokinetic regime.31 Concerning local electroneutrality, our numerical results confirm that even in the entropic electrokinetic regime, the local electroneutrality assumed by previous works26,27,30,32 is fulfilled for mild values of the external field. However, upon increasing the external field our numerical simulations show that local electroneutrality breaks down and charge polarization (QP) sets, leading to the onset of a net dipolar contribution to the electrostatic field. Interestingly, a similar phenomenon has been recently observed for pressure-driven flows across conical pores.33
Results
We study the electro-osmotic flow (see ESI† for the details) of a KCl water solution across a channel with half sectionh(x) = h0 + h1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(2πx/Lx) cos(2πx/Lx) | (1) |
We begin our analysis by focusing on the case of larger ionic concentration ρ0 ≃ 3 M. The density profile of both Cl− and K+ is expected to decay over a length scale comparable to the Debye length,  , where e is the elementary charge, z is the valence of the ions (z = 1, in our case), β = (kBT)−1 with kB the Boltzmann constant and T the absolute temperature and ε = 80·ε0 is the dielectric constant with ε0 the vacuum dielectric constant. For 3 M ionic solution we estimate a Debye length of λD ≃ 1.8 Å and therefore λD ≪ hmin being hmin = h0 − h1 = 6 Å the half-section calculated at channel bottlenecks. In such a case, since there is only a small overlap between the Debye layers of the two facing walls, we do not expect the onset of any entropic electrokinetic effects.31 As expected, the accumulation of Cl− ions at the positively charged solid wall induces and electro-osmotic flow, opposed to the direction of the external electrostatic field, that is almost symmetric with respect to the z-axis (see panels (a and b) of Fig. 2).
, where e is the elementary charge, z is the valence of the ions (z = 1, in our case), β = (kBT)−1 with kB the Boltzmann constant and T the absolute temperature and ε = 80·ε0 is the dielectric constant with ε0 the vacuum dielectric constant. For 3 M ionic solution we estimate a Debye length of λD ≃ 1.8 Å and therefore λD ≪ hmin being hmin = h0 − h1 = 6 Å the half-section calculated at channel bottlenecks. In such a case, since there is only a small overlap between the Debye layers of the two facing walls, we do not expect the onset of any entropic electrokinetic effects.31 As expected, the accumulation of Cl− ions at the positively charged solid wall induces and electro-osmotic flow, opposed to the direction of the external electrostatic field, that is almost symmetric with respect to the z-axis (see panels (a and b) of Fig. 2).
We have then increased the Debye length by reducing the ionic concentration ρ0. For ρ0 ∼ 1 M we have λD ≃ 3.1 Å. Hence, the Debye layers of the facing walls overlaps in the narrower sections (λD/hmin ∼ 1, hmin = 6 Å) highlighting that the system is within the entropic electrokinetic regime.31 Panel (d) of Fig. 2 shows that, for ΔV = 2.4 V, the ionic densities are quite affected by the flow. In particular, Cl− concentration largely increases in the channel bottleneck, see e.g. the region x/Lx ∈ (0.45, 0.5) in Fig. 2d. This increase in Cl− concentration is associated to K+ depletion, that will be discussed more in details in next paragraphs. This feature is associated to the onset of eddies in the electro-osmotic velocity profile, as shown in panels (c and d) of Fig. 2. These eddies form for sufficiently large driving forces. Indeed, while for ΔV = 0.6 V we do not observe major discrepancies with the previous case, for ΔV = 2.4 V two eddies form inside the channel. Interestingly, such eddies break down the left-right symmetry of the channel, for instance, Fig. 2d, shows that the eddies are shifted in the direction of the volumetric fluid flow, i.e. negative x in our reference frame. This occurrence is in contrast to the prediction obtained in linear regime31 for which the eddies center is in the channel center, x/Lx = 0. Then we further increase the Debye length by setting ρ0 ∼ 0.3 M, for which λD ≃ 5.6 Å. In such a regime panels (e and f) of Fig. 2 show that the zone in which K+ are depleted is enhanced as compared to the previous cases. Moreover, comparing panels (e and f) to (c and d) in Fig. 2 we notice that, for ρ0 ∼ 0.3 M, the onset of the eddies occurs for smaller values of the external force as compared to ρ0 ∼ 1 M.
In order to quantitatively capture the accumulation of ions density, we have analyzed the dependence of the ionic densities averaged over the channel section as a function of the longitudinal position. Fig. 3 shows that the dependence of normalized densities profiles ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) ±(x) defined as
±(x) defined as
 | (2) |
q(x) ≡ 2h(x)[![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) +(x) − +(x) − ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) −(x)] −(x)] | (3) |
![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (x) ≡ (x) ≡ ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) +(x) + +(x) + ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) −(x) −(x) | (4) |
![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (x) so that in a plane channel |q(x)| always match the total surface charge of channel walls 2|σ| whereas
(x) so that in a plane channel |q(x)| always match the total surface charge of channel walls 2|σ| whereas ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (x) is the average density that, in the Debye–Hückel regime, determines the local value of the Debye length.‡ Interestingly, Fig. 4a shows that in the limit of small Debye lengths, λD/hmin < 1, local electroneutrality is recovered, i.e. q(x) ≃ 2σ, and the ionic density profile is almost constant along the channel. Only at very high voltages a small deviation is present at x/Lx ≃ 0.4.
(x) is the average density that, in the Debye–Hückel regime, determines the local value of the Debye length.‡ Interestingly, Fig. 4a shows that in the limit of small Debye lengths, λD/hmin < 1, local electroneutrality is recovered, i.e. q(x) ≃ 2σ, and the ionic density profile is almost constant along the channel. Only at very high voltages a small deviation is present at x/Lx ≃ 0.4.
 | ||
Fig. 3
![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) +(x) (blue) and
+(x) (blue) and ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) −(x) (red) curves as a function of the longitudinal position for ρ = 3 M (panel a), ρ = 1 M (panel b) and ρ = 0.3 M (panel c). −(x) (red) curves as a function of the longitudinal position for ρ = 3 M (panel a), ρ = 1 M (panel b) and ρ = 0.3 M (panel c). ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) +(x) and +(x) and ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) −(x) are calculated viaeqn (2). −(x) are calculated viaeqn (2). | ||
 | ||
Fig. 4
![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (x) (red curves) and q(x) (blue curves), defined respectively in eqn (3) and (4), as a function of the longitudinal position for ρ = 3 M (panel a), ρ = 1 M (panel b) and ρ = 0.3 M (panel c). Lighter color stand for large values of the potential drop ΔV, see legend. (x) (red curves) and q(x) (blue curves), defined respectively in eqn (3) and (4), as a function of the longitudinal position for ρ = 3 M (panel a), ρ = 1 M (panel b) and ρ = 0.3 M (panel c). Lighter color stand for large values of the potential drop ΔV, see legend. | ||
In contrast, for larger values of the Debye length, λD/hmin ≃ 1, neither q(x) nor ![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (x) are constant. Such inhomogeneities trigger the onset of modulations in the magnitude of the local electrostatic potential. In particular, an accurate inspection of panels (b and c) of Fig. 4 reveals that weaker values of the external field triggers solely an inhomogeneity in the ionic density but do not affect the local electroneutrality. Interestingly, upon increasing the strength of external electric field local electroneutrality breaks down and on the top of the well-known concentration polarization (CP),26,27,30,32,34 a charge polarization (QP) appears. We remark that charge polarization sets for smaller values of the external force for smaller values of the Debye length. This can be due to finite liquid slippage at solid wall that cannot be disregarded in the regime under study. Indeed, slippage is commonly described in term of the Navier boundary condition that, for a plane channel, reads ut|wall = Ls∂ut/∂n with ut the component of the velocity field tangent to the wall, n the normal to the wall and Ls the slip length.35 Atomistic simulation showed that for smooth hydrophilic and slightly hydrophobic (contact angle <120°) surfaces Ls, hardly exceeds a nanometer.36–38 In addition, the presence of a strong surface charge, further reduces the slip length for hydrophobic surfaces.39 When comparing simulations performed with different ionic concentrations it should be taken into account that the slip length Ls in the three setups may be slightly different. Indeed, the relevant parameter ruling the effect of slippage on the electroosmotic flow is the ratio Ls/λD.40 This feature is emphasized by Fig. S1 in ESI† that shows that the mismatch between the prediction of the analytical model (see ref. 31) and the numerical results increases upon decreasing λD.
(x) are constant. Such inhomogeneities trigger the onset of modulations in the magnitude of the local electrostatic potential. In particular, an accurate inspection of panels (b and c) of Fig. 4 reveals that weaker values of the external field triggers solely an inhomogeneity in the ionic density but do not affect the local electroneutrality. Interestingly, upon increasing the strength of external electric field local electroneutrality breaks down and on the top of the well-known concentration polarization (CP),26,27,30,32,34 a charge polarization (QP) appears. We remark that charge polarization sets for smaller values of the external force for smaller values of the Debye length. This can be due to finite liquid slippage at solid wall that cannot be disregarded in the regime under study. Indeed, slippage is commonly described in term of the Navier boundary condition that, for a plane channel, reads ut|wall = Ls∂ut/∂n with ut the component of the velocity field tangent to the wall, n the normal to the wall and Ls the slip length.35 Atomistic simulation showed that for smooth hydrophilic and slightly hydrophobic (contact angle <120°) surfaces Ls, hardly exceeds a nanometer.36–38 In addition, the presence of a strong surface charge, further reduces the slip length for hydrophobic surfaces.39 When comparing simulations performed with different ionic concentrations it should be taken into account that the slip length Ls in the three setups may be slightly different. Indeed, the relevant parameter ruling the effect of slippage on the electroosmotic flow is the ratio Ls/λD.40 This feature is emphasized by Fig. S1 in ESI† that shows that the mismatch between the prediction of the analytical model (see ref. 31) and the numerical results increases upon decreasing λD.
In conclusion, we have reported on numerical simulations concerning a KCl solution embedded in a varying-section channel under the action of a constant electrostatic field. Our simulations show that, when the Debye length is comparable to the width of the channel bottlenecks, the system is in the entropic electrokinetic regime that is characterized by the onset of eddies.31 In this perspective we observe, in agreement with what has been reported in the literature, the onset of a concentration polarization and local recirculation of the fluid velocity that comes along with the onset of a standing shock in the ionic concentration. Surprisingly, for stronger external fields the local electroneutrality breaks down and an additional charge polarization (QP) sets in. Such a novel phenomena has been observed thanks to our microscopic approach based on Molecular Dynamics simulations in which the ionic densities are not constrained. In this perspective, our results show that for mild external fields local electroneutrality is recovered. This can justify a posteriori the assumption of local electroneutrality in these regimes. However, for larger external fields, local electroneutrality does not hold and a net electric dipole sets in inside the channel.
Conflicts of interest
There are no conflicts to declareAcknowledgements
PM acknowledges Dr Mathijs Janssen for useful discussions. This research used the computational resource from CINECA (NATWE project), and the Swiss National Super-computing Centre (CSCS), project ID sm11. Open Access funding provided by the Max Planck Society.References
- B. Hille, Ion channels of excitable membranes, Sinauer, Sunderland, MA, 2001, vol. 507 Search PubMed.
- E. Weatherall and G. R. Willmott, Analyst, 2015, 140, 3318 RSC.
- H. Bayley, O. Braha and L.-Q. Gu, Adv. Mater., 2000, 12, 139 CrossRef CAS.
- G. Celaya, J. Perales-Calvo, A. Muga, F. Moro and D. Rodriguez-Larrea, ACS Nano, 2017, 11, 5815–5825 CrossRef CAS PubMed.
- E. L. Bonome, R. Lepore, D. Raimondo, F. Cecconi, A. Tramontano and M. Chinappi, J. Phys. Chem. B, 2015, 119, 5815 CrossRef CAS PubMed.
- A. Asandei, A. Ciuca, A. Apetrei, I. Schiopu, L. Mereuta, C. H. Seo, Y. Park and T. Luchian, Sci. Rep., 2017, 7, 6167 CrossRef PubMed.
- M. Chinappi and F. Cecconi, J. Phys.: Condens. Matter, 2018, 30 DOI:10.1088/1361-648X/aababe.
- A. Asandei, I. Schiopu, M. Chinappi, C. H. Seo, Y. Park and T. Luchian, ACS Appl. Mater. Interfaces, 2016, 8, 13166 CrossRef CAS PubMed.
- M. Boukhet, F. Piguet, H. Ouldali, M. Pastoriza-Gallego, J. Pelta and A. Oukhaled, Nanoscale, 2016, 8, 18352–18359 RSC.
- G. Huang, K. Willems, M. Soskine, C. Wloka and G. Maglia, Nat. Commun., 2017, 8, 935 CrossRef PubMed.
- P. Malgaretti, I. Pagonabarraga and J. M. Rubi, Macromol. Symp., 2015, 357, 178 CrossRef CAS.
- P. Malgaretti, I. Pagonabarraga and J. M. Rubi, J. Chem. Phys., 2016, 144, 034901 CrossRef PubMed.
- A. Bolet, G. Linga and J. Mathiesen, Phys. Rev. E, 2018, 97, 043114 CrossRef PubMed.
- W. Guo, Y. Tian and L. Jiang, Acc. Chem. Res., 2013, 46, 2834 CrossRef CAS PubMed.
- S. Balme, T. Ma, E. Balanzat and J.-M. Janot, J. Membr. Sci., 2017, 544, 18 CrossRef CAS.
- J. Experton, X. Wu and C. R. Martin, Nanomaterials, 2017, 7, 445 CrossRef PubMed.
- C. B. Picallo, S. Gravelle, L. Joly, E. Charlaix and L. Bocquet, Phys. Rev. Lett., 2013, 111, 244501 CrossRef PubMed.
- N. Laohakunakorn and U. F. Keyser, Nanotechnology, 2015, 26, 275202 CrossRef PubMed.
- P. Bacchin, Membranes, 2018, 8, 10 CrossRef PubMed.
- E. Kalman, I. Vlassiouk and Z. Siwy, Adv. Mater., 2008, 20, 293 CrossRef CAS.
- G. Chen and S. Das, J. Phys. Chem. B, 2017, 121, 3130 CrossRef CAS PubMed.
- S. R. Maduar, A. V. Belyaev, V. Lobaskin and O. I. Vinogradova, Phys. Rev. Lett., 2015, 114, 118301 CrossRef CAS PubMed.
- I. Rubinstein and B. Zaltzman, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 2238 CrossRef CAS.
- S. Y. Park, C. J. Russo, D. Branton and H. A. Stone, J. Colloid Interface Sci., 2006, 297, 832 CrossRef CAS PubMed.
- A. Mani, T. A. Zangle and J. G. Santiago, Langmuir, 2009, 25, 3898 CrossRef CAS PubMed.
- A. Mani and M. Bazant, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 061504 CrossRef PubMed.
- Y. Green, S. Shloush and G. Yossifon, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89, 043015 CrossRef PubMed.
- T. A. Zangle, A. Mani and J. G. Santiago, Langmuir, 2009, 25, 3909 CrossRef CAS PubMed.
- H.-C. Chang, G. Yossifon and E. A. Demekhin, Annu. Rev. Fluid Mech., 2012, 44, 401 CrossRef.
- V. V. Nikonenko, A. V. Kovalenko, M. K. Urtenov, N. D. Pismenskaya, J. Han, P. Sistat and G. Pourcelly, Desalination, 2014, 342, 85 CrossRef CAS.
- P. Malgaretti, I. Pagonabarraga and J. M. Rubi, Phys. Rev. Lett., 2014, 113, 128301 CrossRef PubMed.
- H. Leese and D. Mattia, Microfluid. Nanofluid., 2014, 16, 711 CrossRef CAS.
- L. Jubin, A. Poggioli, A. Siria and L. Bocquet, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 4063 CrossRef CAS PubMed.
- M. Andersen, K. Wang, J. Schiffbauer and A. Mani, Electrophoresis, 2017, 38, 702 CrossRef CAS PubMed.
- E. Lauga, M. Brenner and H. Stone, Springer handbook of experimental fluid mechanics, Springer, 2007, pp. 1219–1240 Search PubMed.
- M. Chinappi and C. Casciola, Phys. Fluids, 2010, 22, 042003 CrossRef.
- D. M. Huang, C. Sendner, D. Horinek, R. R. Netz and L. Bocquet, Phys. Rev. Lett., 2008, 101, 226101 CrossRef PubMed.
- M. Sega, M. Sbragaglia, L. Biferale and S. Succi, Soft Matter, 2013, 9, 8526–8531 RSC.
- D. M. Huang, C. Cottin-Bizonne, C. Ybert and L. Bocquet, Langmuir, 2008, 24, 1442–1450 CrossRef CAS PubMed.
- L. Bocquet and E. Charlaix, Chem. Soc. Rev., 2010, 39, 1073–1095 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sm01298a |
‡ We stress that according to eqn (4) we have that  where where 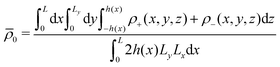 is the density averaged over the full volume. is the density averaged over the full volume. |
| This journal is © The Royal Society of Chemistry 2018 |



