A simple, solvent free method for transforming bio-derived aldehydes into cyclic acetals for renewable diesel fuels†
Orion
Staples
a,
Cameron M.
Moore
*a,
Juan H.
Leal
b,
Troy A.
Semelsberger
b,
Charles S.
McEnally
c,
Lisa D.
Pfefferle
c and
Andrew D.
Sutton
 *a
*a
aChemistry Division, Los Alamos National Laboratory, MS K558, Los Alamos, NM 87545, USA. E-mail: cameron_moore@lanl.gov; adsutton@lanl.gov
bMaterials Physics Applications Division, Los Alamos National Laboratory, MS K793, Los Alamos, NM 87545, USA
cDepartment of Chemical and Environmental Engineering, Yale University, New Haven, CT 06520, USA
First published on 24th September 2018
Abstract
The acetalization of 2,3-butanediol with bio-derived C4–8 aldehydes has yielded a route to substituted 1,3-dioxolanes from small bio-building blocks. The reported reaction system features excellent carbon yields (>93%), atom economy (>89%) and phase separation of the analytically pure product which eliminates elaborate purification processes and facilitates simple catalyst recycling. The 1,3-dioxolanes offer performance advantages over traditional diesel and have the potential to augment petroleum derived fuels.
Significant efforts have been focused on developing new methods to convert non-food biomass feedstocks1 into suitable liquid fuels that meet or exceed the performance and physical properties of gasoline, diesel and/or jet fuels.2 This approach aims to supplant single use carbon with renewable carbon that can quickly be reincorporated into the carbon cycle. Biodiesel has been employed3 since the advent of the diesel engine and traditionally refers to the transesterification products of animal fats, vegetable oils, or recycled restaurant grease with methanol to produce fatty acid methyl ester (FAME) fuels and glycerol.4 These biodiesels have some favourable properties when compared to traditional diesel including significant reduction in aromatic emissions, increased lubricity, and increased cetane number (a measure of the ignitability of a fuel in a compression ignition engine).4–6 Another aspect of these biofuels is the presence of oxygen-containing functional groups;5 in contrast to petroleum diesel which contains <0.6 wt% oxygen.7 This offers a significant benefit for current biofuels over petroleum diesel, as the oxygen helps increase combustion efficiency, reduce NOx emissions and produce significantly less particulate matter.5–7 While these properties encourage further development of oxygenated biodiesel, there are several drawbacks that limit their extensive application. These include a much higher kinematic viscosity than petroleum diesel and a high freezing point, both of which render these fuels less likely to be suitable for cold weather applications.8 To circumvent these issues, significant efforts have been focused on producing advanced renewable diesel fuels which have improved performance over traditional biodiesel.1,2
We have been interested in utilizing ethanol as a bio-derived building block for fuel production. This is due to the high volume and growth of global ethanol production (2.65 × 1010 U.S. gal in 2016) versus its limited applicability in road transport fuels by the current “blend wall” which imposes limits (typically 10–15%) on the volume that is allowed to be blended with gasoline.9 Recently, we described a method by which C4–8 aldehydes could be produced from the condensation of acetaldehyde, the dehydrogenation product of ethanol.10 While there are many currently employed routes to produce aldehydes, including the hydroformylation of propene to generate butyraldehyde11 or the oxidation of alcohols,12 these routes, however, often rely on hydrocarbons derived from petroleum (i.e. propene). Other efforts have focused on the production of hydrocarbons directly from ethanol,13 but these products would require additional upgrading (akin to petroleum)14 to introduce additional beneficial functional groups. By contrast, in order to capitalise on the abundant functional groups ever present in biomass, our previous work uses bioderived precursors to provide access to industrially-relevant aldehydes (i.e. butyraldehyde and 2-ethylhexanal).10 Further stepwise and selective derivatization of these aldehydes (i.e. hydrogenation or acetalization) provides access to both relevant chemical precursors and drop-in renewable fuels.
The aldehyde moiety provides a functional handle through which various reaction pathways could modify the end product to suit a range of fuel applications. A traditional approach would be to perform the hydrodeoxygenation (HDO) of the bio-derived aldehydes to obtain the respective alkanes. However, these hydrocarbons do not have octane or cetane numbers high enough for gasoline or diesel use, respectively, and would decrease the overall atom efficiency of the process.15 To this end, we sought to emulate the chemical structures of some known fuel additives i.e. petroleum ether and diethyl ether. These are typically used to increase the cetane number of diesel fuels and to decrease fuel viscosity (which is a key fuel property directly related to combustion efficiency and fuel economy) while not negatively impacting other relevant fuel properties.16,17 Consequently, we aimed at incorporating additional carbon into the aldehydes via acid catalysed acetalization to obtain fuels that both retain valuable oxygen as well as increased renewable carbon. Rather than extending the carbon chain and subsequently removing all the oxygen via HDO, we instead paid mind to extensive efforts which have been focused on the synthesis of oxygenated hydrocarbons, similar to traditional biodiesel, which burn cleaner than petroleum diesel.18,19 Several advantages of ethers over fatty acids exist; namely, the ethers are less viscous, less likely to freeze and are more resistant to common microbes in the environment.16,20
While we previously showed that bio-derived aldehydes can be readily transformed into diethyl acetals via acid catalysed acetalization with ethanol,10 we decided to investigate the synthesis of 1,3-dioxolane compounds as renewable diesel molecules since they are likely to have higher stability than the corresponding diethyl acetals. Through careful selection of the vicinal diol, it would be possible to incorporate additional renewable carbon into the molecule, while maintaining the oxygen atoms. For example, ethylene glycol (EG) and 2,3-butanediol (BDO) are both vicinal diols, however BDO would incorporate twice as much renewable carbon, is available via fermentation of biomass with Zymomonas mobilis.21
Harvey and coworkers have previously described dioxolanes produced from BDO via a dehydration/acetalization reaction, and dioxolanes produced from condensation of bio-derived methylketones with diols (such as BDO, 1,2-propanediol and ethylene glycol).18,22 In the case of the “BDO-only” work, the dioxolane mixture obtained (2-ethyl-2,4,5-trimethyl-1,3-dioxolane and isomers) had excellent fuel properties for use in spark ignition engines (anti-knock index = 90.1) and could be blended with gasoline without introducing adverse water solubility, however this fuel exhibited a poor derived cetane number (DCN) of 22.9.22 Dioxolane fuels more suitable for compression ignition engines were reported by Harvey and co-workers and were produced from the condensation of methyl ketones with diols (obtaining three dioxolanes 2,4,5-trimethyl-2-undecyl-1,3-dioxolane (H1), 2,4-dimethyl-2-undecyl-1,3-dioxolane (H2), and 2-methyl-2-undecyl-1,3-dioxolane (H3)).18 These fuels showed excellent cetane numbers and viscosities similar to that of biodiesel. While the above reports demonstrate the potential for dioxolanes as renewable fuel blendstocks, alternative sources of renewable carbon and methods for their production should be assessed to fully understand the impact of these potential biofuels. In this work we demonstrate a strategy for producing dioxolane fuels from bio-derived aldehydes using a solvent-free method which produces fuels with excellent suitability for blending with petroleum diesel (Table 1).
| Entry | Fuel | Density (25 °C, g mL−1) | NHOC (MJ L−1) | Viscosity (40 °C, mm2 s−1) | Freezing/MPa (°C) | YSI | DCN |
|---|---|---|---|---|---|---|---|
| a Freezing and melting points were measured by DSC (Ar = 40 sccm, Tramp = 10 K min−1, Trange = 123–323 K). b Taken from ref. 18. c Taken from ref. 23. d Taken from ref. 24, for USA diesel east–west coasts. e Indicates measured Cetane Number (CN). | |||||||
| 1 | 1 | 0.893 | 29.45 | 1.26 | <−100 | 58 | 44.8 |
| 2 | 2 | 0.885 | 30.41 | 1.88 | <−100 | 69 | 64.2 |
| 3 | 3 | 0.894 | 27.67 | 0.94 | <−100 | 36 | 33.4 |
| 4 | 4 | 0.887 | 29.20 | 1.49 | <−100 | 49 | 48.1 |
| 5 | 5 | 0.883 | 30.06 | 2.34 | <−100 | 63 | 68.9 |
| 6 | H1b | 0.868 | 31.99 | 4.44 | −51/−18 | — | 84 |
| 7 | H2b | 0.872 | 31.99 | 5.15 | −48/−14 | — | 91 |
| 8 | H3b | 0.883 | 32.28 | 4.98 | −25/0 | — | 81 |
| 9 | Soy biodieselc | 0.882 | 32.63 | 4.26 | 0/−4 | — | 51.3e |
| 10 | Palm biodieselc | 0.873 | 32.55 | 4.61 | 14/13 | — | 61.9e |
| 11 | Winter dieseld | 0.843–0.830 | 35.96 | 2.51–2.67 | <−21 | 240 | 45–54e |
We examined the acetalization of ethanol-derived C4–8 aldehydes with BDO using the commercially available solid acid catalyst, Amberlyst 15. Initially, we investigated the reaction of 2-ethylbutyraldehyde and BDO, by stirring with Amberlyst 15 in the absence of any solvent. The synthesis is outlined in Fig. 1A, where the reaction is conducted in a slight BDO molar excess (ca. 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
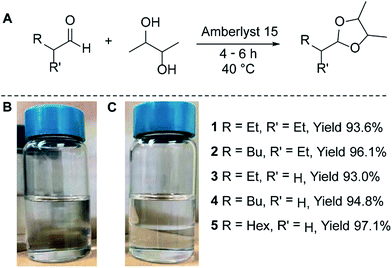
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v). The two compounds are miscible when mixed, Fig. 1B. After stirring for 5 h under mild (≤40 °C) temperatures the reaction was complete and a distinct phase separation was observed, Fig. 1C. The organic phase contained only the desired 1,3-dioxolane while the unreacted BDO and the produced water formed an aqueous layer. This phase separation, along with the absence of any solvent, allowed the reaction product to be simply decanted and the remaining BDO could then be used for additional cycles, with no additional workup or purification. The initial reaction of 2-ethylbutyraldehyde and BDO yielded compound 1, in 93.6% isolated yield. To prove the generality of this method to other aldehydes, we investigated the acetalization of BDO with 2-ethylhexanal and butyraldehyde to obtain 2 (96.1% yield) and 3 (93.0% yield), respectively. Given the availability of both branched and straight chain aldehydes as starting materials, we wished to investigate the effect of branching on cetane number. To this end, we reacted hexanal and octanal with BDO to obtain the straight chain acetals 4 (94.8% yield) and 5 (97.1% yield), respectively.
1; v/v). The two compounds are miscible when mixed, Fig. 1B. After stirring for 5 h under mild (≤40 °C) temperatures the reaction was complete and a distinct phase separation was observed, Fig. 1C. The organic phase contained only the desired 1,3-dioxolane while the unreacted BDO and the produced water formed an aqueous layer. This phase separation, along with the absence of any solvent, allowed the reaction product to be simply decanted and the remaining BDO could then be used for additional cycles, with no additional workup or purification. The initial reaction of 2-ethylbutyraldehyde and BDO yielded compound 1, in 93.6% isolated yield. To prove the generality of this method to other aldehydes, we investigated the acetalization of BDO with 2-ethylhexanal and butyraldehyde to obtain 2 (96.1% yield) and 3 (93.0% yield), respectively. Given the availability of both branched and straight chain aldehydes as starting materials, we wished to investigate the effect of branching on cetane number. To this end, we reacted hexanal and octanal with BDO to obtain the straight chain acetals 4 (94.8% yield) and 5 (97.1% yield), respectively.
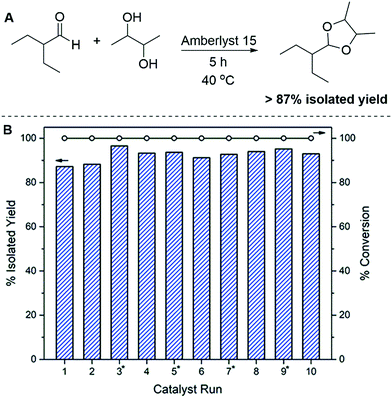
Given that the only loss in atom economy is water, we achieve quantitative conversion with atom economy of greater than 88.9% for all five reactions, with carbon yields equalling the isolated yield. The purity of each of the acetals was determined via1H NMR, and shown to be a mixture of three diastereomers. In these spectra, no water was observed suggesting low or zero solubility. As such, no toxic side products have been identified and the water could potentially be separated from the remaining BDO via distillation. Additionally, each reaction proceeds to completion, and while no carbon is lost in the form of unidentifiable side products, the carbon balance is 100% for every example presented in this work. Due to the requirement of a slight BDO excess and generation of water as a side-product, it was imperative to investigate the recyclability of the catalyst system. In a similar reaction system, it was previously reported23 that during acetone condensation, Amberlyst CH28 was deactivated due to water build up on the catalyst surface. Therefore, we assessed the stability of Amberlyst 15 for the acetalization of compounds 1–5. To test the catalyst recyclability, we chose the reaction of 2-ethylbutyraldehyde with BDO, which produces 1, Fig. 2A. After 10 catalyst recycle runs, the reaction was still producing >87% isolated yield of 1, with 100% conversion observed for each of ten catalyst recycle runs, Fig. 2B.
The solubility of water in BDO likely prevents competitive inhibition on the catalyst surface and affords the high degree of catalyst recyclability evidenced above. It is imperative to note that the conversion for each of the ten catalyst recycle runs was 100% and the discrepancy between the conversion and isolated yield is due to the workup procedure. The product was simply decanted away from the aqueous phase to the best of our ability, and the ability to remove all of the product was limited due to our desire to eliminate the use of an organic solvent for extraction purposes. While the isolated yields are not unity, the absence of additional solvents or purification steps demonstrate the simplicity and sustainability of this method. This property, as well as the employment of a solid acid catalyst could allow this method to easily be translated to a continuous flow reactor, for large scale production.
The efficient acquisition of five distinct dioxolanes merits further investigation to determine if they would make suitable fuel components.4,5,8,16,18–20 The first metric we considered was viscosity. While many current biodiesels24 are significantly more viscous than their petroleum counterparts,25 compounds 1–5 exhibited kinematic viscosities significantly lower than biodiesel, H1–H3, and petroleum diesel at temperatures ranging from −20 to 40 °C, Table 1. Viscosity is a key fuel property because if the viscosity falls outside of the ASTM D 975 specification (>4 mm2 s−1), it leads to poor atomization of the fuel which results in poor combustion and a subsequent loss of power and decrease in fuel economy.17 In this respect, compounds 1–5 could be used to thin biodiesel to meet standards for petroleum diesel.
In addition to high viscosities, many current biodiesels have high freezing points,24 which lie at temperatures near or even above the freezing point of water, which is another critical drawback to their extensive application. Compounds 1–5 have freezing points below −100 °C, which is likely due to each of the compounds existing as a mixture of three diastereomers, which is a significant improvement over both biodiesel, H1, H2, H3, and petroleum diesel. Additionally, compounds 1–5 have molecular weights that are between 49 and 68% that of soy biodiesel,24 for the C8 and C12 acetals, respectively. This lower molecular weight likely contributes to the low viscosity and freezing point of compounds 1–5.
Two other important considerations for diesel fuels are the density and net heat of combustion (NHOC). For a chemical compound to be able to be blended with diesel it must not decrease the density of the fuel mixture below 0.82 g mL−1, because the energy content of the fuel brought into the engine is dependent on the density of the fuel.17,25 Compounds 1–5 maintain densities which are slightly higher than biodiesel, H1–H3, and petroleum diesel. A related metric is the NHOC, which is a measure of the energy contained in a given quantity of a compound. While compounds 1–5 do not have NHOC's comparable to winter diesel, this is only one metric of fuel performance, and other measurements are potentially more important. Petroleum diesel is prone to producing a high level of soot during combustion.26 One method of determining how cleanly a fuel burns is via determination of the yield sooting index (YSI), which provides a measure of soot formation rates.27,28 When compared to petroleum diesel, compounds 1–5 display much lower YSI values, and therefore will likely burn cleaner.
One of the most important measures of the quality of a diesel fuel is the cetane number (CN), which is a dimensionless measurement typically ranging from 0–100 (though measurements outside this range are possible), and measures how readily a fuel autoignites in a compression ignition engine. Using ignition quality testing (IQT), we obtained the derived cetane number (DCN, ASTM D689D), which is an excellent predictor of CN. It was observed than only compound 3 did not meet diesel standards (minimum cetane number of 40 required for most of North America),25 while compounds 1, 2, 4 and 5 have DCN values comparable to, or exceeding that of winter diesel. This suggests that compounds 1–5 (excluding 3) could be readily blended with either petroleum diesel or biodiesel to reduce the viscosity and help to depress the freezing point, without hampering the combustion performance of the fuel. Additionally, the cetane number of the branched acetals was approximately 4 units lower than their corresponding straight chain counterparts. While this is expected, it is not significant and indicates that increasing carbon number is a more efficient way to increase cetane number, which is evident when comparing compounds 1–5 to dioxolanes H1–H3 reported by Harvey and co-workers.
The atom economic acetalization of bio-derived aldehydes with BDO affords a simple method to substituted 1,3-dioxolanes. This work represents a catalytic transformation which operates at mild temperatures (<40 °C), using the commercially available solid acid Amberlyst-15. The solvent-free reaction system provides easy isolation of the product through phase separation and subsequent decantation, which allows for simple synthesis of potential renewable diesel molecules. While the energy density of compounds 1, 2, 4 and 5 is lower than petroleum diesel, several other properties indicate that blending these compounds would be beneficial. These include an increased cetane number and density, as well as decreased viscosity, freezing point and soot formation. Given that both BDO and ethanol can be produced industrially, combining this work with our previous report of ethanol upgrading provides a feasible avenue by which this process could be combined to be conducted at a single biorefinery. In addition, the method described herein for the formation of 1,3-dioxolanes could be used for the simultaneous production and extraction of renewable diesel additives directly from BDO fermentation broths using bio-derived aldehydes.29 As such, this work provides a potential opportunity to further diversify the use of ethanol as a renewable diesel fuel and provide performance advantages to the consumer.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded through the Office of Energy Efficiency & Renewable Energy (EERE) Bioenergy Technology Office (BETO) through the ChemCatBio: Chemical Catalysis for Bioenergy Consortium (LANL) and the Co-Optimization of Fuels & Engines initiative (Co-Optima; LANL & Yale). Additional support (Yale) was provided by the NSF sustainable Chemistry program (award CBET 16044983). Los Alamos National Laboratory is operated by Los Alamos National Security, LLC, for the National Nuclear Security Administration of the U.S. Department of Energy under contract DE-AC5206NA25396.Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106(9), 4044 CrossRef CAS PubMed.
- P. Fairley, Nature, 2011, 474, S2 CrossRef CAS PubMed.
- R. Henriques, Angew. Chem., 1898, 11(30), 697 CrossRef.
- R. L. McCormick, A. Williams, J. Ireland, M. Brimhall and R. R. Hayes, Effects of biodiesel Blends on Vehicle Emissions, Milestone Report NREL/MP-540-40554, National Renewable Energy Laboratory, 2006 Search PubMed.
- B. S. Sazzad, M. A. Fazal, A. S. M. A. Haseeb and H. H. Masjuki, RSC Adv., 2016, 6(65), 60244 RSC.
- R. W. Jenkins, C. M. Moore, T. A. Semelsberger, C. J. Chuck, J. C. Gordon and A. D. Sutton, ChemSusChem, 2016, 9(9), 922–923 CrossRef CAS PubMed.
- D. Singh, K. A. Subramanian, M. Juneja, K. Singh, S. Singh, R. Badola and N. Singh, Environ. Prog. Sustainable Energy, 2017, 36(1), 214 CrossRef CAS.
- G. Knothe and K. R. Steidley, Fuel, 2005, 84(9), 1059 CrossRef CAS.
- Building Partnerships | Growing Markets 2017 Ethanol Industry Outlook, Renewable Fuels Association, http://www.ethanolrfa.org/wpcontent/uploads/2017/02/Ethanol-Industry-Outlook-2017.pdf, accessed April 2018.
- C. M. Moore, O. Staples, R. W. Jenkins, T. J. Brooks, T. A. Semelsberger and A. D. Sutton, Green Chem., 2017, 19(1), 169 RSC.
- W. Bertleff, M. Roeper and X. Sava, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KgaA, 2007 Search PubMed.
- A. W. Franz, H. Kronemayer, D. Pfeiffer, R. D. Pilz, G. Reuss, W. Disteldorf, A. O. Gamer and A. Hilt, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KgaA, 2016 Search PubMed.
- K. K. Ramasamy and Y. Wang, Catal. Today, 2014, 237, 89 CrossRef CAS.
- M. Schlaf, Dalton Trans., 2006, 39, 4645 RSC.
- Please refer to ref. 10 for discussion of fuel properties of alkanes derived from HDO of aldehydes produced from acetaldehyde..
- K. Górski and M. Przedlacki, Energy Fuels, 2014, 28(4), 2608 CrossRef.
- Chevron Corporation, Diesel Fuels Technical Review, 2007 Search PubMed.
- K. W. Harrison and B. G. Harvey, Sustainable Energy Fuels, 2018, 2(2), 367 RSC.
- H. Liu, X. Bi, M. Huo, C.-f. F. Lee and M. Yao, Energy Fuels, 2012, 26(3), 1900 CrossRef CAS.
- G. Sørensen, D. V. Pedersen, A. K. Nørgaard, K. B. Sørensen and S. D. Nygaard, Bioresour. Technol., 2011, 102(8), 5259 CrossRef PubMed.
- S. Yang, A. Mohagheghi, M. A. Franden, Y.-C. Chou, X. Chen, N. Dowe, M. E. Himmel and M. Zhang, Biotechnol. Biofuels, 2016, 9(1), 189 CrossRef PubMed.
- B. G. Harvey, W. W. Merriman and R. L. Quintana, ChemSusChem, 2016, 9, 1814 CrossRef CAS PubMed.
- S. Talwalkar and S. Mahajani, Appl. Catal., A, 2006, 302(1), 140 CrossRef CAS.
- S. K. Hoekman, Renewable Sustainable Energy Rev., 2012, 16(1), 143 CrossRef CAS.
- Infinium International, Ltd, Worldwide Winter Diesel fuel Quality Survey 2016, Infineum International, Ltd., Abingdon, U.K., 2016 Search PubMed.
- D. D. Das, C. S. McEnally, T. A. Kwan, J. B. Zimmerman, J. W. Cannella, C. J. Mueller and L. D. Pfefferle, Fuel, 2017, 197, 445 CrossRef CAS.
- D. D. Das, P. C. St. John, C. S. McEnally, S. Kim and L. D. Pfefferle, Combust. Flame, 2018, 190, 349 CrossRef CAS.
- C. S. McEnally, Y. Xuan, P. C. St. John, D. D. Das, A. Jain, S. Kim, T. A. Kwan, L. K. Tan, J. Zhu and L. D. Pfefferle, Proc. Combust. Inst., 2018 Search PubMed , in press..
- Y. Li, Y. Wu, J. Zhu, J. Liu and Y. Shen, J. Saudi Chem. Soc., 2016, 20, S495 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00371h |
| This journal is © The Royal Society of Chemistry 2018 |