On the development of a proton conducting solid polymer electrolyte using poly(ethylene oxide)†
Sudeshna
Patra
a,
Anand B.
Puthirath
 a,
Thazhe Veettil
Vineesh
a,
Sreekanth
Narayanaru
a,
Bhaskar
Soman
a,
Shruti
Suriyakumar
b,
A. Manuel
Stephan
b and
Tharangattu N.
Narayanan
a,
Thazhe Veettil
Vineesh
a,
Sreekanth
Narayanaru
a,
Bhaskar
Soman
a,
Shruti
Suriyakumar
b,
A. Manuel
Stephan
b and
Tharangattu N.
Narayanan
 *a
*a
aTata Institute of Fundamental Research – Hyderabad, Sy. No. 36/P, Gopanapally Village, Serilingampally Mandal, Ranga Reddy District, Hyderabad 500107, India. E-mail: tnn@tifrh.res.in; tn_narayanan@yahoo.com
bCSIR-Central Electrochemical Research Institute, Karaikudi – 630003, Tamilnadu, India
First published on 26th June 2018
Abstract
By mimicking the polymer backbone assisted ‘hop and lock’ lithium ion transport in lithium solid polymer (SP) electrolytes, a new type of proton (H+) transport membrane cum separator is designed which is found to work even in pure water electrolysis. An inexpensive H+ transporting SP membrane (HPEOP) is formulated using perchloric acid (HClO4) as the proton source with a poly(ethylene oxide) (PEO) and polydimethylsiloxane blend as the host structure. The H+ coordinated PEO backbone via the solvation of HClO4 allows easy transport of H+ through PEO segmental motion and inter-segmental hopping. Humidity dependent ionic conductivity measurements on the optimized HPEOP membrane show higher values in comparison to those of Nafion 117, and a considerable ionic conductivity was shown by HPEOP even in an anhydrous environment (3.165 ± 0.007 mS cm−1) unlike Nafion 117 (∼10−7 mS cm−1). Lowering the melting temperature of PEO through HClO4 ‘salting in’ is found to have a considerable effect in enhancing the conductivity of this SP membrane, while addition of HClO4 also modifies the microstructure and mechanical strength of the membrane. Water electrolysis ‘H’ cells are constructed with both pure and protonated water using both HPEOP and Nafion separators (membranes), and studies show the possibilities of highly efficient low cost water electrolysis and fuel cells devoid of expensive Nafion membranes.
1 Introduction
Proton (H+) conducting solid membranes are receiving tremendous scientific attention due to their widespread applications in proton exchange membrane fuel cells, water electrolysers, electrochemical sensors, and reactors.1–6 In these various developed membranes, Nafion – a sulfonated tetrafluoroethylene based fluoropolymer-copolymer by M/s. DuPont, is considered as a benchmarked polyelectrolyte membrane separator in fuel cells and water electrolysis due to its high ionic conductivity in relatively high humidity.7–11 It has a robust structure, high proton conductivity (0.13 S cm−1 at 75 °C and 100% relative humidity (RH)), chemical stability and longevity (>60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h) in the context of a typical fuel cell environment.12 High manufacturing cost, poor performance at high temperatures (above 80 °C) and/or low humidity (below ∼80% relative humidity (RH)) hinder its commercial applicability, and thus open new endeavours for alternate materials.13–15 Such novel materials developed during the recent past include coordination polymers (CPs),16,17 metal organic frameworks (MOFs),18–21 polyoxometallates,22,23 and covalent organic frameworks.24,25 Although chemical modification of the membrane backbone to create more proton hopping sites is the core part of the synthesis of efficient proton conducting materials,26,27 poor physicochemical stability and difficulties in large scale processability hinder widespread commercialization of such reported membrane materials.28,29
000 h) in the context of a typical fuel cell environment.12 High manufacturing cost, poor performance at high temperatures (above 80 °C) and/or low humidity (below ∼80% relative humidity (RH)) hinder its commercial applicability, and thus open new endeavours for alternate materials.13–15 Such novel materials developed during the recent past include coordination polymers (CPs),16,17 metal organic frameworks (MOFs),18–21 polyoxometallates,22,23 and covalent organic frameworks.24,25 Although chemical modification of the membrane backbone to create more proton hopping sites is the core part of the synthesis of efficient proton conducting materials,26,27 poor physicochemical stability and difficulties in large scale processability hinder widespread commercialization of such reported membrane materials.28,29
Along similar lines to solid membranes for H+ conduction, lithium ion (Li+) conduction through solid membranes is another burgeoning field of research. Solid state electrolytes provide ample opportunities to alleviate the potential risks like volatilization, flammability, and explosion of liquid electrolyte based Li ion batteries.30–32 Among various Li transport membranes, inorganic solid electrolytes, solid polymer electrolytes (SPEs), and composite solid electrolytes are the main classes having a high Li transport number and ionic conductivity.32 SPEs are emerging as an appealing platform for Li+ batteries due to their excellent safety, mechanical stability and flexibility.33 Recently, the authors had shown the high ionic conductivity of a Li+ transport membrane developed using lithium perchlorate (LiClO4) in a poly(ethylene oxide) (PEO)–polydimethylsiloxane (PDMS) blend.34 It is proven that a salt having low lattice energy and a host polymer possessing a high dielectric constant are the main constituents of an optimal solid polymer electrolyte system.35 Due to this, PEO is considered as a Li+ host polymer since its discovery due to its low cost, easy fabrication, and good electrochemical stability,36–38 but due to its decelerated polymer chain dynamics upon crystallinity at room temperature (melting temperature, Tm ∼ 67 °C), PEO has low ionic conductivity (10−8 to 10−4 S cm−1) at room temperature.39,40 Hence amorphization of the PEO matrix completely by the suppression of Tm below room temperature by various means was instigated by many researchers.38,41–44 Our recent work shows that LiClO4 solvated in PEO has a salting-in effect, where it lowers the Tm of the PEO to below room temperature, and PDMS acts as a plasticizer in the PEO matrix.34 These synergistic effects lead to high Li+ conducting transparent flexible Li transport membranes which are directly applied to solid state batteries.34
Here we report such a transport mechanism for H+ ions using solvated perchloric acid (HClO4) in PEO, where one could develop inexpensive mechanically robust H+ transport solid state membranes (HPEOPs) where their conductivity properties are found to be better than those of commercially available Nafion 117 membranes. Understanding the proton transport through a coordinated water environment is highly challenging and various mechanisms were proposed for the proton transport through solid state membranes.45 Here, the transport mechanism of H+ ions is proposed to be through the backbones of PEO chains, where H+ ions coordinate with the oxygen radicals (lock) in PEO, and the segmental motion of the amorphized PEO matrix along with the inter-segmental hopping of ions among different sites (PEO segments) aids the ion transport through the polymer matrix. This ‘hop and lock’ mechanism is in tune with the recent theoretical predictions on proton transfer through hydrated environments by Ali Hassanali et al., where they propose that proton and hydroxide diffusion occurs through periods of intense activity involving concerted proton hopping followed by periods of rest.46 Such ‘hop and lock’ mechanism assisted H+ ion transport membranes have not been attempted in the past, to the best of our knowledge. PDMS ensures the structural integrity of these solid membranes. A water electrolysis ‘H’ cell is setup to study the temperature dependent conductivity performance of the HPEOP membranes with varying HClO4 content and pure water (resistivity of 18.2 MΩ cm) electrolysis is even found to be feasible with these membranes.
2 Results and discussion
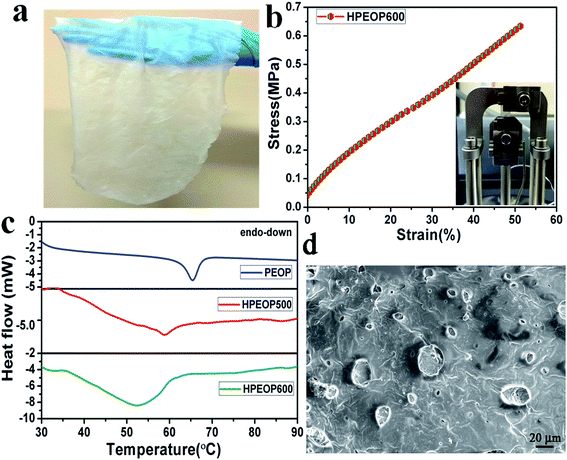
Detailed synthesis procedures for various proton conducting solid polymer electrolytes (HPEOPs) are given in the Experimental section and also shown in ESI (Fig. S1†). After optimizing the synthesis, three types of H+ membranes were subjected to detailed studies, namely, HPEOP400, HPEOP500, and HPEOP600 with varying HClO4 content as shown in Table 1. Fig. 1a shows the formation of a large area uniform, thin (thickness ∼90–100 μm) flexible proton transport membrane using a doctor blade film applicator (coating) method. By adjusting the blade-platform gap in the applicator, the thickness was varied up to 190 μm, which is used to compare the conductivity with that of Nafion 117 (thickness 190 μm). Fourier transform-infrared (FTIR) and micro-Raman spectra of the HPEOP600 are shown in Fig. S2(a) and (b) (and Fig. S3†) and the characteristics peaks are listed in Table S1.† The analyses show the presence of characteristics of PEO, PDMS and HClO4 in the membranes.| HClO4 (μL) | PEO (mg) | PDMS (mL) | Cross-linker (μL) | |
|---|---|---|---|---|
| a The abbreviation HPEOP stands for the following constituents: H (HClO4), PEO (polyethylene oxide), P (PDMS). | ||||
| HPEOP400 | 400 | 100 | 1 | 100 |
| HPEOP500 | 500 | 100 | 1 | 100 |
| HPEOP600 | 600 | 100 | 1 | 100 |
The mechanical properties of the membranes are important for their commercial applicability. Static tensile test measurements using a dynamic mechanical analyzer (DMA) are shown in Tables 2 and S1.† It can be seen from both the tables that as the HClO4 loading increases, the films become stiffer with higher tensile stress and have lower elongation at break (Fig. S4†). The tensile stress of HPEOP600 is found to be 0.62 MPa (Fig. 1b, the inset shows the HPEOP600 clamped with a tensile clamp of DMA) with the ultimate tensile strain of ∼50%. The changes in the mechanical properties of membranes with the HClO4 amount can be attributed to the reaction of PDMS with concentrated HClO4 forming oligomers with different numbers of dimethylsiloxane subunits [–(CH3)2SiO].47 There is also a possibility of high degree of ion aggregation48 with high HClO4 content leading to its precipitation (as seen in micro-Raman analyses where some part of the membranes showed the presence of unsolvated HClO4, though this won't participate in the H+ conduction which is discussed in the later part, Fig. S5†) which eventually makes the polymer brittle.
| Material | Tensile stress, σ (MPa) | Elongation at break (%) |
|---|---|---|
| HPEOP400 | 0.24 ± 0.11 | 187 ± 3 |
| HPEOP500 | 0.86 ± 0.35 | 112 ± 2 |
| HPEOP600 | 0.62 ± 0.01 | 50 ± 2 |
In order to study the effect of HClO4 on PEO crystallization temperature (Tm), differential scanning calorimetric (DSC) studies are conducted (10° min−1 scan rate, Fig. 1c). The PEO–PDMS system devoid of HClO4 shows a clear endothermic peak at 67 °C. This is in accordance with the reported values of Tm of PEO.49 But, upon addition of HClO4, the Tm is found to be consistently shifting to lower temperatures (59 °C for HPEOP500 and 52 °C for HPEOP600) along with the broadening of transition indicating the amorphization of the matrix. This study indeed shows the ‘salting in’ effect of HClO4, similar to that reported with the salt LiClO4 in the PEO matrix. A relatively high intense broad peak of HPEOP600 might be due to the clumps of distorted PEO at this site, but a consistent temperature shift to a lower value is evident in this sample too. Further, a scanning electron microscope (SEM) image of the HPEOP600 is shown in Fig. 1d. The microstructure modification of the membrane is found to occur where its structure shows a stark contrast from the pure PEO–PDMS matrix (Fig. S3d†). This has been previously observed as the effect of ‘salting in’ in polymers, where crystallization induced gelation boundary shifting and porosity formation in polymers are reported.50
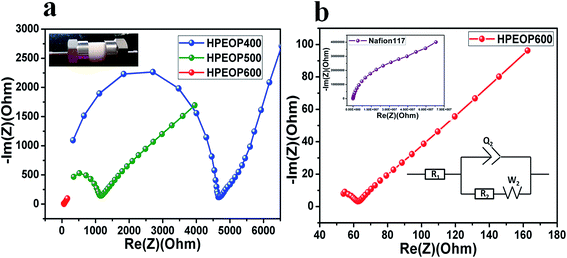
The ionic conductivities of the membranes are calculated using impedance spectroscopy (EIS, details of the measurement are given in the Methods section) studies (Nyquist plots), which are conducted by sandwiching the membrane in a two-electrode (stainless steel blocking electrodes of 12 mm diameter) parallel plate capacitor configuration using a Swagelok cell (results are shown in Fig. 2). All the measurements are carried out on completely dried samples, where the films are dried overnight at 60 °C on a hot plate before each measurement. Nafion 117 is also studied for conductivity measurements using the same setup under similar conditions. It can be seen that the bulk impedances (Rb) for HPEOP400 and HPEOP500 are quite high in comparison to that of HPEOP600 (Fig. 2a). The Rb of Nafion 117 (Fig. 2b) is found to be quite high (∼107 Ω) indicating the very low conductivity of Nafion under anhydrous conditions. The Randles circuit used for calculating Rb is shown in the inset. The tabulated conductivities are shown in Table 3.
| Membrane | Conductivity (S cm−1) at 25 °C |
|---|---|
| Nafion 117 | (4.77 ± 0.028) × 10−10 |
| HPEOP600 | (3.165 ± 0.007) × 10−3 |
| HPEOP500 | (1.08 ± 0. 028) × 10−4 |
| HPEOP400 | (1.9 ± 0. 2) × 10−5 |
Further, humidity dependent conductivity variations of different membranes are determined using a humidity chamber (Jeiotech TH-G 180) with a two electrode setup, and the results are shown in Fig. 3. The results show a systematic enhancement in the conductivity of the membranes with humidity, from 20% to 80%. The Nafion 117 conductivity values corresponding to different humidities are similar to earlier reports.51 It is to be noted that HPEOP 600 has very high ionic conductivity (∼0.01 S cm−1) even at lower humidity (30%) where Nafion 117 showed a low conductivity of ∼0.001 S cm−1. This indicates the presence of transportable protons in HPEOP 600 which can augment the hydronium ion transport under hydrated conditions (with reasonable humidity values).
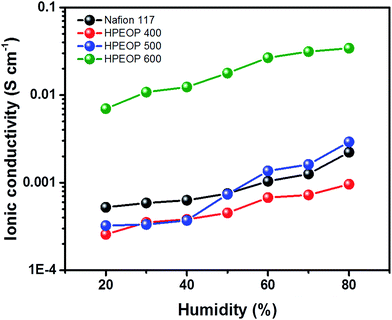 | ||
| Fig. 3 Humidity dependent (relative humidity, %) ionic conductivity variations of different membranes at 30 °C. | ||
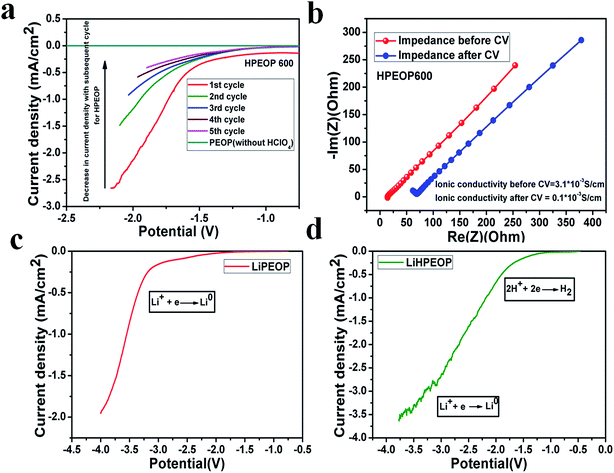
To further probe the existence of protons in HPEOP 600 dried membranes and the transport of these ions through the dried (60 °C) membrane (polymer matrix), the ion precipitation (reduction) measurements are conducted using linear sweep voltammetry (LSV) measurements using a Swagelok cell (two electrode). The potential sweep is carried out using a potentiostat in a window of 0 to −3 V (two electrode setup) with a scan rate of 50 mV s−1 (Fig. 4a). A drastic enhancement in the current density (current density calculated considering the geometrical area of the steel electrode) for HPEOP600 is found to occur at ∼−1.5 V, and this has subsequently decreased in the further cycles. The conductivity of the cycled and uncycled samples is calculated using impedance spectra (Fig. 4b), and prior to the cycling, the conductivity value (0.003 S cm−1) decreased to 0.001 S cm−1 after 5 cycles. This indicates the utilization of mobile H+ ions in the membrane, and the consistent decrease in the current density shows the limited availability of H+ in the membrane. This is in tune with the recent theoretical predictions that proton transport happens through the formed ‘proton wires’, where the utilisation of these protons affects the temporal and spatial movement of protons.46 No such current enhancement is observed for the PEO–PDMS membrane (PEOP, devoid of HClO4) in the entire potential window, as seen in Fig. 4a. Further, a Li+ ion conducting membrane is also developed with the same method with LiClO4 (0.1 g) instead of HClO4, to show its transport through the membrane and precipitation (reduction) at the blocking electrode, which happens at a much higher potential than that of protons. The LSV measurements on the same are shown in Fig. 4c, and the enhancement in current density is found to occur at ∼−3.2 V, corresponding to the precipitation of Li (reduction of Li+ to Li). The reduction of Li+ is in tune with the well reported values of the same process.52 Further, the films taken out from the cell have black deposits on the metal surface (Swagelok cell), indicating the formation of lithium oxide/hydroxide as evident from the Raman analyses, upon the exposure of plated lithium to an ambient atmosphere.
Further, the possibilities of multi-ion carrier capacities of such membranes are studied by solvating both HClO4 (600 μL) and LiClO4 (0.75 g) in the PEO–PDMS matrix. The LSV measurement shows enhancement in the current density from −1.5 V onwards to much higher values up to −3.5 V (Fig. 4d), showing the presence of mobile H+ and Li+ ions and their reduction processes at the respective potentials. It is to be noted that controlled experiments are conducted by soaking the PEO–PDMS (PEOP) membranes in HClO4 for 24 hours (drying at 60 °C on a hot plate overnight), and also making PDMS–HClO4 based membranes (devoid of PEO). Both the cases show negligible/nil H+ conductivity, ruling out the possibilities of trapped HClO4 in HPEOP towards conduction and contribution of PDMS in HPEOP membranes to conduction. All these precipitation studies show that movable ions (H+ and/or Li+) can be inserted into such a PEO matrix and these ion channels are important while using these membranes in water electrolysis cells or Li ion conducting batteries.
To further study the H+ ion transport through this membrane (HPEOP600) in a real water electrolysis cell (Fig. 4a inset), a cell is designed in the form of the character ‘H’ and a magnified picture of the same is shown in Fig. S6.† The anode and cathode are made of platinum (Pt) wire, and the electrochemical surface area of the Pt is calculated for current density calculations (the details are given in ESI, Fig. S7†). The two compartments (arms) of the ‘H’ cells are separated using the HPEOP600 membrane (Nafion 117 in respective studies), and leak proof nature (electrolyte transport/leaking among the compartments) is confirmed prior to each test. Comparisons are made with similar thick HPEOP and Nafion 117 membranes, and the electrode positions are kept fixed and kept close to the membranes to decrease the solution resistance in all measurements.
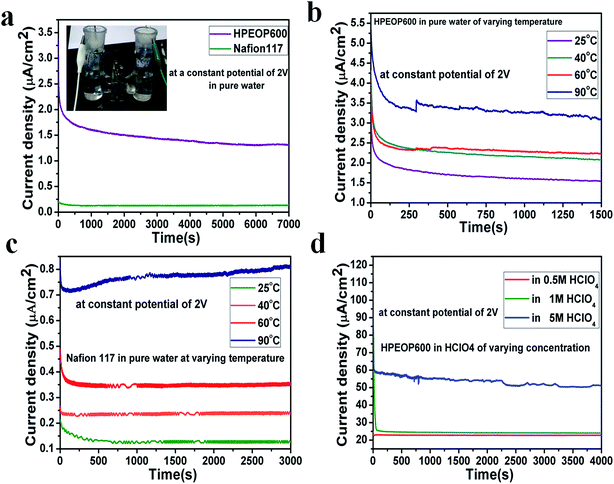
Fig. 5a shows the chronoamperometric curve of ultra-pure water (type I water, 18.2 MΩ cm resistivity) electrolysis. Recent studies show that the H+ transport mechanism in Nafion membranes is due to the collective response of both structural and dynamical properties of the water network instead of the simple Grotthuss mechanism,46 whereas long range ion motion and hopping are the principle transport phenomena in solid polymer electrolytes. There is no/little bare H+ in Millipore (type I) water which can enter the hydrophilic regions around the clusters of sulfonated side chains in Nafion. Eventually there is only negligible current density (0.18 μA cm−2) for Nafion, and at the same time there is considerable (1.5 μA cm−2) increase in current density for the HPEOP600 membrane. A stable current density was observed with the HPEOP600 membrane in pure water and the stability was tested up to 12 hours (Fig. S8† data show up to 8 hours). Prior to this work, the work which showed pure water electrolysis was that of Li Xia et al. using an alkaline polymer electrolyte (APE) where their strategy was increasing the cationic groups (self-cross-linking ammonia polysulfone) attached to the polymer chain in the APE membrane.53 In HPEOP membranes, the electric field assisted motion of the H+ ions within the membrane followed by the diffusion of generated H+ from the anode compartment to the cathode might be the reason for the longstanding performance of the cell with constant current density.
The hopping mechanism and segmental motion are more favoured at higher temperatures. Temperature dependent conductivity measurements show the consistent enhancement in the current densities of both the membranes (Fig. 5b and c), with much higher current densities for HPEOP600 than Nafion. The H+ transport of the HPEOP600 is also tested using HClO4 and H2SO4 electrolytes (Fig. 5d and S9†), and the results show much enhanced current densities (an order magnitude higher) in both media and different concentrations with stable performance. These studies show the efficacy of the developed HPEOP membranes as proton exchange membranes with better performance than expensive commercial membranes, and can also be used in pure water electrolysis. A membrane electrode stacked cell assembly setup study will help to quantify the generated gases and these studies are in progress. The cost analysis shows that, with laboratory grade reagents, the HPEOP600 can cost (without the labour charge) ∼$ 1.31 for 225 cm2 area, while that of Nafion 117 is ∼$ 65 (ref. 54) (Table S2†). A mechanistic insight into the proton transport through these membranes is still lacking and molecular dynamics based studies are in progress to unravel the mechanism.
3 Conclusions
A new method for the development of an inexpensive ($ 1.31 per 225 cm2 area) proton transport membrane is proposed, and the developed HPEOP600 membrane is tested for its pure water and protonated (acidified) water electrolysis efficacies. The mechanism of H+ transport in HPEOP membranes is proposed to be similar to that of solid polymer electrolytes of lithium, where the segmental motion of the polymer chains along with the inter-segmental hopping of ions among different sites aids the conduction, as it is evident from this study that amorphization of the PEO matrix improved the conductivity. The HPEOP600 membrane's ionic conductivity at 30 °C is found to systematically vary from ∼0.01 S cm−1 to 0.1 S cm−1 while changing the humidity from 20% to 80%, reaching the reported values of Nafion 117 conductivity at 100% humidity and 75 °C. Pure water electrolysis using HPEOP600 is demonstrated using a homemade ‘H’ cell, where it showed a stable (12 hours) current density of 1.75 μA cm−2 at 2 V (whereas that of Nafion is 0.18 μA cm−2). The H+ transport efficacy of HPEOP600 in protonated electrolytes (acid) is much higher and the ‘H’ cell current densities with acid electrolytes are an order higher with HPEOP membranes. This work shows the possibilities of revolutionizing water electrolysis and proton exchange fuel cells with inexpensive new membranes and separators which are devoid of expensive benchmarked Nafion or other membranes.4 Experimental section
4.1 Preparation of HPEOP membranes
The reagents used for H+ transport membrane preparation are perchloric acid (HClO4), poly(ethylene oxide) (PEO; average Mv 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, CAS number 25322-68-3, Sigma Aldrich), and polydimethylsiloxane (PDMS, viscosity-15–40 mPa s, CAS number 63148-57-2). Initially, 0.1 g of PEO is dissolved in varying concentrations of HClO4 (70%) (as given in Table 1). 1 mL of PDMS is added to this mixture followed by the addition of 100 μL of curing agent. The details of the cross-linking mechanism are explained in the ESI.†55 The whole slurry is blended rigorously by mortaring it for 15–20 minutes. Finally, the viscous slurry is transferred to a doctor blade film applicator for getting a large area, uniform thin film. After curing at 90° for 24 hours, the solid polymer electrolyte membranes are formed and they are named HPEOP400, HPEOP 500, HPEOP600 (naming corresponds to the addition of HClO4). It is to be noted that HPEOP films cured even at higher temperature (130 °C) showed similar conductivity values. The other concentrations tested for mechanical analyses are mentioned in the ESI.†
000, CAS number 25322-68-3, Sigma Aldrich), and polydimethylsiloxane (PDMS, viscosity-15–40 mPa s, CAS number 63148-57-2). Initially, 0.1 g of PEO is dissolved in varying concentrations of HClO4 (70%) (as given in Table 1). 1 mL of PDMS is added to this mixture followed by the addition of 100 μL of curing agent. The details of the cross-linking mechanism are explained in the ESI.†55 The whole slurry is blended rigorously by mortaring it for 15–20 minutes. Finally, the viscous slurry is transferred to a doctor blade film applicator for getting a large area, uniform thin film. After curing at 90° for 24 hours, the solid polymer electrolyte membranes are formed and they are named HPEOP400, HPEOP 500, HPEOP600 (naming corresponds to the addition of HClO4). It is to be noted that HPEOP films cured even at higher temperature (130 °C) showed similar conductivity values. The other concentrations tested for mechanical analyses are mentioned in the ESI.†
4.2 Preparation of a LiPEOP membrane
Another kind of membrane comprised of Li+ ions was prepared in a similar manner by dissolving a desired quantity of LiClO4 (battery grade, CAS number 7791-03-9, Sigma Aldrich) in an appropriate amount of ethanol (CAS number 64-17-5). The remaining procedure is identical to that discussed for HPEOP.4.3 Preparation of LiHPEOP
Sample preparation begins with dissolving PEO in a mixture of HClO4 and LiClO4 followed by the addition of PDMS and the cross-linker. Later the aforementioned procedure for HPEOP is followed here also.4.4 Characterization
The composition of the solid polymer electrolyte is analyzed by FTIR with the help of a Bruker FTIR spectrophotometer. Micro-Raman spectra of the membranes are recorded using a Renishaw inVia Raman spectrometer at a laser excitation of 514.5 nm.DSC is performed using a Shimadzu DSC-60 (Shimadzu Corporation) setup under a nitrogen atmosphere at a constant heating rate of 10 °C min−1.
4.5 Dynamic mechanical analysis
A dynamic mechanical analyzer (DMA Q800, TA Instruments) is employed for static mechanical testing of the solid electrolyte membranes. The test samples of a specified width (5.30 mm) and thickness (0.09 mm) are mounted using film tension clamps with a clamp compliance of about 0.290 μm N−1. Static tensile tests are performed in controlled force mode with a preload of 0.01 N and a force ramp rate of 0.2 N min−1. The ultimate tensile stress and elongation at break are evaluated for each sample.4.6 Electrochemical characterization
The ionic conductivity of solid electrolyte is evaluated using electrochemical impedance spectroscopy (EIS) on a Biologic SP300 potentiostat, accomplished by applying an AC potential over the frequency range from 100 mHz to 7 MHz with an amplitude of the sine wave ∼50 mV.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Tata Institute of Fundamental Research, Hyderabad for the financial support. The authors also thank Prof. Karthik Chethan V. at BITS Pilani Hyderabad campus for helping with the DSC measurements. The authors thank Mr Naresh Shyaga at the University of Hyderabad, India for helping in SEM measurements.Notes and references
- P. Colomban, Proton Conductors: Solids, Membranes and Gels—materials and Devices. Series: Chemistry of Solid State Materials, Cambridge University Press, Cambridge, UK, 1992 Search PubMed.
- K. D. Kreuer, Chem. Mater., 1996, 8, 610 CrossRef.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637 CrossRef PubMed.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubersa, Chem. Soc. Rev., 2009, 38, 226 RSC.
- L. C. Malavasi, A. J. Fisherb and M. S. Islam, Chem. Soc. Rev., 2010, 39, 4370 RSC.
- J. Peron, Z. Shi and S. Holdcroft, Energy Environ. Sci., 2011, 4, 1575 RSC.
- D. E. Moilanen, D. B. Spry and M. D. Fayer, Langmuir, 2008, 24, 3690 CrossRef PubMed.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535 CrossRef PubMed.
- S. S. Ivanchev, Russ. J. Appl. Chem., 2008, 81, 569 CrossRef.
- T. D. Gierke, G. E. Munn and F. C. Wilson, J. Polym. Sci., Part B: Polym. Phys., 1981, 19, 1687 Search PubMed.
- F. Bauer, S. Denneler and M. Willert-Porada, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 786 CrossRef.
- J. Rozière and D. J. Jones, Annu. Rev. Mater. Res., 2003, 33, 505 CrossRef.
- J. Malis, P. Mazúr, M. Paidar, T. Bystron and K. Bouzek, Int. J. Hydrogen Energy, 2016, 41, 2177 CrossRef.
- X. Meng, H. N. Wang, S. Y. Song and H. J. Zhang, Chem. Soc. Rev., 2017, 46, 464 RSC.
- R. Devanathan, Energy Environ. Sci., 2008, 1, 101 RSC.
- T. Yamada, K. Otsubo, R. Makiura and H. Kitagawa, Chem. Soc. Rev., 2013, 42, 6655 RSC.
- M. Inukai, S. Horike, T. Itakura, R. Shinozaki, N. Ogiwara, D. Umeyama, S. Nagarkar, Y. Nishiyama, M. Malon, A. Hayashi, T. Ohhara, R. Kiyanagi and S. Kitagawa, J. Am. Chem. Soc., 2016, 138, 8505 CrossRef PubMed.
- C. Maxim, S. Ferlay, H. Tokoro, S. I. Ohkoshid and C. Train, Chem. Commun., 2014, 50, 5629 RSC.
- J. M. Taylor, S. Dekura, R. Ikeda and H. Kitagawa, Chem. Mater., 2015, 27, 2286 CrossRef.
- J. M. Taylor, K. W. Dawson and G. K. H. Shimizu, J. Am. Chem. Soc., 2013, 135, 1193 CrossRef PubMed.
- S. Bureekaew, S. Horike, M. Higuchi, M. Mizuno, T. Kawamura, D. Tanaka, N. Yanai and S. Kitagawa, Nat. Mater., 2009, 8, 831 CrossRef PubMed.
- O. Nakamura, T. Kodama, I. Ogino and Y. Miyake, Chem. Lett., 1979, 17 CrossRef.
- G. J. Cao, J. D. Liu, T. T. Zhuang, X. H. Cai and S. T. Zheng, Chem. Commun., 2015, 51, 2048 RSC.
- S. Chandra, T. Kundu, S. Kandambeth, R. Babarao, Y. Marathe, S. M. Kunjir and R. Banerjee, J. Am. Chem. Soc., 2014, 136, 6570 CrossRef PubMed.
- Y. X. Ye, L. Q. Zhang, Q. F. Peng, G. E. Wang, Y. C. Shen, Z. Y. Li, L. H. Wang, X. L. Ma, Q. H. Chen, Z. J. Zhang and S. C. Xiang, J. Am. Chem. Soc., 2015, 137, 913 CrossRef PubMed.
- J. A. Hurd, R. Vaidhanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski and G. K. H. Shimizu, Nat. Chem., 2009, 1, 705 CrossRef PubMed.
- G. K. H. Shimizu, J. M. Taylor and S. Kim, Science, 2013, 341, 354 CrossRef PubMed.
- P. Samanta, A. V. Desai, B. Anothumakkool, M. M. Shirolkar, A. Karmakar, S. Kurungot and S. K. Ghosh, J. Mater. Chem. A, 2017, 5, 13659 RSC.
- W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H. C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126 CrossRef PubMed.
- P. Adelhelm and J. Janek, Handbook of Lithium Ion Batteries, ed. R. Korthauer, Springer, 2013, p. 199 Search PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef PubMed.
- R. Chen, W. Qu, X. Guo, L. Liab and L. Wu, Mater. Horiz., 2016, 3, 487 RSC.
- H. Ben youcef, O. G. Calvo, N. Lago, S. Devaraj and M. Armand, Electrochim. Acta, 2016, 220, 587 CrossRef.
- A. B. Puthirath, S. Patra, S. Pal, M. Manoj, A. P. Balan, S. Jayalekshmi and N. N. Tharangattu, J. Mater. Chem. A, 2017, 5, 11152 RSC.
- W. H. Meyer, Adv. Mater., 1998, 10, 439 CrossRef PubMed.
- D. E. Fenton, J. M. Parker and P. V. Wright, Polymer, 1973, 14, 589 CrossRef.
- P. V. Wright, Br. Polym. J., 1975, 7, 319–327 CrossRef.
- Z. Xue, D. He and X. Xie, J. Mater. Chem. A, 2015, 3, 19218 RSC.
- F. B. Dias, L. Plomp and J. B. Veldhuis, J. Power Sources, 2000, 88, 169 CrossRef.
- E. Quartarone and P. Mustarelli, Chem. Soc. Rev., 2011, 40, 2525 RSC.
- L. R. A. K. Bandara, M. A. K. L. Dissanayake and B. E. Mellander, Electrochim. Acta, 1998, 43, 1447 CrossRef.
- S. Srivastava, J. L. Schaefer, Z. Yang, Z. Tu and L. A. Archer, Adv. Mater., 2014, 26, 201 CrossRef PubMed.
- Z. Wen, T. Itoh, Y. Ichikawa, M. Kubo and O. Yamamoto, Solid State Ionics, 2000, 134, 281 CrossRef.
- K. Murata, S. Izuchi and Y. Yoshihisa, Electrochim. Acta, 2000, 45, 1501 CrossRef.
- G. A. Ludueña, T. D. Kühne and D. Sebastiani, Chem. Mater., 2011, 23, 1424 CrossRef.
- A. Hassanali, F. Giberti, J. Cuny, T. D. Kühne and M. Parrinello, Proc. Natl. Acad. Sci., 2013, 110, 13723 CrossRef PubMed.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544 CrossRef PubMed.
- A. Tomaszewska, E. Zygadlo-Monikowska, Z. Florjanczyk, Abs. 531, 206th Meeting, The Electrochemical Society, Inc, ©, 2004 Search PubMed.
- B. K. Money and J. Swenson, Macromoleculess, 2013, 46, 6949 CrossRef.
- R. D. Lundberg, F. E. Bailey and R. W. Callard, J. Polym. Sci., Part B: Polym. Phys., 1966, 4, 1563 CrossRef.
- D. S. Kim, K. H. Shin, H. B. Park and Y. M. Lee, Macromol. Res., 2004, 12, 413 CrossRef.
- R. Huston and J. N. Butler, J. Phys. Chem., 1968, 72, 4263 CrossRef.
- L. Xiao, S. Zhang, J. Pan, C. Yang, M. He, L. Zhuang and J. Lu, Energy Environ. Sci., 2012, 5, 7869 RSC.
- http://www.fuelcellstore.com .
- A. Genest, D. Portinha, E. Fleury and F. Ganachaud, Prog. Polym. Sci., 2017, 72, 61 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00262b |
| This journal is © The Royal Society of Chemistry 2018 |