Preparation and photocatalytic activity of Au/TiO2 lyogels for hydrogen production†
Lester
Martínez
a,
Mónica
Benito
b,
Ignasi
Mata
 bc,
Lluís
Soler
bc,
Lluís
Soler
 a,
Elies
Molins
b and
Jordi
Llorca
a,
Elies
Molins
b and
Jordi
Llorca
 *a
*a
aInstitute of Energy Technologies, Department of Chemical Engineering and Barcelona Research Center in Multiscale Science and Engineering, Universitat Politècnica de Catalunya, EEBE, 08019 Barcelona, Spain. E-mail: jordi.llorca@upc.edu
bInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus UAB, 08193 Bellaterra, Spain
cDepartament de Geologia, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain
First published on 3rd August 2018
Abstract
High surface area titania lyogels have been prepared, thermally transformed into TiO2 polymorphs at 400–850 °C, and decorated by ball milling with preformed Au nanoparticles of ca. 2 nm to ensure the same contact points at the metal–support interphase. The best performance in the photogeneration of hydrogen from gaseous water–ethanol under dynamic conditions in a fixed bed reactor has been obtained with the lyogel calcined at 550 °C, with a hydrogen photoproduction rate of 19.8 mmol H2 g−1 h−1 under an irradiance of 80 mW cm−2 and GHSV = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. This photocatalyst contains 84% anatase and 16% rutile polymorphs, very similar to the standard P25. A series of photocatalysts prepared by lyophilization of a mixture of the titania gel and the preformed Au nanoparticles and calcination under the same conditions has resulted in samples with Au nanoparticles up to 18 nm strongly interacting with TiO2. The presence of Au nanoparticles in the composite lyogel has strongly retarded the transformation of anatase into rutile. The results have shown that the TiO2 polymorph has a greater influence than the Au nanoparticle size on the photoproduction of hydrogen.
000 h−1. This photocatalyst contains 84% anatase and 16% rutile polymorphs, very similar to the standard P25. A series of photocatalysts prepared by lyophilization of a mixture of the titania gel and the preformed Au nanoparticles and calcination under the same conditions has resulted in samples with Au nanoparticles up to 18 nm strongly interacting with TiO2. The presence of Au nanoparticles in the composite lyogel has strongly retarded the transformation of anatase into rutile. The results have shown that the TiO2 polymorph has a greater influence than the Au nanoparticle size on the photoproduction of hydrogen.
1. Introduction
The photocatalytic generation of hydrogen is considered one of the best environmentally friendly methods to produce hydrogen because of its simplicity and mild conditions required.1–4 Contrarily to catalytic reforming technologies and electrochemical processes, photocatalysis can be carried out under ambient conditions using direct sunlight. The main drawback of photocatalytic processes, however, is their low efficiency. For that reason, a great deal of effort is addressed toward the formulation of an active and stable photocatalyst. Titanium dioxide (TiO2) is used widely because of its inert character, low price and availability.5 When TiO2 is exposed to UV light, electron–hole pairs are produced, which can be used to split water into its fundamental components, H2 and O2. Since the recombination of the electron–hole pairs is faster than the redox reactions, usually hole scavengers are employed to consume the holes present in the valence band, and metal nanoparticles are employed to retain the electrons in the conduction band to reduce protons into H2 more efficiently. In the literature there are abundant reports regarding the effect of the TiO2 polymorph and of the metal nanoparticles added to TiO2 on the photoproduction of hydrogen, with Pt and Au being the most effective.6–10 However, a reliable comparison of the photocatalytic performance between the different photocatalysts is difficult because very rarely the metal nanoparticles are comparable, since the conventional methods used for their preparation and anchoring on the TiO2 surface (impregnation, photodeposition, and deposition–precipitation) originate metal nanoparticles with different sizes, morphologies and metal–support interactions, which are of paramount importance in any catalytic process.11 Also, the textural properties of the different TiO2 polymorphs usually differ considerably.12,13 Herein we report on the preparation of Au/TiO2 photocatalysts using TiO2 lyogels and preformed Au nanoparticles and on their photoactivity in the generation of hydrogen from gaseous water–ethanol in a fixed bed photoreactor under dynamic conditions. On one hand, the use of lyogels allows starting from an amorphous, porous material with Ti–O bonds, which can evolve under controlled conditions into crystalline TiO2 polymorphs14–16 and, on the other hand, the use of preformed Au metal nanoparticles as a cocatalyst ensures that the same contact points will be present in all photocatalysts independent of the polymorph. We unambiguously show that the TiO2 polymorph has a greater influence on the photoproduction of hydrogen from water–ethanol than the Au nanoparticle size. In contrast, the photocatalysts prepared by calcination of a lyogel prepared by premixing the TiO2 gel and Au nanoparticles do not allow the control of the architecture of the photocatalyst and lower hydrogen production rates are obtained.2. Experimental methods
2.1. Materials
Titanium(IV) isopropoxide (97%), gold(III) chloride hydrate (99.999% trace metal basis) and tert-butanol (99%) were purchased from Sigma-Aldrich. Nitric acid 65% technical grade was supplied by Panreac AppliChem. Milli-Q water (Millipore, conductivity of 18.2 MΩ cm at 25 °C) was used during the synthesis. All the chemicals were used without further purification.2.2. Synthesis
2.3. Materials characterization
Powder X-ray diffraction patterns were recorded using a Siemens D5000 diffractometer using Cu radiation (CuKα = 1.5418 Å, 45 kV, 35 mA) in Bragg–Brentano geometry. The diffraction patterns were recorded in the 2θ range of 15–65° in steps of 0.02° per second and 1 second per step. Crystalline phases were identified using the database (PDF-4+ 2016) from the International Centre for Diffraction Data. The ratio between anatase and rutile phases was calculated according to the method described in ref. 19, which uses the equation % rutile = 100/[(A/R) × 0.884 + 1], where A is the peak area for the anatase (101) reflection at 25.3° and R is the peak area for the rutile (110) reflection at 27.4°. The average crystallite sizes were determined by the Debye–Scherrer equation. Raman spectra were recorded with a Renishaw inVia Qontor spectrometer using the green line of a diode-pumped solid state laser (532.1 ± 0.3 nm) in the micro-Raman configuration. Diffuse reflectance UV-Vis absorbance spectra were collected over the wavelength range of 290–800 nm using a Shimadzu UV3600 UV-vis/NIR spectrophotometer equipped with a diffuse reflectance cell and BaSO4 as the reference standard. The band gap values (Eg) were calculated by using Tauc plots. Infrared spectroscopy (FT-IR) was performed with a Nicolet 6700 instrument equipped with a CsI detector. Bright field transmission electron microscopy (TEM) and scanning transmission electron microscopy in high-angle annular dark field mode (HAADF-STEM) were used to analyze the changes in TiO2 morphology and the size distribution of the Au nanoparticles. Samples were prepared by dispersing the catalysts in hexane. A drop of the suspension was then allowed to evaporate on a carbon coated copper grid. TEM was conducted with a JEOL 1210 instrument operating at an accelerating voltage of 120 kV. HAADF-STEM was carried out with a FEI TECNAI F20 S/TEM instrument equipped with a field emission electron source operated at 200 kV. The specific surface area was measured on a Micromeritics ASAP 2000 instrument using N2 as an adsorbent at liquid nitrogen temperature. The materials were initially degassed at 150 °C for 20 h in order to desorb impurities. X-ray photoelectron spectroscopy (XPS) was performed with a SPECS system using an Al X-ray source (150 W) and a 9-channel Phoibos detector at a pressure below 10−6 Pa. Quantification was carried out using Shirley baselines and Gaussian–Lorentzian line shapes.2.4. Photocatalytic reaction for hydrogen production
The photocatalytic tests were performed at room temperature and atmospheric pressure in a tubular glass photoreactor under dynamic conditions. An argon stream (20 ml min−1) was bubbled into a Drechsel bottle containing a liquid mixture of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water in order to obtain a gaseous reactant mixture of EtOH
water in order to obtain a gaseous reactant mixture of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (molar basis), which was directly introduced into the reactor. The partial pressure of ethanol was 0.30 kPa. The UV-light source (from SACOPA, S.A.U.) consisted of four LEDs emitting at 365 ± 5 nm and a synthetic quartz glass cylindrical lens that transmitted the light to the photocatalyst.18 The catalyst samples (4 mg) were dispersed in ethanol and ultrasonicated, and the resultant suspension was poured onto a circular porous cellulose membrane (from Albet LabScience, pore size 35–40 μm, 80 g m−2, thickness 0.18 mm). The impregnated membrane was dried in an oven at 50 °C and weighed to check for the amount of supported photocatalyst and then placed in the photoreactor. The gas hourly space velocity was ca. 26
9 (molar basis), which was directly introduced into the reactor. The partial pressure of ethanol was 0.30 kPa. The UV-light source (from SACOPA, S.A.U.) consisted of four LEDs emitting at 365 ± 5 nm and a synthetic quartz glass cylindrical lens that transmitted the light to the photocatalyst.18 The catalyst samples (4 mg) were dispersed in ethanol and ultrasonicated, and the resultant suspension was poured onto a circular porous cellulose membrane (from Albet LabScience, pore size 35–40 μm, 80 g m−2, thickness 0.18 mm). The impregnated membrane was dried in an oven at 50 °C and weighed to check for the amount of supported photocatalyst and then placed in the photoreactor. The gas hourly space velocity was ca. 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The irradiance over the sample was 80 ± 2 mW cm−2 as measured with an UV-A sensor (model PMA 2110, Solar Light Co.), which registered the UV radiation within spectral response 320–400 nm, connected to a radiometer (model PMA2200, Solar Light Co.). The outlet of the photoreactor was connected to a GC (micro gas chromatograph Agilent 490), which was equipped with MS 5 Å, Plot U and Stabilwax columns for a complete analysis of the photoreaction products. Analyses were performed on-line every 4 minutes for a total reaction time of 90 min.
000 h−1. The irradiance over the sample was 80 ± 2 mW cm−2 as measured with an UV-A sensor (model PMA 2110, Solar Light Co.), which registered the UV radiation within spectral response 320–400 nm, connected to a radiometer (model PMA2200, Solar Light Co.). The outlet of the photoreactor was connected to a GC (micro gas chromatograph Agilent 490), which was equipped with MS 5 Å, Plot U and Stabilwax columns for a complete analysis of the photoreaction products. Analyses were performed on-line every 4 minutes for a total reaction time of 90 min.
3. Results and discussion
3.1. Characterization results
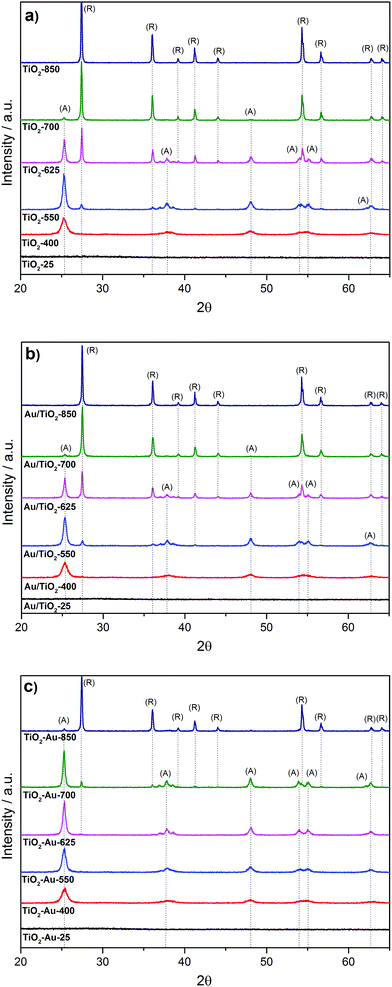
Fig. 1a shows the XRD patterns of the as-prepared TiO2 lyogels (TiO2-25, TiO2-400, TiO2-550, TiO2-625, TiO2-700, and TiO2-850). Initially, the untreated TiO2 lyogel contained the amorphous phase but calcination at 400 °C originated anatase as the unique polymorph, with a crystallite size of about 12 nm. As expected, the crystallite size increased with increasing calcination temperature and anatase was progressively transformed into rutile,15,16 obtaining pure rutile at 850 °C with a crystallite size of about 50 nm. The anchoring of the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures (photocatalysts Au/TiO2-25, Au/TiO2-400, Au/TiO2-550, Au/TiO2-625, Au/TiO2-700, and Au/TiO2-850) did not result in significant changes in the characteristics of the TiO2 supports, as deduced from the XRD analysis (Fig. 1b), and similar phase distributions and crystallite sizes were encountered (Table 1).| Sample | S BET (m2 g−1) | Pore V (cm3 g−1) |
A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ra (%) Ra (%) |
TiO2 size (nm) | Au sizeb (nm) | Au/Tic (at/at) | E g (eV) |
|---|---|---|---|---|---|---|---|
a The distribution of anatase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rutile (A rutile (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R) and the TiO2 crystallite size were calculated by XRD.
b The Au particle size was determined by TEM and HAADF-STEM.
c The surface Au/Ti atomic ratio was calculated from XPS.
d The band gap energy values (Eg) were determined by diffuse reflectance UV-Vis (Tauc plots). R) and the TiO2 crystallite size were calculated by XRD.
b The Au particle size was determined by TEM and HAADF-STEM.
c The surface Au/Ti atomic ratio was calculated from XPS.
d The band gap energy values (Eg) were determined by diffuse reflectance UV-Vis (Tauc plots).
|
|||||||
| Au/TiO2-25 | 519 | 0.84 | — | — | 2.1 ± 0.6 | 0.002 | 3.37 |
| Au/TiO2-400 | 91 | 0.41 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
12 (A) | 1.8 ± 0.4 | 0.002 | 3.21 |
| Au/TiO2-550 | 44 | 0.29 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
19 (A), 39 (R) | 1.8 ± 0.4 | 0.004 | 3.12 |
| Au/TiO2-625 | 20 | 0.20 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
27 (A), 48 (R) | 2.1 ± 0.6 | 0.009 | 3.05 |
| Au/TiO2-700 | 6 | 0.07 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 96 |
40 (A), 51 (R) | 2.0 ± 0.5 | 0.027 | 3.05 |
| Au/TiO2-850 | 3 | 0.01 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
52 (R) | 1.9 ± 0.5 | 0.052 | 3.03 |
| TiO2–Au-25 | 479 | 1.15 | — | — | 1.9 ± 0.4 | 0.002 | 3.21 |
| TiO2–Au-400 | 117 | 0.63 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
12 (A) | 4.3 ± 0.9 | 0.002 | 3.26 |
| TiO2–Au-550 | 72 | 0.49 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
15 (A) | 6 ± 1 | 0.002 | 3.25 |
| TiO2–Au-625 | 45 | 0.40 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
22 (A), 51 (R) | 10 ± 2 | 0.002 | 3.24 |
| TiO2–Au-700 | 32 | 0.30 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
29 (A), 56 (R) | 14 ± 4 | 0.002 | 3.20 |
| TiO2–Au-850 | 5 | 0.05 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 96 |
48 (R) | 18 ± 5 | 0.006 | 3.03 |
In Fig. 1c the XRD patterns of the lyogel photocatalysts obtained by dispersion of preformed gold nanoparticles during the synthesis of the titania gels and calcination at different temperatures are shown (samples TiO2–Au-25, TiO2–Au-400, TiO2–Au-550, TiO2–Au-625, TiO2–Au-700, and TiO2–Au-850). Interestingly, rutile phase appearance is strongly retarded with respect to the photocatalysts prepared by anchoring preformed Au nanoparticles onto the TiO2 lyogels previously calcined at each temperature (Table 1). In particular, the rutile polymorph starts appearing at a calcination temperature of 700 °C, but only at 850 °C it is the dominant phase. Therefore, the presence of Au nanoparticles, even at a low loading of 1 wt%, has a strong effect on the dynamics of the anatase–rutile transformation, with the anatase phase being strongly stabilized. Regarding Au, diffraction peaks were not observed in any XRD patterns due to the low metal loading; in addition, there is an overlap between the most intense reflection of gold (111) at 38.2° and anatase reflections (004) and (112) at 37.8 and 38.6°, respectively.
The specific surface area and the pore volume were found to decrease sharply with increasing the calcination temperature, as shown in Table 1. However, the specific surface area was related not only to the calcination temperature, but also to the TiO2 polymorph present in the samples. For a given calcination temperature, the specific surface area of the photocatalysts containing anatase was always higher than those containing rutile. On the other hand, the grinding method used to graft the preformed Au nanoparticles onto TiO2 lyogels had no effect on the textural characteristics of the samples as similar specific surface areas (within 2% deviation) were recorded for the lyogels calcined at different temperatures before and after Au nanoparticle anchoring.
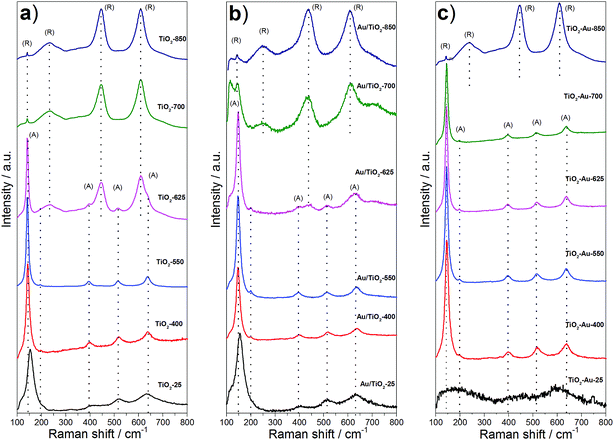
Fig. 2 shows the Raman spectra corresponding to the lyogel samples and the photocatalysts. Anatase shows six Raman active modes at around 144 cm−1 (Eg), 197 cm−1 (Eg), 399 cm−1 (B1g), 513 cm−1 (A1g), 519 cm−1 (B1g) and 639 cm−1 (Eg), while rutile has four vibrational modes at 145 cm−1 (B1g), 445 cm−1 (Eg), 610 cm−1 (A1g) and 826 cm−1 (B2g), and a multi-photon process at 240 cm−1.20–23 The as prepared TiO2 lyogel exhibited broad bands close to those corresponding to anatase (Fig. 2a), but slightly shifted according to its amorphous/poorly crystalline nature. By increasing the calcination temperature of the TiO2 lyogel up to 400 and 550 °C, bands of anatase became well-defined, in accordance with the XRD results discussed above. At a calcination temperature of 625 °C and higher, the bands of rutile became progressively more intense at the expense of anatase, as expected. Similar Raman bands were recorded over the photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures (Fig. 2b), except for a slight shift to higher wavenumbers and broadening with respect to the bare lyogels calcined at the same temperatures, which is an indication of the electronic interaction between titania and gold.24 In contrast, different proportions of anatase and rutile polymorphs were observed in the Raman spectra corresponding to the photocatalysts prepared from the TiO2–Au composite lyogel calcined at the same temperatures (Fig. 2c). In this case, anatase was the only polymorph identified for calcination temperatures up to 700 °C, and a sudden transformation into rutile occurred at 850 °C. These results are completely in accordance with the data recorded by XRD and, again, highlight the role of Au nanoparticles in the retardation of the transformation of anatase into rutile. Based on these results we believe that the strong metal–support interaction that takes place between anatase and the Au nanoparticles during the calcination of the composite TiO2–Au lyogel is responsible for the stabilization of the anatase phase against a structural transformation into rutile.
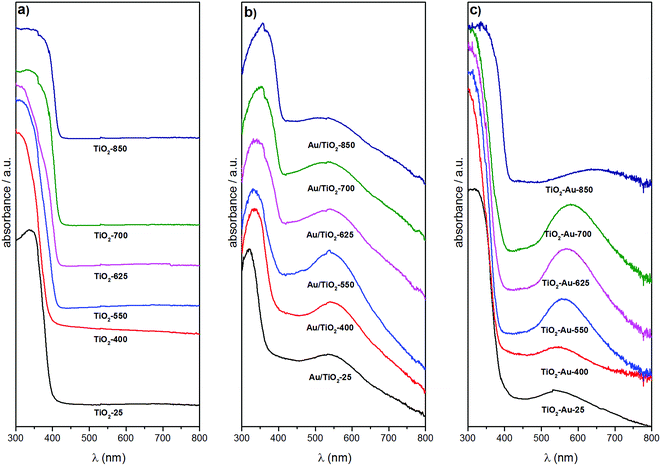
Fig. 3 shows the diffuse reflectance UV-Vis spectra of the TiO2 lyogels calcined at different temperatures (Fig. 3a) and of the photocatalyst samples (Fig. 3b and c). The spectra of the TiO2 lyogels were dominated by a strong absorption at 300–400 nm due to the band gap of TiO2. In addition to this band, a broad band at about 540–660 nm corresponding to the surface plasmon resonance of Au nanoparticles was identified in the photocatalyst samples. In all cases there was a good correspondence between the TiO2 polymorph (anatase, rutile or a combination of both) and the edge of the TiO2 absorption band. As reported in the literature, the edge of the absorption band of rutile is redshifted with respect to that of anatase.25 Therefore, the spectra of samples with high rutile content, such as TiO2-700 and TiO2-850 in Fig. 3a, Au/TiO2-700 and Au/TiO2-850 in Fig. 3b and TiO2–Au-850 in Fig. 3c, exhibit TiO2 absorption bands that extend to higher wavelengths. Accordingly, for the TiO2 lyogels calcined at different temperatures and for the photocatalysts prepared by anchoring preformed Au nanoparticles onto them, the TiO2 absorption band progressively shifted to higher wavelengths as the calcination temperature increased, due to the progressive transformation of anatase to rutile (Fig. 3a and b). In contrast, the TiO2 absorption band of the different calcined TiO2–Au composite lyogel photocatalysts remained similar until a calcination temperature of 850 °C was reached, where a drastic transition from anatase to rutile took place, as shown in Fig. 3c. This is consistent with the retardation effect of the anatase–rutile transformation induced by the Au nanoparticles as discussed above from the XRD and Raman data. The band gap energies for the different photocatalysts were determined from the corresponding Tauc plots and they ranged from 3.26 eV for the samples with only anatase down to 3.03 eV for samples with only rutile (Table 1), which compare well with data reported in the literature for the pure polymorphs.25,26 The incorporation of Au nanoparticles had a slight effect on the band gap energy of TiO2 lyogels, as reported for other Au/TiO2 systems.27 In particular, the band gap energy of the photocatalyst Au/TiO2-550 was significantly higher than that of the TiO2-550 lyogel prior to the grafting of Au nanoparticles (3.12 vs. 3.03 eV, respectively). Also, the band gap energy values of the series of photocatalysts prepared by calcining the TiO2–Au composite lyogel were consistently higher than those of the photocatalysts prepared by anchoring the preformed Au nanoparticles on calcined lyogels (i.e. 3.26 vs. 3.21 eV for TiO2–Au-400 and Au/TiO2-400, respectively). Given that in both samples the TiO2 crystallites are anatase and they have the same particle size (∼12 nm), the difference observed in the band gap energy values may be related to the Au nanoparticle size (4.3 vs. 1.8 nm for TiO2–Au-400 and Au/TiO2-400, respectively, as will be shown later by TEM).
The surface plasmon resonance (SPR) of Au nanoparticles was found at about 540–550 nm for the photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures (Fig. 3b). The similar position of the SPR band in this series of photocatalysts is a direct proof that the same size of Au nanoparticles was present in all samples, which is in accordance with the preparation method used. The decoration of TiO2 lyogels with preformed Au nanoparticles after the calcination step ensured a similar number of contact points between the Au nanoparticles and the TiO2 support, independent of the specific surface area exposed by the photocatalyst and the TiO2 polymorph. The same location of the SPR band was found in the UV-Vis spectrum of the sample before calcination (Au/TiO2-P25). In contrast, the scene was completely different for the photocatalysts prepared from the TiO2–Au composite lyogel calcined at different temperatures, as illustrated in Fig. 3c, where the SPR band of the Au nanoparticles showed a strong dependence on the calcination temperature. In this series of photocatalysts, the position of the SPR band was progressively shifted toward higher wavelengths as the calcination temperature of the sample was increased, varying from about 540–550 nm for the sample calcined at 400 °C up to 650–660 nm for the highest calcination temperature of 850 °C. Moreover, in addition to the redshift of the SPR band, there was a simultaneous increase in bandwidth. These observations clearly demonstrate that the size of the Au nanoparticles progressively augmented as the calcination temperature of the TiO2–Au lyogel composite was increased. In this case, the number and quality of the contact points between the Au nanoparticles and the TiO2 support were not preserved. The same applies obviously to the metal–support interaction.
Fig. 4 shows TEM and HAADF-STEM images corresponding to the photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures. The images reveal that the nanostructures of the lyogels suffered important changes during the thermal treatments. In particular, TiO2 crystallites, which were not present in the uncalcined sample, were clearly observed in the images of the calcined lyogels and their size increased from about 10–15 nm up to ca. 25–50 nm as the calcination temperature increased from 400 to 625 °C. A strong grain growth of TiO2 was observed after calcination at 700 and 850 °C coincident with the transformation of anatase into rutile, with TiO2 crystallites of about 50–100 and 80–200 nm, respectively. This is in good agreement with the mean size of the TiO2 crystallites obtained by XRD and the specific surface area data reported in Table 1. Regarding the Au nanoparticles, their size was maintained at about 2.0 ± 0.5 nm as a result of the preparation method, where preformed Au nanoparticles of the same size were anchored onto the TiO2 lyogels previously calcined at the different temperatures. The similar size of Au nanoparticles measured by TEM in all the photocatalysts prepared by this way (Table 1) is in accordance with the UV-Vis spectra discussed above (Fig. 3b), where the SPR band was similar for the different samples.
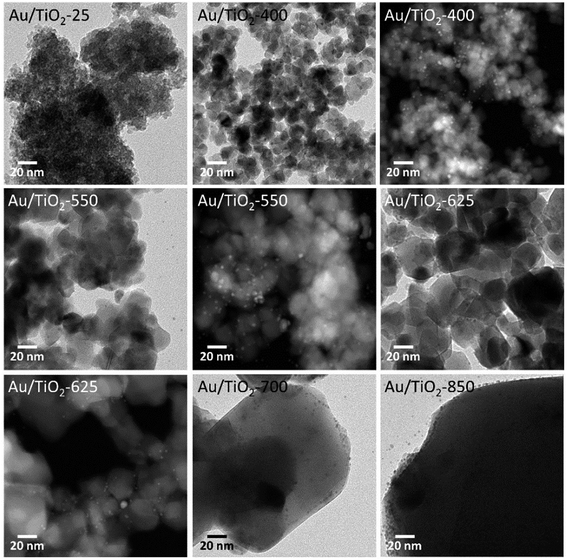 | ||
| Fig. 4 TEM and HAADF-STEM images of Au/TiO2 photocatalysts, prepared by anchoring preformed Au nanoparticles on TiO2 lyogels previously calcined at different temperatures. | ||
The changes in the morphology of TiO2 for the TiO2–Au composite lyogels at increasing calcination temperature are shown in Fig. 5 and followed exactly the same trend, from amorphous TiO2 to well faceted crystallites, except that a severe grain growth of the TiO2 crystallites occurred only at 850 °C due to the retardation effect of Au nanoparticles on the anatase–rutile transformation, as discussed above. For this series of photocatalysts, however, the size of the Au nanoparticles increased progressively from ca. 2.0 ± 0.5 nm to 18 ± 5 nm following the increase in the calcination temperature, as reported in Table 1. In the preparation of these photocatalysts, the preformed Au nanoparticles were added during the synthesis of the Au–TiO2 lyogel composite, so they were exposed directly to the subsequent calcination temperatures. This progressive growth of the Au particle size observed by TEM explains well the progressive shift toward higher wavelengths and simultaneous broadening of the SPR bands shown in Fig. 3c as the calcination temperature was increased.
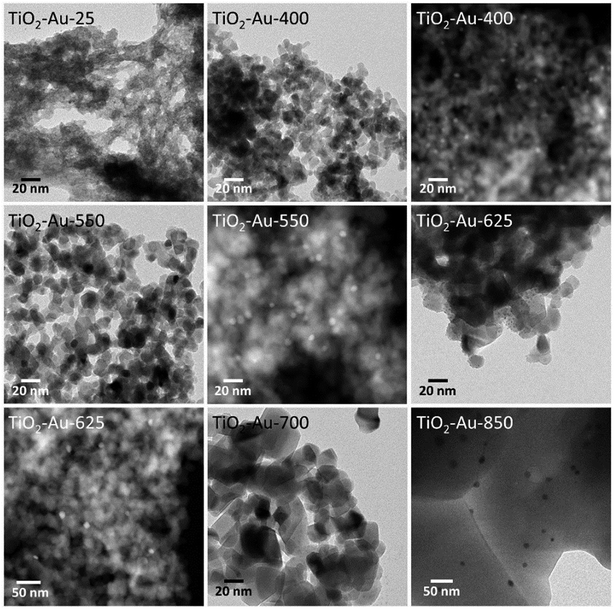 | ||
| Fig. 5 TEM and HAADF-STEM images of TiO2–Au photocatalysts, prepared by calcining a TiO2–Au composite lyogel at different temperatures. | ||
Finally, the photocatalysts were also characterized by X-ray photoelectron spectroscopy (XPS). The Ti 2p and Au 4f spectra are shown in Fig. S1† and the surface atomic Au/Ti ratios are compiled in Table 1. The Ti 2p spectra showed two main peaks corresponding to Ti4+ (Ti 2p3/2 at 458.8 eV), with an almost inexistent residual contribution of Ti3+ at lower binding energies. The Au 4f spectra showed, in all cases, two bands at 83.5 ± 0.2 and 87.1 ± 0.2 eV, which correspond well to the binding energies of the 4f7/2 and 4f5/2 bands of metallic Au, respectively, as expected from the preparation method using preformed Au0 nanoparticles. The interpretation of the surface Au/Ti atomic ratios deserves a careful analysis since they are affected by both the size of the Au nanoparticles and the surface area of the TiO2 support. For the series of Au/TiO2 photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures the size of the Au nanoparticles was maintained constant at about 2 nm. Taking into account the photon energy used for collecting the spectra (Al X-ray source) it can be assumed that the escape depth of the photoemitted electrons exceeds to a large extent the size of the Au nanoparticles, and therefore the Au/Ti atomic ratio should be constant if other parameters do not interfere with the measurement. However, from Table 1 it is clear that the surface area of this series of photocatalysts decreased strongly with the calcination temperature (from 91 down to 3 m2 g−1 when the calcination temperature increased from 400 up to 850 °C) due to the progressive increase of the TiO2 crystallite size. For that reason, the surface atomic Au/Ti ratio obtained by XPS increased with the calcination temperature of the TiO2 lyogel because, whereas the signal originating from the Au nanoparticles remained constant, the contribution of the Ti signal progressively decreased as the surface area decreased. Interestingly, there is an almost perfect indirect relationship between the photocatalyst surface area and the Au/Ti atomic ratio. This, again, can be considered as an indication of the homogeneous dispersion of Au nanoparticles with the same size over the TiO2 support in this series of photocatalysts. For the series of photocatalysts prepared from the TiO2–Au composite lyogels calcined at different temperatures, the interpretation is not that straightforward. In this case, in addition to a decrease in surface area upon calcination at increasing temperature, there is also an increase of the Au nanoparticle size (from about 2 to 18 nm, Table 1). For that reason, the surface Au/Ti atomic ratio is kept quite similar in all samples of this series (Au/Ti = 0.002–0.006). It can be noticed that these values are lower than the Au/Ti ratios measured in the series of photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures; this is a direct consequence of the different size of the Au nanoparticles.
3.2. Photocatalytic H2 production
Table 2 shows the steady-state hydrogen photoproduction rates (mmol H2 gcat−1 h−1) obtained over the different photocatalysts from gaseous water–ethanol (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 9
EtOH = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar) under dynamic conditions at GHSV = 26
1 molar) under dynamic conditions at GHSV = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 and 80 mW cm−2. The only products of the photocatalytic process measured by gas chromatography were hydrogen and acetaldehyde in equal amounts, in accordance with literature data.4,6 The photocatalytic mechanism of Au/TiO2 for the generation of hydrogen from water–ethanol mixtures has been already described in detail previously4 and will not be discussed further here. No signs of deactivation were detected over the duration of the photocatalytic tests (90 min). No photogeneration of hydrogen was obtained by using visible light and no enhancement of activity was observed by combining UV and visible light, thus ruling out any possible effect originating from SPR.
000 h−1 and 80 mW cm−2. The only products of the photocatalytic process measured by gas chromatography were hydrogen and acetaldehyde in equal amounts, in accordance with literature data.4,6 The photocatalytic mechanism of Au/TiO2 for the generation of hydrogen from water–ethanol mixtures has been already described in detail previously4 and will not be discussed further here. No signs of deactivation were detected over the duration of the photocatalytic tests (90 min). No photogeneration of hydrogen was obtained by using visible light and no enhancement of activity was observed by combining UV and visible light, thus ruling out any possible effect originating from SPR.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 using a gaseous mixture of H2O
000 h−1 using a gaseous mixture of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 9
EtOH = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar) under dynamic conditions
1 (molar) under dynamic conditions
| Sample | mmol H2 gcat−1 h−1 | mmol H2 manatase−2 h−1 |
|---|---|---|
| Au/TiO2-25 | 1.1 | — |
| Au/TiO2-400 | 16.5 | 0.13 |
| Au/TiO2-550 | 19.8 | 0.29 |
| Au/TiO2-625 | 7.2 | 0.37 |
| Au/TiO2-700 | 2.4 | 1.56 |
| Au/TiO2-850 | 0.9 | — |
| TiO2–Au-25 | 2.3 | — |
| TiO2–Au-400 | 15.0 | 0.10 |
| TiO2–Au-550 | 18.0 | 0.17 |
| TiO2–Au-625 | 13.8 | 0.19 |
| TiO2–Au-700 | 12.0 | 0.24 |
| TiO2–Au-850 | 0.6 | — |
| Au/TiO2-P25 | 18.6 | 0.30 |
From Table 2 it is inferred that the samples containing amorphous TiO2 lyogels, namely Au/TiO2-25 and TiO2–Au-25, were poorly active in the photoproduction of hydrogen (1.1–2.3 mmol H2 gcat−1 h−1). These results demonstrate that crystalline TiO2 is a requirement for the efficiency of the photoprocess, which is in accordance with previous studies where the recombination of electron–hole pairs has been related to the crystallinity of TiO2, with non-ordered structures being more prone to recombination and, hence, less photoactive.28,29 Accordingly, despite the high specific surface areas recorded for the amorphous lyogels, 480–520 m2 g−1 (Table 1), the hydrogen photoproduction rates were low. In addition, the band gap energy values measured for the amorphous lyogels, 3.21 and 3.37 eV for samples TiO2–Au-25 and Au/TiO2-25, respectively (Table 1), were significantly higher than those of crystalline TiO2 (3.0 and 3.2 eV for rutile and anatase, respectively) which, together with a fast electron–hole recombination rate, explains the low hydrogen photoproduction rates obtained over the amorphous TiO2 lyogel samples.
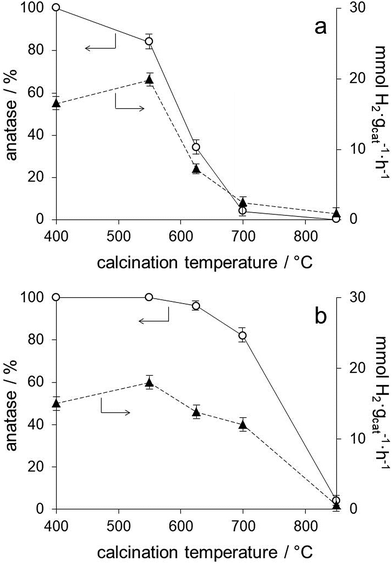
The series of photocatalysts prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogels calcined at different temperatures (Au/TiO2-400, Au/TiO2-550, Au/TiO2-625, Au/TiO2-700 and Au/TiO2-850) allows for a precise analysis of the effect of the TiO2 polymorph on the photoproduction of hydrogen because they contain, not only the same Au loading, but Au nanoparticles with exactly the same size (Table 1) and electronic properties, as deduced from the SPR bands in their UV-Vis spectra (Fig. 3b) and XPS data (Table 1 and Fig. S1†). Therefore, the number of contact points between Au and TiO2 is kept constant independent of the TiO2 polymorph and of the TiO2 particle size. From the photocatalytic results compiled in Table 2 for this series of photocatalysts it is clear that the hydrogen photoproduction rates strongly depend on the TiO2 polymorph. From XRD and Raman spectra we have shown that the sample Au/TiO2-400 contains anatase as the only crystalline TiO2 polymorph, and that anatase was progressively transformed into rutile in Au/TiO2-550, Au/TiO2-625 and Au/TiO2-700, with rutile being the only TiO2 polymorph present in Au/TiO2-850 (Fig. 1 and 2). In general, the hydrogen photoproduction rates were clearly higher in samples containing mostly anatase (Au/TiO2-400 and Au/TiO2-550) with respect to those rich in rutile. The obtained results followed this trend nicely except that the sample Au/TiO2-550, which contained 84% anatase and 16% rutile, performed best (19.8 mmol H2 gcat−1 h−1), as shown in Fig. 6a. It is interesting to note that the hydrogen photoproduction rate recorded over the sample Au/TiO2-550 is similar to that of Au/TiO2-P25 (19.8 vs. 18.6 mmol H2 gcat−1 h−1, respectively, or 0.29 vs. 0.30 mmol H2 manatase−2 h−1), which contains also a mixture of anatase and rutile in similar proportions (84.7% anatase and 16.3% rutile).30 The positive effect of the anatase–rutile heterojunction has been largely reported in the literature and has been ascribed essentially to a combined effect between the slower electron–hole pair recombination rate of anatase and the lower band gap energy of rutile.19,31 The hydrogen photoproduction rate normalized by the anatase surface area (taking into account the distribution of TiO2 polymorphs and their crystallite size calculated by XRD) is included in Table 2. It is clear that the hydrogen photoproduction normalized by the surface area of anatase (the most photoactive TiO2 polymorph) is not constant and increases with the lyogel calcination temperature prior to the incorporation of the preformed Au nanoparticles. Taking into account that the TiO2 particle size has no effect on alcohol photoreforming,31 our results can be interpreted in terms of a cooperative effect between anatase and rutile or, alternatively, as an effect of the crystallinity of TiO2. The higher the calcination temperature the higher the crystallinity of TiO2 and the higher the density of oxygen vacancy clusters, as reported in the literature from positron annihilation spectroscopy studies.20 These oxygen vacancy clusters have been related to a lower recombination rate of the electron–hole pairs, which would result in an enhanced photoactivity.
The series of photocatalysts prepared by calcining the TiO2–Au composite lyogel at different temperatures (TiO2–Au-400, TiO2–Au-550, TiO2–Au-625, TiO2–Au-700, and TiO2–Au-850) allows us to confirm the role of the TiO2 polymorph and to infer the effect of Au nanoparticle size. As shown in Fig. 6b, samples TiO2–Au-400, TiO2–Au-550 and TiO2–Au-625 exhibit similar hydrogen photoproduction rates. These samples contain 96–100% anatase (Table 1) but differ in their Au nanoparticle size (from about 2 to 6.2 nm). The fact that similar photoactivities are encountered indicates that the TiO2 polymorph has a greater influence than the Au nanoparticle size. Also, the hydrogen photoproduction rates recorded over these samples are similar to that of the photocatalyst of the previous series prepared by anchoring the preformed Au nanoparticles onto the TiO2 lyogel calcined at 400 °C, which contains 100% anatase (13.8–18.0 vs. 16.5 mmol H2 gcat−1 h−1, respectively). On the other hand, the retardation effect on the transformation of anatase into rutile with increasing the calcination temperature of the TiO2–Au composite lyogel induced by the presence of the Au nanoparticles has a clear consequence on the hydrogen photoproduction rates. This is nicely seen in Fig. 6, where the samples of the TiO2–Au composite lyogel calcined at high temperatures (625 and 700 °C) perform better (Fig. 6b) than their counterparts prepared by decorating with preformed Au nanoparticles the TiO2 lyogel previously calcined at the same temperatures (Fig. 6a). Once more, the essential role of anatase in the photoproduction of hydrogen is highlighted in front of the role of the Au nanoparticle size. This is in accordance with previous studies, which demonstrated that the photoreforming of ethanol over Au/TiO2 was independent of the Au nanoparticle size in the 3–12 nm range.9 Interestingly, the hydrogen photoproduction rate normalized by the surface area of anatase exposed is markedly different for the samples calcined at 550 °C (0.29 vs. 0.17 mmol H2 manatase−2 h−1 for Au/TiO2-550 and TiO2–Au-550, respectively), even if they show similar hydrogen photoproduction rates on a weight basis (19.8 and 18.0 mmol H2 gcat−1 h−1). This is explained because the sample Au/TiO2-550 contains a mixture of anatase and rutile, whereas the sample TiO2–Au-550 is 100% anatase.
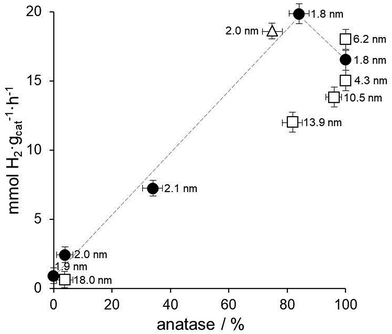
Fig. 7 shows the relationship between the amount of anatase, Au nanoparticle size and hydrogen photoproduction rate over all the Au/TiO2 photocatalysts prepared in this work. As expected from the precedent discussion, the Au/TiO2 photocatalysts prepared by anchoring preformed Au nanoparticles on TiO2 lyogels previously calcined at different temperatures (black circles in Fig. 7) show a clear positive trend between hydrogen photoproduction rates and the amount of anatase in those samples containing both anatase and rutile. For the sample containing only anatase lower hydrogen photoproduction rates are recorded due to the absence of the anatase–rutile heterojunction (see the dashed line in Fig. 7). Since these samples contain preformed Au nanoparticles with exactly the same size and contact points, this relationship is independent of the Au nanoparticle size and of the metal–support interaction. The same applies to the sample prepared with commercial titania, Au/TiO2-P25 (triangle in Fig. 7), which shows a similar hydrogen photoproduction rate and anatase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rutile content to the sample Au/TiO2-550.
rutile content to the sample Au/TiO2-550.
The effect of Au nanoparticle size can be discussed by comparing this series with the series of photocatalysts prepared by calcining the TiO2–Au composite lyogel at different temperatures (squares in Fig. 7). In this case it is observed that the photoproduction of hydrogen over TiO2–Au-400 and TiO2–Au-550 samples is similar to that of Au/TiO2-400. All these samples contain anatase as the only crystalline TiO2 polymorph, but they contain Au nanoparticles ranging from 1.8 up to 6.2 nm, which is a clear indication that the Au nanoparticle size does not play an important role in the photoproduction of hydrogen. However, when the hydrogen photoproduction rates are normalized by the Au surface area these values translate into 10.6 mmol H2 mAu−2 h−1 for Au/TiO2-400 but into 20.7 and 35.9 mmol H2 mAu−2 h−1 for TiO2–Au-400 and TiO2–Au-550, respectively. This suggests that there is a better metal–support interaction when the composite lyogel containing both Au and TiO2 is calcined, favoring the photocatalytic process with respect to the photocatalysts prepared by ball grinding, where a weak metal–support interaction is likely to occur. It should be recalled that in all cases the XP spectra indicated the sole presence of metallic Au, thus ruling out the possibility that the oxidation state of Au could influence the photoproduction of hydrogen and, in turn, be influenced by the size of the Au nanoparticles. Finally, the different hydrogen photoproduction rates of samples TiO2–Au-700 and Au/TiO2-550, which contain Au nanoparticles of 13.9 and 1.8 nm, respectively, but a similar amount of anatase (82 and 84%), can be satisfactorily explained taking into account the crystallite size of anatase (29 nm for TiO2–Au-700 and 19 nm for Au/TiO2-550) because their hydrogen photoproduction rates normalized by the surface area of anatase exposed are similar, 0.24 and 0.29 mmol H2 manatase−2 h−1 for TiO2–Au-700 and Au/TiO2-550, respectively. In contrast, their hydrogen photoproduction rates normalized by the surface area of Au are markedly different, 53.7 vs. 12.7 mmol H2 mAu−2 h−1 for TiO2–Au-700 and Au/TiO2-550, respectively, which again suggests that the metal–support interaction is an important factor to consider.
4. Conclusions
The photogeneration of hydrogen from ethanol–water was studied over Au/TiO2 photocatalysts prepared from lyogels. One series of samples were prepared by anchoring preformed Au nanoparticles of about 2 nm over TiO2 lyogels calcined at different temperatures. The resulting materials contained different proportions of TiO2 polymorphs with different crystallinity and dimensions depending on the calcination temperature of the TiO2 lyogel, but a constant Au loading and Au nanoparticle size. This ensured a constant number and quality of contact points between Au nanoparticles and TiO2, thus allowing a careful analysis of the effect of the TiO2 polymorph on the photoactivity. Photocatalysts containing crystalline mixtures of anatase and rutile exhibited the highest photoactivity, followed by samples containing only anatase. Samples containing rutile or amorphous phases were poorly active. On the other hand, the photocatalysts prepared by calcining a TiO2–Au composite lyogel at different temperatures yielded Au nanoparticles ranging from 2 up to 18 nm with a strong metal–support interaction. The results indicated that the TiO2 polymorph has a greater influence than the Au nanoparticle size on the photoproduction of hydrogen.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work has been carried out through the MINECO/FEDER grant ENE2015-63969. JL is a Serra Hunter Fellow and is grateful to the ICREA Academia program and grant GC 2017 SGR 128. MB, IM and EM are grateful to the MINECO/FEDER grant SEV2015-0496. LM is grateful to CONACYT México for the PhD grant no. 409809.References
- G. Colón, Towards the hydrogen production by photocatalysis, Appl. Catal., A, 2016, 518, 48–59 CrossRef
.
- J. Qi, W. Zhang and R. Cao, Solar-to-Hydrogen Energy Conversion Based on Water Splitting, Adv. Energy Mater., 2018, 8, 1701620 CrossRef
.
- N. Armaroli and V. Balzani, Solar Electricity and Solar Fuels: Status and Perspectives in the Context of the Energy Transition, Chem.–Eur. J., 2016, 22, 32–57 CrossRef PubMed
.
- M. Murdoch, G. I. N. Waterhouse, M. A. Nadeem, J. B. Metson, M. A. Keane, R. F. Howe, J. Llorca and H. Idriss, The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles, Nat. Chem., 2011, 3, 489–492 CrossRef PubMed
.
- A. Primo, A. Corma and H. García, Titania supported gold nanoparticles as photocatalyst, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC
.
- E. Taboada, I. Angurell and J. Llorca, Dynamic photocatalytic hydrogen production from ethanol-water mixtures in an optical fiber honeycomb reactor loaded with Au/TiO2, J. Catal., 2014, 309, 460–467 CrossRef
.
- H. Alghamdi, K. Katsiev, A. K. Wahab, J. Llorca and H. Idriss, Up-conversion luminescence coupled to plasmonic gold nanorods for light harvesting and hydrogen production, Chem. Commun., 2017, 53, 13051–13054 RSC
.
- B. Gupta, A. A. Melvin, T. Matthews, S. Dash and A. K. Tyagi, TiO2 modification by gold (Au) for photocatalytic hydrogen (H2) production, Renewable Sustainable Energy Rev., 2016, 58, 1366–1375 CrossRef
.
- D. G. Nocera, Solar fuels and solar chemicals industry, Acc. Chem. Res., 2017, 50, 616–619 CrossRef PubMed
.
- A. A. Ismail and D. W. Bahnemann, Photochemical splitting of water for hydrogen production by photocatalysis: a review, Sol. Energy Mater. Sol. Cells, 2014, 128, 85–101 CrossRef
.
- L. Soler, A. Casanovas, A. Urrich, I. Angurell and J. Llorca, CO oxidation and COPrOx over preformed Au nanoparticles supported over nanoshaped CeO2, Appl. Catal., B, 2016, 197, 47–55 CrossRef
.
- J. B. Priebe, J. Radnik, A. J. J. Lennox, M.-M. Pohl, M. Karnahl, D. Hollmann, K. Grabow, U. Bentrup, H. Junge, M. Beller and A. Brückner, Solar Hydrogen Production by Plasmonic Au–TiO2 Catalysts: Impact of Synthesis Protocol and TiO2 Phase on Charge Transfer Efficiency and H2 Evolution Rates, ACS Catal., 2015, 5, 2137–2148 CrossRef
.
- G. L. Chiarello, M. V. Dozzi and E. Selli, TiO2-based materials for photocatalytic hydrogen production, J. Energy Chem., 2017, 26, 250–258 CrossRef
.
- A. Pons, L. Casas, E. Estop, E. Molins, K. D. M. Harris and M. Xu, A new route to aerogels: monolithic silica cryogels, J. Non-Cryst. Solids, 2012, 358, 461–469 CrossRef
.
- X. Wang and R. A. Caruso, Enhancing photocatalytic activity of titania materials by using porous structures and the addition of gold nanoparticles, J. Mater. Chem., 2011, 21, 20–28 RSC
.
- S. R. Mukai, H. Nishihara, S. Shichi and H. Tamon, Preparation of Porous TiO2 Cryogel Fibers through Unidirectional Freezing of Hydrogel Followed by Freeze-Drying, Chem. Mater., 2004, 16, 4987–4991 CrossRef
.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC
.
- E. Molins, M. Benito, I. Mata, L. Martinez, L. Soler and J. Llorca, Au/TiO2 Lyogels for Hydrogen Production, MRS Adv., 2017, 2, 3499–3504 CrossRef
.
- Z. H.
N. Al-Azri, W.-T. Chen, A. Chan, V. Jovic, T. Ina, H. Idriss and G. I. N. Waterhouse, The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures, J. Catal., 2015, 329, 355–367 CrossRef
.
- J. Yan, G. Wu, N. Guan, L. Li, Z. Li and X. Cao, Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: anatase versus rutile, Phys. Chem. Chem. Phys., 2013, 15, 10978–10988 RSC
.
- A. Wypych, I. Bobowska, M. Tracz, A. Opasinska, S. Kadlubowski, A. Krzywania-Kaliszewska, J. Grobelny and P. Wojciechowski, Dielectric properties and characterisation of titanium dioxide obtained by different chemistry methods, J. Nanomater., 2014, 2014, 124814 Search PubMed
.
- A. Alagarasi, P. U. Rajalakshmi, K. Shanthi and P. Selvam, Ordered mesoporous nanocrystalline titania: a promising new class of photocatalytic materials, Catal. Today, 2018, 309, 202–211 CrossRef
.
- H. L. Ma, J. Y. Yang, Y. Dai, Y. B. Zhang, B. Lu and G. H. Ma, Raman study of phase transformation of TiO2 rutile single crystal irradiated by infrared femtosecond laser, Appl. Surf. Sci., 2007, 253, 7497–7500 CrossRef
.
- A. A. Melvin, K. Illath, T. Das, T. Raja, S. Bhattacharyya and C. S. Gopinath, M–Au/TiO2 (M = Ag, Pd, and Pt) nanophotocatalyst for overall solar water splitting: role of interfaces, Nanoscale, 2015, 7, 13477–13488 RSC
.
- A. L. Linsebigler, G. Lu and J. T. Yates, Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results, Chem. Rev., 1995, 95, 735–758 CrossRef
.
- V. Jovic, W.-T. Chen, D. Sun-Waterhouse, M. G. Blackford, H. Idriss and G. I. N. Waterhouse, Effect of gold loading and TiO2 support composition on the activity of Au/TiO2 photocatalysts for H2 production from ethanol–water mixtures, J. Catal., 2013, 305, 307–317 CrossRef
.
- J. Fang, S.-W. Cao, Z. Wang, M. M. Shahjamali, S. C. J. Loo, J. Barber and C. Xue, Mesoporous plasmonic Au–TiO2 nanocomposites for efficient visible-light-driven photocatalytic water reduction, Int. J. Hydrogen Energy, 2012, 37, 17853–17861 CrossRef
.
- P. Calza, E. Pelizzetti, K. Mogyorósi, R. Kun and I. Dékány, Size dependent photocatalytic activity of hydrothermally crystallized titania nanoparticles on poorly adsorbing phenol in absence and presence of fluoride ion, Appl. Catal., B, 2007, 72, 314–321 CrossRef
.
- B. Ohtani, Y. Ogawa and S. Nishimoto, Photocatalytic activity of amorphous-anatase mixture of titanium(IV) oxide particles suspended in aqueous solutions, J. Phys. Chem. B, 1997, 101, 3746–3752 CrossRef
.
- S. Bakardjieva, J. Šubrt, V. Štengl, M. J. Dianez and M. J. Sayagues, Photoactivity of anatase-rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase, Appl. Catal., B, 2005, 58, 193–202 CrossRef
.
- M. A. Nadeem, M. Murdoch, G. I. N. Waterhouse, J. B. Metson, M. A. Keane, J. Llorca and H. Idriss, Photoreaction of ethanol on Au/TiO2 anatase: comparing the micro to nanoparticle size activities of the support for hydrogen production, J. Photochem. Photobiol., A, 2010, 216, 250–255 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00293b |
| This journal is © The Royal Society of Chemistry 2018 |