A flexible membrane electrode with an electrolyte-affinity surface for energy storage: effects of amphiphilic block copolymers and membrane thickness†
Fen
Ran
 *ab,
Hezhen
Yang
b,
Xiaoning
Zhao
b,
Xiaoqin
Niu
*c,
Yuhong
Chen
c and
Lingbin
Kong
*ab,
Hezhen
Yang
b,
Xiaoning
Zhao
b,
Xiaoqin
Niu
*c,
Yuhong
Chen
c and
Lingbin
Kong
 ab
ab
aState Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: ranfen@163.com; ranfen@lut.cn
bSchool of Material Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, P. R. China
cCollege of Petrochemical Technology, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: niuxiaoqin@163.com
First published on 26th June 2018
Abstract
We fabricate a porous and flexible membrane electrode composed of nano-nickel hydroxide as an electrochemically active material, a polymer as a substrate material, and a copolymer as a modification additive. A series of amphiphilic block copolymers of PAA-b-PAN-b-PAA, F127, PDMC-b-PAN-b-PDMC, and PVP-b-PAN-b-PVP are prepared, and their effects on the thickness, surface structure, and electrochemical performance of the electrode materials are investigated. It is discovered that the hydrophilic chains of the amphiphilic block copolymer contribute a lot to improving the surface properties of electrolyte-affinity. The results indicate that the electrode membrane with a membrane thickness of 60 μm modified by PAA-b-PAN-b-PAA demonstrates the highest specific capacitance of 3090.0 F g−1 at a current density of 0.5 A g−1. An asymmetric supercapacitor based on the fabricated electrode membrane using Ni(OH)2 as the positive active material and activated carbon as the negative electrode obtains a capacitance of 114.1 F g−1 at 0.5 A g−1. The device shows good cycling stability with a capacitance retention of up to 90.7% after 5000 cycles at 1.0 A g−1. The maximum energy density of the asymmetrical supercapacitor reached 40.6 W h kg−1 at a power density of 400.0 W kg−1, and a high power density of 4000.0 W kg−1 was obtained at 23.5 W h kg−1 in 6 M KOH aqueous electrolyte.
1. Introduction
To meet the increasing market demand for portable and multifunctional devices, many efforts have been devoted to developing energy storage devices that are flexible, lightweight, small, and even wearable.1–5 Supercapacitors are recognized as being among the most promising energy storage devices due to their rapid charging/discharging ability, superior power density, long cycle life, and good eco-friendliness.6,7 Therefore, flexible supercapacitors have received wide attention over the past few years.8–10 In general, a flexible supercapacitor device is made up of a polymer electrolyte sandwiched between two flexible electrodes.11–13 As one of the key components, electrode materials have been studied much for obtaining high-performance flexible devices. For example, Yu et al. fabricated a three-dimensional (3D) nitrogen-doped activated nanofiber derived from pyrolyzed bacterial cellulose (A-p-BC-N) that was used as a flexible electrode material. Based on the A-p-BC-N, the assembled flexible all-solid-state supercapacitor device achieved a maximum power density of 390.53 W kg−1 and exhibited good cycling stability of 95.9% after 5000 cycles.14 Li et al. used flexible graphene/MnO2 composite papers as electrodes, and the specific capacitance reached 256 F g−1 at 500 mA g−1.15 Jin et al. reported a novel reduced graphene sheet patched carbon nanotube/MnO2 composite paper as a flexible electrode, and the specific capacitance was 486.6 F g−1.16As is well known, energy density (E) depends on specific capacitance (C) and voltage (V). Hence, an efficient method has been proposed to develop porous and nano-structured electrode materials for improving capacitance.17 A porous and nano-structured configuration can shorten the diffusion path of electrolyte ions, simultaneously increasing the surface area of materials and improving the electrode/electrolyte contact area to improve the performance of supercapacitors.18–20 Meanwhile, Ni(OH)2 is more attractive owing to its high specific capacitance, low lost, and easy synthesis.21–23 For instance, Li et al. synthesized Co3O4/Ni(OH)2 composite mesoporous nanosheet networks grown on a conductive substrate for supercapacitor application, achieving a high specific capacitance of 1144 F g−1 at 5 mV s−1 and long-term cyclability. The electrochemical performance was much better than that of single Co3O4 or Ni(OH)2.24 These advantages can be attributed to porous network structures that favor the diffusion of active species, while a mesoporous structure ensures fast proton transfer and provides a large OH− and ion accessible area. Su and co-workers reported the preparation of amorphous Ni(OH)2@Ni core–shell nanostructures for a pseudocapacitor, which showed a large specific capacitance of 2868 F g−1 at a scan rate of 1 mV s−1 and a specific capacitance of 96% could be retained after 3000 cycles.25 This architecture provides numerous express pathways for fast electron transport and high interfacial area.
In addition, various chemical and structural modifications have been employed to improve the surface properties at the electrode/electrolyte interface and the electrochemical performance of the electrode materials.26–28 According to some reports, bringing in heterogeneous atoms such as N, O, and P and surface oxygenous acid groups such as –OH and –COOH can largely improve the electrolyte-affinity on the electrode material surface.29–35 In our previous work, hydrophilic polymer chains were introduced into the surface of electrode materials.36 This is beneficial to the penetration of electrolyte and to maintaining sufficient contact with the electrochemically active material, and the electrolyte-affinity properties can decrease the ion transport resistance in the charging and discharging process.
Herein, we prepared a porous and flexible membrane electrode with an electrolyte-affinity surface through introducing a series of amphiphilic triblock copolymers,37,38 in which polyethersulfone was used as a substrate material to provide flexibility and Ni(OH)2 was the electrochemically active material. The effects of the amphiphilic block copolymer type and membrane thickness on the surface structure and electrochemical properties of the electrode materials were investigated. An asymmetric supercapacitor was assembled using a Ni(OH)2-based hybrid membrane as the positive electrode and an activated carbon membrane39 as the negative electrode. The device showed an outstanding electrochemical performance with high specific capacitance and energy density, and a long cycle life.
2. Experimental
2.1. Chemicals
Polyether sulfone (PES, Ultrason E6020P) was obtained from BASF, Germany. Activated carbon was purchased from Shenyang Kejing Auto-instrument Co. Ltd. Vinylcyanide (AN), acrylic acid (AA), methacryloyloxyethyl trimethyl ammonium chloride (DMC), and N-vinyl pyrrolidone (NVP) from Sinopharm Chemical Reagent Co. Ltd. were purified by distillation prior to use. NiCl2·6H2O was purchased from Tianjin Beichen Founder Reagent Factory and NH3·H2O was obtained from Tianjin BASF Chemical Co., Ltd. Azo-bis-isobutyronitrile (AIBN) was purchased from Tianjin Tianhe Chemical Reagent Factory, China. A reversible addition–fragmentation chain transfer polymerization (RAFT) agent was synthesized according to the literature.40 Pluronic® F-127 (PEO–PPO–PEO block copolymer) was purchased from Sigma-Aldrich Co. Ltd. All the other chemicals (analytical grade) were obtained from Sinopharm Chemical Reagent Co. Ltd, China, and used without further purification.2.2. Synthesis of nickel hydroxide (Ni(OH)2)
Ni(OH)2 was prepared by a facile improved precipitation method. In a typical procedure, 40 g of NiCl2·6H2O was dissolved in 141 mL of deionized water in a glass beaker with magnetic stirring at room temperature. Then the NiCl2·6H2O solution was slowly adjusted to pH = 9 by dropwise addition of 5 wt% NH3·H2O at a temperature of 20 °C, controlling the addition time to more than 2 h. The resulting suspension was stirred at this temperature for another 3 h. Finally, the solid was filtered and washed with distilled water, and dried at 60 °C for 6 h.2.3. Synthesis of amphiphilic block copolymers
The general procedure for synthesis of polyacrylic acid (PAA) was as follows: AA, the RAFT agent, AIBN, and DMF were added into a tube. After bubbling for 30 min with nitrogen, the reaction mixture was allowed to warm under a nitrogen atmosphere to 75 °C, and the polymerization lasted 4 h. After precipitating in ethyl ether, the product was dried under vacuum at 60 °C overnight.The synthesis of PAA-b-PAN-b-PAA was also carried out in a sealed tube as follows: acrylonitrile (AN), the macro-RAFT agent (PAA), AIBN, and DMF were added into a tube and stirred for 10 min. After bubbling with nitrogen for 30 min the reaction mixture was allowed to warm to 75 °C under a nitrogen atmosphere and polymerization was carried out for 12 h. After precipitation in ethyl ether the product was dried under vacuum at 60 °C overnight. A similar process was used to synthesize PDMC-b-PAN-b-PDMC and PVP-b-PAN-b-PVP.
2.4. Preparation of the electrode membrane
The membrane was prepared by a phase-separation technique. In a typical synthesis, Ni(OH)2 (1.7 g), acetylene black (0.15 g), and conductive graphite (0.15 g) were firstly dispersed in dimethylacetamide (DMAC) under magnetic stirring and ultrasonication to obtain a homogeneous turbid solution. Then, PES (0.45 g) and PAA-b-PAN-b-PAA (0.05 g) were dissolved in the above-prepared solution, which was stirred for 24 h. The casting solution was prepared as a membrane by spin coating at 20 °C, and it was immediately immersed in a coagulation bath of deionized water at the same temperature. Then, the hybrid polymer–nickel hydroxide membrane was transferred to a water bath for 24 h to remove the residual solvent. Finally, the flexible membrane was dried in a vacuum oven at 60 °C for 24 h and named FME-PAA. The FMEs containing the block copolymers F127, PDMC-b-PAN-b-PDMC, and PVP-b-PAN-b-PVP were fabricated in a similar way, and named FME-F127, FME-PDMC, and FME-PVP, respectively. In addition, the hybrid polymer-activated carbon membrane was also prepared under the same conditions as those used for FME-AC. The membrane thicknesses were controlled by changing the concentration of the casting solution or rotation rate during the membrane preparation process.2.5. Materials characterization
The microstructures and morphologies were characterized by field emission scanning electron microscopy (SEM, JSM-6701F, JEOL, Japan). Photographs of the membrane were taken using a camera (PowerShot S100V, Canon, Japan). The surface area was calculated using the Brunauer–Emmett–Teller (BET) equation. The pore size distribution was calculated by the Barrett–Joyner–Halenda (BJH) method using the adsorption branch of the isotherm. The surface chemical composition of the sample was analyzed by X-ray photoelectron spectroscopy (XPS, Physical Electronics UK). 1H nuclear magnetic resonance spectroscopy (1H NMR) was performed in DMSO-d6 with a Bruker Varian (FT-80A) NMR instrument. Fourier transform infrared spectroscopy (FTIR) spectra of KBr tablets were measured with an FTIR Nexus 670 instrument.2.6. Electrode preparation
For the preparation of FME-0, FME-PAA, FME-F127, FME-PDMC, FME-PVP, and FME-AC electrodes, a size of about 1 × 1 cm2 of the membrane was cut out and pressed between two foam nickels (Chang Sha Lyrun New Material Co. Ltd., 90 PPI, 2 mm) of 1.5 × 2 cm size (the nickel foam functions as the current collector) at 10 MPa. The capacitance values were calculated based on the mass of Ni(OH)2 and AC in the membranes, respectively.2.7. Electrochemical characterization
All electrochemical measurements were conducted in a classical three-electrode glass cell setup at room temperature. The synthesized sample, a platinum foil electrode (1.5 × 1.5 cm), and a saturated calomel electrode (SCE) served as the working electrode, counter electrode, and reference electrode, respectively. The electrochemical performance was tested using cyclic voltammetry (CV), galvanostatic charging–discharging (GCD), and electrochemical impedance spectroscopy (EIS) on a CHI660E (Shanghai, China) electrochemical workstation. The cycling stability test was carried out on a Land cell tester.Electrochemical measurements of the asymmetric supercapacitor were carried out in a two-electrode setup, where FME-PAA-60 and FME-AC were used as the positive electrode and negative electrode, respectively. The mass ratio of the positive and negative electrodes (FME-PAA-60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FME-AC) was estimated to be 0.22 from the specific capacitance and potential window obtained from their galvanostatic charging–discharging curves. All of the above electrochemical measurements were performed in 6 M KOH aqueous solution electrolytes.
FME-AC) was estimated to be 0.22 from the specific capacitance and potential window obtained from their galvanostatic charging–discharging curves. All of the above electrochemical measurements were performed in 6 M KOH aqueous solution electrolytes.
| C = I × t/(ΔV × m) | (1) |
For an asymmetric supercapacitor, charge storage on the positive and negative electrodes follows the relationship q+ = q−. To balance the charge storage, the mass matching of the positive and negative electrodes was optimized using the following equation:
| m+/m− = (C− × ΔV−)/(C+ × ΔV+) | (2) |
The energy and power density of the device are calculated from the discharging curves at different current densities using the following equations:
| E = C × V2/(2 × 3.6) | (3) |
| P = E × 3600/(Δt) | (4) |
3. Results and discussion
A series of amphiphilic block copolymers were synthesized via reversible addition–fragmentation chain transfer polymerization (RAFT), in which –PAN was used as hydrophobic chains and –PAA, –PVP, and –PDMC were introduced as hydrophilic chains. The structure illustration and characterization results are shown in Fig. 1. In order to obtain the block structure, the monomers of AN and NVP or AA or DMC were added sequentially, and the obtained amphiphilic block copolymers copolymerized with NVP, AA, and DMC were named PVP-b-PAN-b-PVP, PAA-b-PAN-b-PAA, and PDMC-b-PAN-b-PDMC (Fig. 1a). FTIR data of the macro-RAFT agent of –PAA and block copolymer of PAA-b-PAN-b-PAA are shown in Fig. 1b. The bands at 3440, 1733, and 1252 cm−1 were attributed to the hydroxyl stretching vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and C–O stretching vibration, respectively. All these functional groups came from –PAA chains. After the AN monomer was induced into the macro-RAFT agent of –PAA, a peak at 2245 cm−1 was observed from the sample PAA-b-PAN-b-PAA, which was ascribed to the C
O stretching vibration, and C–O stretching vibration, respectively. All these functional groups came from –PAA chains. After the AN monomer was induced into the macro-RAFT agent of –PAA, a peak at 2245 cm−1 was observed from the sample PAA-b-PAN-b-PAA, which was ascribed to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration. In the 1H NMR spectrum of PAA-b-PAN-b-PAA (Fig. 1c), the characteristic signals at 1.011, 1.446, and 1.698 ppm were ascribed to the AA repeating units, and those at 1.993 and 3.352 ppm were due to the AN repeating units in the block copolymers.
N stretching vibration. In the 1H NMR spectrum of PAA-b-PAN-b-PAA (Fig. 1c), the characteristic signals at 1.011, 1.446, and 1.698 ppm were ascribed to the AA repeating units, and those at 1.993 and 3.352 ppm were due to the AN repeating units in the block copolymers.
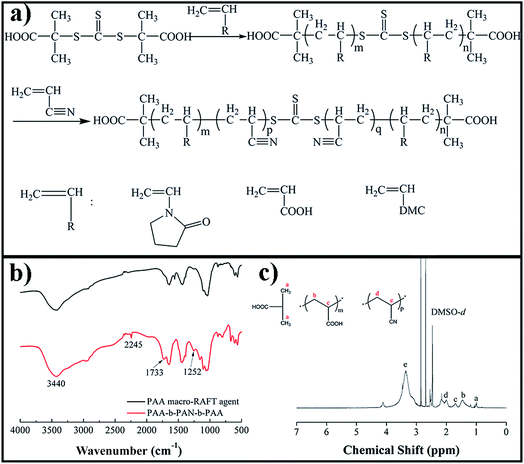 | ||
| Fig. 1 (a) Synthesis approach and characterization of the amphiphilic block copolymers: (b) FTIR and (c) 1H NMR (DMSO-d6). | ||
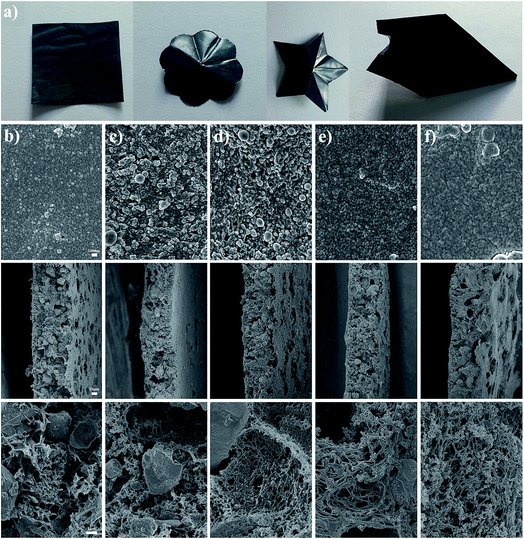
We used the synthesized block copolymers to endow the flexible membrane electrodes (FMEs) with an electrolyte-affinity surface. The FMEs prepared via the phase separation method were smooth and showed outstanding mechanical strength and flexibility ascribed to the use of the membrane substrate material, PES, a kind of engineering plastic. It can be cut into various shapes, such as squares, triangles, pentagrams, circles, or any other shapes, and bent to a large angle, as shown in Fig. 2a. Besides the synthesized block copolymers, commercial F127 was also employed here to fabricate a flexible membrane electrode. The FME prepared without copolymer addition was named FME-0, and those prepared with PAA-b-PAN-b-PAA, F127, PDMC-b-PAN-b-PDMC, and PVP-b-PAN-b-PVP, as mentioned above were named FME-PAA, FME-F127, FME-PDMC, and FME-PVP, respectively. The SEM images of the prepared electrode membranes revealed that all of the electrode membranes exhibited the characteristic morphologies of the polymeric membrane fabricated through liquid to liquid phase separation, and the morphologies of the electrode membranes modified by block copolymers were almost the same as that of the unmodified one. However, there were also some differences among these electrode membranes. For example, the roughness of the surface of the modified FMEs (FME-PAA, FME-F127, FME-PDMC, and FME-PVP) increased compared with that of FME-0. There were many polymer particles enriched on the surface of FMEs attributed to the migration and self-assembly of the block copolymers. From the cross-sectional views of FMEs, it was found that the thickness of the membranes was about 60 μm. It should be noted that the thickness of FMEs could be controlled easily by adjusting the concentration of the casting solution and the speed of spin coating during the fabrication process of the membranes. The thickness of all electrode membranes was controlled at 60 μm. It was interesting to see that the active material of Ni(OH)2 particles was uniformly embedded in the electrode membrane. In the higher magnification images, it could be observed that there were a large number of pores formed in the membrane. The hierarchical porous structure could provide an excellent passageway and a large surface area for enhanced electrode/electrolyte interfacial contact during the electrochemical process.
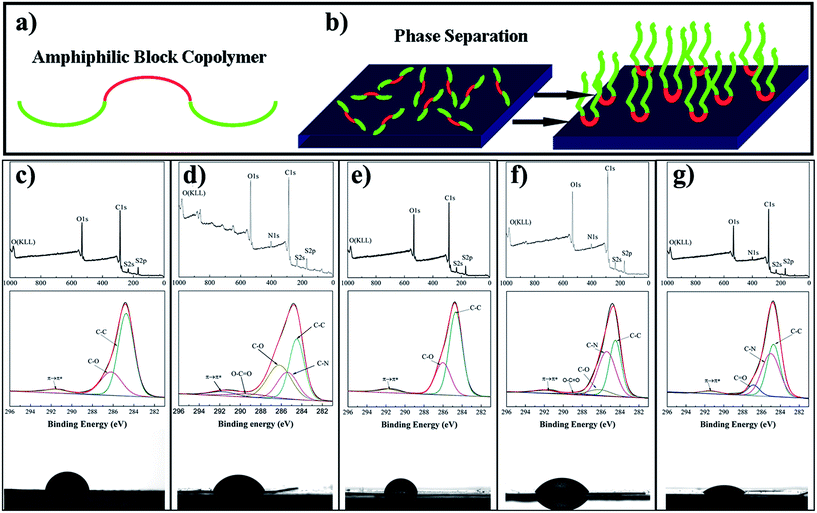
It should be noted that the surface segregation of the amphiphilic block copolymers in the surface of the electrode membrane played an important role in its surface modification and the electrochemical performance. All the copolymers consisted of hydrophobic and hydrophilic components on the macromolecules as presented in Fig. 3a. During the phase separation, the active material of Ni(OH)2 was surrounded by PES macromolecules, and the amphiphilic block copolymers underwent a migration and self-assembly process.36 With the fast solvent exchange of DMAC and water, copolymers migrated to the surface of the membrane, where they, more importantly, self-assembled with the hydrophilic block directed on the surface of the membrane, while the hydrophobic block became embedded in the membrane substrate after the phase separation process, as shown in Fig. 3b. As a result, this kind of membrane would show improved surface affinity due to the hydrophilic chains, and high stability during practical repeated use due to the hydrophobic chains in the block copolymer.
The surface structure and affinity of FMEs were characterized using X-ray photoelectron spectroscopy (XPS) and water contact angle measurements (Fig. 3c–g). There were no N 1s peaks in the full spectrum of FME-0 and FME-F127 owing to the absence of N element. Strong peaks for N 1s were obtained at 403 eV in other FMEs, such as FME-PAA, FME-PDMC, and FME-PVP because the amphiphilic block copolymers included PAN segments with N element. The C 1s spectrum exhibited five contributions by fitting to C–C, C–N, C–O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and π–π* species, which were located at 284.5, 285.4, 286.2, 289.7, and 291.9 eV, respectively.41 The π–π* peak in all FMEs were attributed to the addition of conductive additives (acetylene black and conductive graphite). These results suggested that we successfully prepared flexible membranes containing different amphiphilic block copolymers on the membrane surface. PME-0 showed a large water contact angle (88.4°), and notably, when the amphiphilic block copolymers were assembled on the membrane surface, the values decreased to 68.5, 85, 65.3, and 53.7° for FME-PAA, FME-F127, FME-PDMC, and FME-PVP, respectively. The high contact angles of FME-PAA and FME-F127 may be caused by the surface roughness of the membranes although the functional chains have been grafted and observed from XPS data (the surface roughness of the membranes is shown in Fig. 2c and d).
O, and π–π* species, which were located at 284.5, 285.4, 286.2, 289.7, and 291.9 eV, respectively.41 The π–π* peak in all FMEs were attributed to the addition of conductive additives (acetylene black and conductive graphite). These results suggested that we successfully prepared flexible membranes containing different amphiphilic block copolymers on the membrane surface. PME-0 showed a large water contact angle (88.4°), and notably, when the amphiphilic block copolymers were assembled on the membrane surface, the values decreased to 68.5, 85, 65.3, and 53.7° for FME-PAA, FME-F127, FME-PDMC, and FME-PVP, respectively. The high contact angles of FME-PAA and FME-F127 may be caused by the surface roughness of the membranes although the functional chains have been grafted and observed from XPS data (the surface roughness of the membranes is shown in Fig. 2c and d).
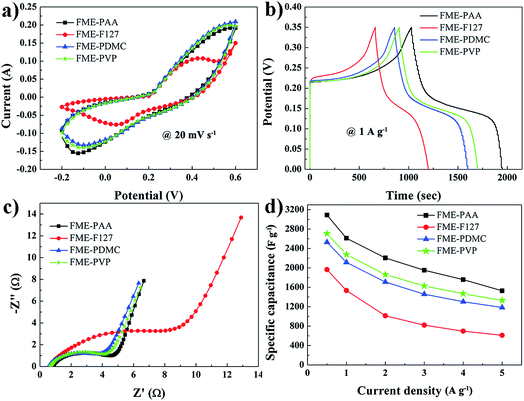
Fig. 4 displays the electrochemical properties of the fabricated flexible membrane electrodes with different amphiphilic block copolymers in 6 mol L−1 KOH aqueous electrolyte. The CV measurements of all four samples were investigated within a potential window of −0.2–0.6 V at a scan rate of 20 mV s−1.42,43 A pair of obvious redox peaks was observed in the CV curves of all samples, exhibiting the pseudocapacitive mechanism of the electrode materials arising from Ni(OH)2 (Fig. 4a), corresponding to the reversible redox reaction of Ni(II) ↔ Ni(III). This reaction can be elaborated as Ni(OH)2 + OH− ↔ NiOOH + H2O + e−, which involves the intercalation and deintercalation of protons.44 The galvanostatic charging–discharging (GCD) curves of all samples at a current density of 1 A g−1 were acquired to explain their specific capacitance (Fig. 4b). From the GCD results, all electrodes had charging–discharging platforms, exhibiting a typical pseudocapacitive behavior, attributed to the characteristics of Ni(OH)2. The specific capacitances were calculated using the equation C = I × t/(ΔV × m), where C (F g−1) is the specific capacitance, I (A) is the discharging current, t (s) is the discharging time, ΔV (V) is the potential drop during the discharging process, and m (g) is the mass of the active material. The specific capacitance of FME-PAA, FME-F127, FME-PDMC, and FME-PVP were 2611.4, 1533.4, 2113.1, and 2275.1 F g−1, respectively. Moreover, there was no obvious voltage drop, demonstrating that all samples had small internal resistances. Electrochemical impedance spectroscopy (EIS) is an efficient technique to study the charge transport process of different electrode materials. The data generated by EIS can be described as a Nyquist plot constituting three regions, a semicircle in the high frequency range, a high-to-medium frequency region and a nearly vertical line along the imaginary axis at low frequency, representing the interface resistance, charge transfer resistance, and capacitive behavior, respectively.45,46 The Nyquist plot of FME-PAA showed a smaller semicircle compared with the other plots, thus identifying a smaller interface resistance and better electrical conductivity (Fig. 4c). The charge transfer resistance was related to the good interaction between the electrode and the electrolyte at their interface. Based on the excellent surface affinity of FME-PAA (Fig. S1†), from the dynamic contact angles for FME-PAA-60, it can be inferred that the electrode membrane of FME-PAA was well wetted by the electrolyte in the electrochemical process, implying a better surface affinity of FME-PAA. Furthermore, the specific capacitances versus current densities were estimated based on the charging/discharging tests (Fig. 4d). It was clearly seen that the specific capacitance of FME-PAA was higher than that of the other three samples at various current densities, and its maximum specific capacitance reached 3090.0 F g−1 at a current density of 0.5 A g−1. Its capacitance remained at 1528.6 F g−1 when the current density was increased to 5 A g−1, implying a capacitance retention of 49.5%, far higher than that of FME-F127, FEM-PDMC, and FME-PVP (31.1, 46.8, and 49.1%, respectively), confirming its good rate capability.
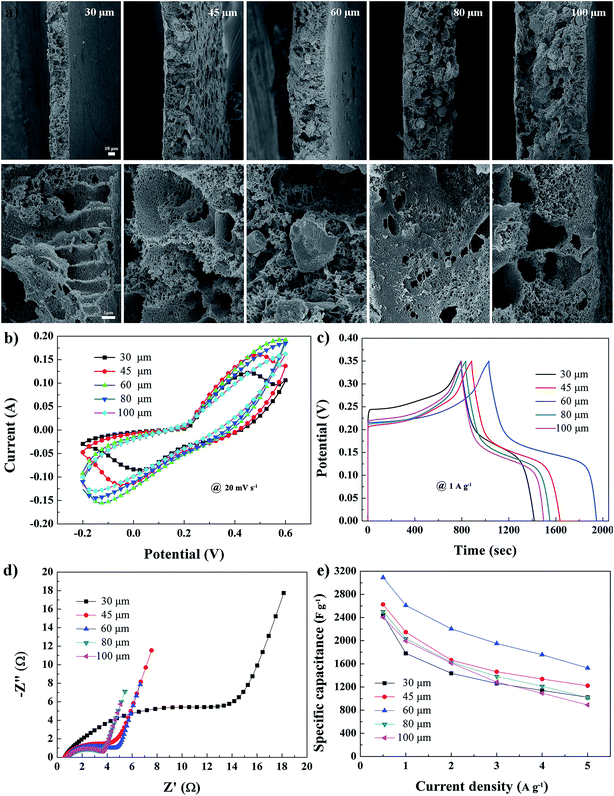
The electrochemical performance of the electrode membrane also depended on its thickness. Therefore, we fabricated FME-PAAs with various thicknesses, and the electrode membranes with thicknesses of 30, 45, 60, 80, and 100 μm were termed FME-AA-30, FME-AA-45, FME-AA-60, FME-AA-80, and FME-AA-100, respectively. From the SEM morphologies shown in Fig. 5a, one can see that the shape and morphology of these membranes were similar but not the thickness. Ni(OH)2 particles were dispersed homogeneously in FME-PAAs, and abundant pore structures were formed. In contrast, BET analyses of FME-PAAs with different thicknesses were also performed (Fig. S2†). The N2 adsorption–desorption isotherms of all samples showed a type-IV isotherm, indicating the existence of mesopores and macropores within the materials. The pore size distribution was calculated by the BJH method, which revealed a wide pore size distribution from micropores to macropores. These pores provided numerous express pathways for fast electron transport and high interfacial area. The CV curves of FME-PAAs at a scan rate of 20 mV s−1 exhibited distinctive redox peaks within the potential window from −0.2 to 0.6 V, which revealed the pseudocapacitive feature of the electrode materials (Fig. 5b). One can also see that the area under the FME-PAA-60 curve was larger than that of the other samples, illustrating a higher specific capacitance. Accurate specific capacitances were calculated from GCD analysis at 1 A g−1 (Fig. 5c), and the specific capacitances of the electrode membranes with thicknesses of 30, 45, 60, 80 and 100 μm ranged from 1781.4, 2147.4, 2611.4, and 2037.7 to 1997.1 F g−1, respectively. From the Nyquist plot (Fig. 5d), it was deduced that all samples had a similar diffusion impedance, with charge transfer impedance showing a gradually decreasing trend with the increase of thickness. Based on the above analysis, it was demonstrated that membrane thickness had an obvious influence on the electrochemical performance. Although both FME-PAA-80 and FME-PAA-100 had smaller charge transfer impedance than FME-PAA-60, their specific capacitance was lower. This suggested that when membrane thickness approaches a certain level, it can produce an effect on the utilization of active materials in the membrane. Fig. 5e shows the specific capacitance at different current densities. Obviously, FME-PAA-60 exhibited better specific capacitance compared with the other electrodes at various current densities. Detailed electrochemical performance of FME-PAA-60 is illustrated in Fig. S3.† The maximum specific capacitance calculated from GCD tests was 3090.0 F g−1 at a current density of 0.5 A g−1 and the capacitance retention was 77.3% after 5000 cycles. Besides, the CV curves of FME-PAA-60 at different bending states were also tested (Fig. S4†). No great changes from the curves can be found, which indicated good flexibility at all bending states. Hence, we used FME-PAA-60 as a positive electrode to assemble the asymmetric supercapacitor device as follows.
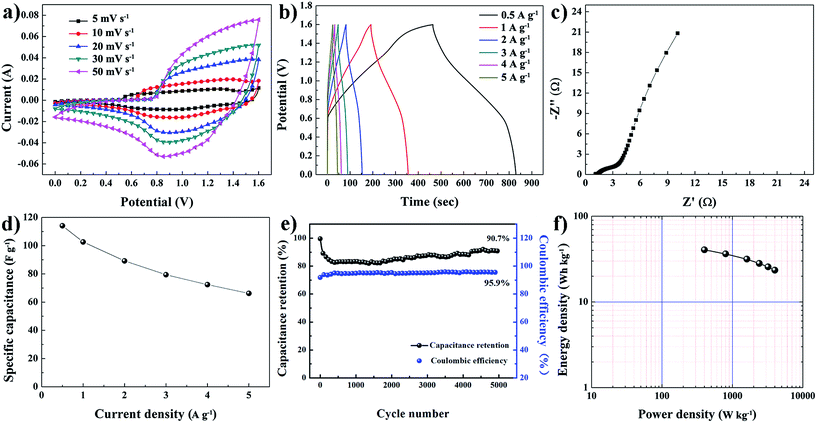
The asymmetric supercapacitor device was assembled using the FME-PAA-60 membrane as the positive electrode, and the similar membrane electrode of activated carbon (FME-AC) as the negative electrode in 6 M KOH electrolyte. The optimized mass ratio of the active materials in the positive and negative electrodes was 0.22 (FME-PAA-60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FME-AC). The electrochemical performance of this device, FME-PAA-60‖FME-AC, is exhibited in Fig. 6. The CV curves of the device exhibited a pair of redox peaks within the potential window from 0 to 1.6 V at various scan rates, implying that the pseudocapacitive feature was caused by the positive electrode (Fig. 6a). It should be noted that the specific capacitance of the device was mainly attributed to the positive electrode. GCD curves of the device at different current densities are shown in Fig. 6b. All the charging and discharging curves were nearly symmetrical, confirming good electrochemical reversibility. The calculated specific capacitances of the device based on the mass of the active materials at 0.5, 1, 2, 3, 4 and 5 A g−1 were 114.1, 101.6, 89.2, 79.4, 72.4 and 66.2 F g−1, further indicating a good fast charging–discharging performance. The EIS curve demonstrated a small semicircle diameter and short Warburg impedance, suggesting its low ionic resistance and high conductivity (Fig. 6c). Fig. 6d shows the rate capability of the ASC device at various current densities from 0.5 to 5 A g−1. A high specific capacitance of 114.1 F g−1 was obtained at 0.5 A g−1, while its capacitance reached 66.2 F g−1 when the current density was increased to 5 A g−1, implying a capacitance retention of 58.0 %. The cycling stability and coulombic efficiency of the asymmetric supercapacitor were further investigated at a current density of 1 A g−1 (Fig. 6e). After 5000 charging–discharging cycles, the capacitance retention of our FME-PAA-60‖FME-AC asymmetric supercapacitor remained at 90.7%, demonstrating an excellent cycling stability. And the coulombic efficiency remained at about 95.9%, which indicated an excellent electrochemical reversibility. Fig. 6f presents the Ragone plot (energy density vs. power density) of the device derived from its discharging curves at various charging–discharging currents, and we found that the maximum energy density of the device reached 40.6 W h kg−1 at a power density of 400.0 W kg−1, and a maximum power density of 4000.0 W kg−1 was obtained at 23.5 W h kg−1. This value was significantly higher than those of asymmetric supercapacitors based on Ni(OH)2 reported previously (Table S1†), such as CNT/Ni(OH)2‖rGO (35.0 W h kg−1 at 1800 W kg−1),47 Ni(OH)2/AC/CNT‖AC (32.3 W h kg−1 at 504.8 W kg−1),48 Co3O4@Ni(OH)2‖AC (40.0 W h kg−1 at 346.9 W kg−1),49 and Ni(OH)2@3D Ni‖AC (21.8 W h kg−1 at 660 W kg−1).25 These results demonstrate that our asymmetric supercapacitor exhibits good electrochemical performance and it is likely to provide new opportunities for developing high-performance flexible supercapacitor electrodes.
FME-AC). The electrochemical performance of this device, FME-PAA-60‖FME-AC, is exhibited in Fig. 6. The CV curves of the device exhibited a pair of redox peaks within the potential window from 0 to 1.6 V at various scan rates, implying that the pseudocapacitive feature was caused by the positive electrode (Fig. 6a). It should be noted that the specific capacitance of the device was mainly attributed to the positive electrode. GCD curves of the device at different current densities are shown in Fig. 6b. All the charging and discharging curves were nearly symmetrical, confirming good electrochemical reversibility. The calculated specific capacitances of the device based on the mass of the active materials at 0.5, 1, 2, 3, 4 and 5 A g−1 were 114.1, 101.6, 89.2, 79.4, 72.4 and 66.2 F g−1, further indicating a good fast charging–discharging performance. The EIS curve demonstrated a small semicircle diameter and short Warburg impedance, suggesting its low ionic resistance and high conductivity (Fig. 6c). Fig. 6d shows the rate capability of the ASC device at various current densities from 0.5 to 5 A g−1. A high specific capacitance of 114.1 F g−1 was obtained at 0.5 A g−1, while its capacitance reached 66.2 F g−1 when the current density was increased to 5 A g−1, implying a capacitance retention of 58.0 %. The cycling stability and coulombic efficiency of the asymmetric supercapacitor were further investigated at a current density of 1 A g−1 (Fig. 6e). After 5000 charging–discharging cycles, the capacitance retention of our FME-PAA-60‖FME-AC asymmetric supercapacitor remained at 90.7%, demonstrating an excellent cycling stability. And the coulombic efficiency remained at about 95.9%, which indicated an excellent electrochemical reversibility. Fig. 6f presents the Ragone plot (energy density vs. power density) of the device derived from its discharging curves at various charging–discharging currents, and we found that the maximum energy density of the device reached 40.6 W h kg−1 at a power density of 400.0 W kg−1, and a maximum power density of 4000.0 W kg−1 was obtained at 23.5 W h kg−1. This value was significantly higher than those of asymmetric supercapacitors based on Ni(OH)2 reported previously (Table S1†), such as CNT/Ni(OH)2‖rGO (35.0 W h kg−1 at 1800 W kg−1),47 Ni(OH)2/AC/CNT‖AC (32.3 W h kg−1 at 504.8 W kg−1),48 Co3O4@Ni(OH)2‖AC (40.0 W h kg−1 at 346.9 W kg−1),49 and Ni(OH)2@3D Ni‖AC (21.8 W h kg−1 at 660 W kg−1).25 These results demonstrate that our asymmetric supercapacitor exhibits good electrochemical performance and it is likely to provide new opportunities for developing high-performance flexible supercapacitor electrodes.
4. Conclusions
In summary, we have successfully developed a porous flexible nickel hydroxide membrane electrode containing an amphiphilic block copolymer by a phase separation method (FME-PAA-60), which displayed outstanding flexibility and excellent electrochemical behavior. The FME-PAA-60‖FME-AC asymmetric supercapacitor showed a maximum energy density of 40.6 W h kg−1 and a high power density of 4000.0 W kg−1. Moreover, the device exhibited a high capacitance retention of 90.7% after 5000 charging–discharging cycles at 1.0 A g−1. This excellent electrochemical performance of the asymmetric supercapacitor should be ascribed to the porous structure, good conductivity, and favorable electrolyte-affinity of the electrode membrane prepared by a liquid–liquid phase-separation method and the migration and self-assembly of the amphiphilic block copolymer. Our work provided a good candidate for developing a flexible supercapacitor electrode material by a feasible, low-cost, and safe way.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (51203071, 51363014, 51463012, and 51763014), the China Postdoctoral Science Foundation (2014M552509 and 2015T81064), the Natural Science Funds of Gansu Province (1506RJZA098), and the Program for Hongliu Distinguished Young Scholars in Lanzhou University of Technology (J201402).References
- X. Dong, Z. Guo and Y. Song, et al., Flexible and Wire-shaped micro-supercapacitor based on Ni(OH)2-nanowire and ordered mesoporous carbon electrodes, Adv. Funct. Mater., 2014, 24(22), 3405–3412 CrossRef.
- Y. He, W. Chen and X. Li, et al., Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes, ACS Nano, 2013, 7(1), 174 CrossRef PubMed.
- L. Li, K. S. Hui and K. N. Hui, et al., High-performance solid-state flexible supercapacitor based on reduced graphene oxide/hierarchical core-shell Ag nanowire@NiAl layered double hydroxide film electrode, Chem. Eng. J., 2018, 348, 338–349 CrossRef.
- Y. Huang, M. Zhong and Y. Huang, et al., Multifunctional energy storage and conversion devices, Adv. Mater., 2016, 28(38), 8344–8364 CrossRef PubMed.
- H. Li, C. Han and Y. Huang, et al., An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte, Energy Environ. Sci., 2018, 11(4), 941–951 RSC.
- Y. Yang, L. Zhao and K. Shen, et al., Ultra-small vanadium nitride quantum dots embedded in porous carbon as high performance electrode materials for capacitive energy storage, J. Power Sources, 2016, 333, 61–71 CrossRef.
- Y. Wang, Y. Song and Y. Xia, Electrochemical capacitors: mechanism, materials, systems, characterization and applications, Chem. Soc. Rev., 2016, 45(21), 5925–5950 RSC.
- K. Wang, X. Zhang and C. Li, et al., Chemically crosslinked hydrogel film leads to integrated flexible supercapacitors with superior performance, Adv. Mater., 2015, 27(45), 7451 CrossRef PubMed.
- M. Zhong, Y. Huang and Y. Huang, et al., A highly durable, transferable, and substrate-versatile high-performance all-polymer micro-supercapacitor with plug-and-play function, Adv. Mater., 2017, 29(16), 1605137 CrossRef PubMed.
- Y. Huang, M. Zhong and Y. Huang, et al., A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte, Nat. Commun., 2015, 6, 10310 CrossRef PubMed.
- L. Zang, Q. Liu and J. Qiu, et al., Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel, ACS Appl. Mater. Interfaces, 2017, 9(39), 33941–33947 CrossRef PubMed.
- D. Zhao, C. Chen and Q. Zhang, et al., High performance, flexible, solid-state supercapacitors based on a renewable and biodegradable mesoporous cellulose membrane, Adv. Energy Mater., 2017, 7(18), 1700739 CrossRef.
- Y. Zhu, N. Li and T. Lv, et al., Ag-doped PEDOT:PSS/CNT composites for thin-film all-solid-state supercapacitors with a stretchability of 480%, J. Mater. Chem. A, 2018, 6(3), 941–947 RSC.
- L. F. Chen, Z. H. Huang and H. W. Liang, et al., Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose, Energy Environ. Sci., 2013, 6(11), 3331–3338 RSC.
- Z. Li, Y. Mi and X. Liu, et al., Flexible graphene/MnO2 composite papers for supercapacitor electrodes, J. Mater. Chem., 2011, 21(38), 14706–14711 RSC.
- Y. Jin, H. Chen and M. Chen, et al., Graphene-patched CNT/MnO2 nanocomposite papers for the electrode of high-performance flexible asymmetric supercapacitors, ACS Appl. Mater. Interfaces, 2013, 5(8), 3408–3416 CrossRef PubMed.
- T. Lv, M. Liu and D. Zhu, et al., Nanocarbon-based materials for flexible all-solid-state supercapacitors, Adv. Mater., 2018, 30(17), 1705489 CrossRef PubMed.
- J. Ji, L. L. Zhang and H. Ji, et al., Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor, ACS Nano, 2013, 7(7), 6237–6243 CrossRef PubMed.
- Y. Tan, Y. Liu and L. Kong, et al., Supercapacitor electrode of nano-Co3O4 decorated with gold nanoparticles via in situ reduction method, J. Power Sources, 2017, 363, 1–8 CrossRef.
- M. Shi, M. Cui and L. Kang, et al., Porous Ni3(NO3)2(OH)4 nano-sheets for supercapacitors: Facile synthesis and excellent rate performance at high mass loadings, Appl. Surf. Sci., 2018, 427, 678–686 CrossRef.
- Z. Tang, C. H. Tang and H. Gong, A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes, Adv. Funct. Mater., 2012, 22(6), 1272–1278 CrossRef.
- J. Li, W. Zhao and F. Huang, et al., Single-crystalline Ni(OH)2 and NiO nanoplatelet arrays as supercapacitor electrodes, Nanoscale, 2011, 3(12), 5103–5109 RSC.
- L. Wang, X. Li and T. Guo, et al., Three-dimensional Ni(OH)2 nanoflakes/graphene/nickel foam electrode with high rate capability for supercapacitor applications, Int. J. Hydrogen Energy, 2014, 39(15), 7876–7884 CrossRef.
- J. H. Zhong, A. L. Wang and G. R. Li, et al., Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications, J. Mater. Chem., 2012, 22(12), 5656 RSC.
- Y. Z. Su, K. Xiao and N. Li, et al., Amorphous Ni(OH)2@three-dimensional Ni core–shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors, J. Mater. Chem. A, 2014, 2(34), 13845–13853 RSC.
- A. T. Chidembo, K. I. Ozoemena and B. O. Agboola, et al., Nickel(II) tetra-aminophthalocyanine modified MWCNTs as potential nanocomposite materials for the development of supercapacitors, Energy Environ. Sci., 2010, 3(2), 228–236 RSC.
- A. Faye, G. Dione and M. M. Dieng, et al., Usefulness of a composite electrode with a carbon surface modified by electrosynthesized polypyrrole for supercapacitor applications, J. Appl. Electrochem., 2010, 40(11), 1925–1931 CrossRef.
- Z. Wang, Y. Tan and Y. Liu, et al., New amphiphilic block copolymer-modified electrodes for supercapacitors, New J. Chem., 2018, 42(2), 1290–1299 RSC.
- J. N. Kim, M. K. Choi and R. Ryoo, Synthesis of mesoporous carbons with controllable N-content and their supercapacitor properties, Bull. Korean Chem. Soc., 2008, 29(2), 413–416 CrossRef.
- R. P. Rietra, T. Hiemstra and W. H. van Riemsdijk, Electrolyte anion affinity and its effect on oxyanion adsorption on goethite, J. Colloid Interface Sci., 2000, 229(1), 199–206 CrossRef PubMed.
- Y. Deng, Y. Xie and K. Zou, et al., Review on recent advances in nitrogen-doped carbons: preparations and applications in supercapacitors, J. Mater. Chem. A, 2016, 4(4), 1144–1173 RSC.
- M. P. Bichat, E. Raymundo-Piñero and F. Béguin, High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte, Carbon, 2010, 48(15), 4351–4361 CrossRef.
- J. Zhao, H. Lai and Z. Lyu, et al., Hydrophilic Hierarchical Nitrogen-Doped Carbon Nanocages for Ultrahigh Supercapacitive Performance, Adv. Mater., 2015, 27(23), 3541–3545 CrossRef PubMed.
- F. Ran, Z. Wang and Y. Yang, et al., Nano vanadium nitride incorporated onto interconnected porous carbon via the method of surface-initiated electrochemical mediated ATRP and heat-treatment approach for supercapacitors, Electrochim. Acta, 2017, 258, 405–413 CrossRef.
- Y. Wu and F. Ran, Vanadium nitride quantum dot/nitrogen-doped microporous carbon nanofibers electrode for high-performance supercapacitors, J. Power Sources, 2017, 344, 1–10 CrossRef.
- X. Zhao, Y. Yang and J. Wu, et al., A polymer-supported electrolyte-affinity hybrid membrane and modification of the amphiphilic block copolymer for use as a super-high flexible and high-performance supercapacitor, Sustainable Energy Fuels, 2017, 1(5), 1074–1081 RSC.
- F. Ran, S. Nie and W. Zhao, et al., Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone), Acta Biomater., 2011, 7(9), 3370 CrossRef PubMed.
- F. Ran, X. Niu and H. Song, et al., Toward a highly hemocompatible membrane for blood purification via a physical blend of miscible comb-like amphiphilic copolymers, Biomater. Sci., 2014, 2(4), 538–547 RSC.
- X. Zhao, F. Ran and K. Shen, et al., Facile fabrication of ultrathin hybrid membrane for highly flexible supercapacitors via in situ phase separation of polyethersulfone, J. Power Sources, 2016, 329, 104–114 CrossRef.
- J. T. Lai, A. Debby Filla and R. Shea, Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents, Macromolecules, 2002, 35(18), 6754–6756 CrossRef.
- H. L. Fan, F. Ran and X. X. Zhang, et al., Easy fabrication and high electrochemical capacitive performance of hierarchical porous carbon by a method combining liquid-liquid phase separation and pyrolysis process, Electrochim. Acta, 2014, 138(25), 367–375 CrossRef.
- Q. Ke, M. Zheng and H. Liu, et al., 3D TiO2@Ni(OH)2 Core-shell arrays with tunable nanostructure for hybrid supercapacitor application, Sci. Rep., 2015, 5, 13940 CrossRef PubMed.
- K. Lu, J. Zhang and Y. Wang, et al., Interfacial deposition of three-dimensional nickel hydroxide nanosheet-graphene aerogel on Ni wire for flexible fiber asymmetric supercapacitors, ACS Sustainable Chem. Eng., 2017, 5(1), 821–827 CrossRef.
- K.-W. Nam, K.-H. Kim and E.-S. Lee, et al., Pseudocapacitive properties of electrochemically prepared nickel oxides on 3-dimensional carbon nanotube film substrates, J. Power Sources, 2008, 182(2), 642–652 CrossRef.
- B. Y. Chang and S. M. Park, Electrochemical impedance spectroscopy, Annu. Rev. Anal. Chem., 2010, 3(1), 207 CrossRef PubMed.
- C. Arbizzani, M. Mastragostino and L. Meneghello, Characterization by impedance spectroscopy of a polymer-based supercapacitor, Electrochim. Acta, 1995, 40(13), 2223–2228 CrossRef.
- R. R. Salunkhe, J. Lin and V. Malgras, et al., Large-scale synthesis of coaxial carbon nanotube/Ni(OH)2 composites for asymmetric supercapacitor application, Nano Energy, 2015, 11(59), 211–218 CrossRef.
- L. Sui, S. Tang and Y. Chen, et al., An asymmetric supercapacitor with good electrochemical performances based on Ni(OH)2/AC/CNT and AC, Electrochim. Acta, 2015, 182, 1159–1165 CrossRef.
- X. Bai, Q. Liu and J. Liu, et al., Hierarchical Co3O4@Ni(OH)2 core-shell nanosheet arrays for isolated all-solid state supercapacitor electrodes with superior electrochemical performance, Chem. Eng. J., 2017, 315, 35–45 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00223a |
| This journal is © The Royal Society of Chemistry 2018 |